Abstract
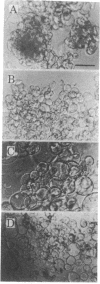
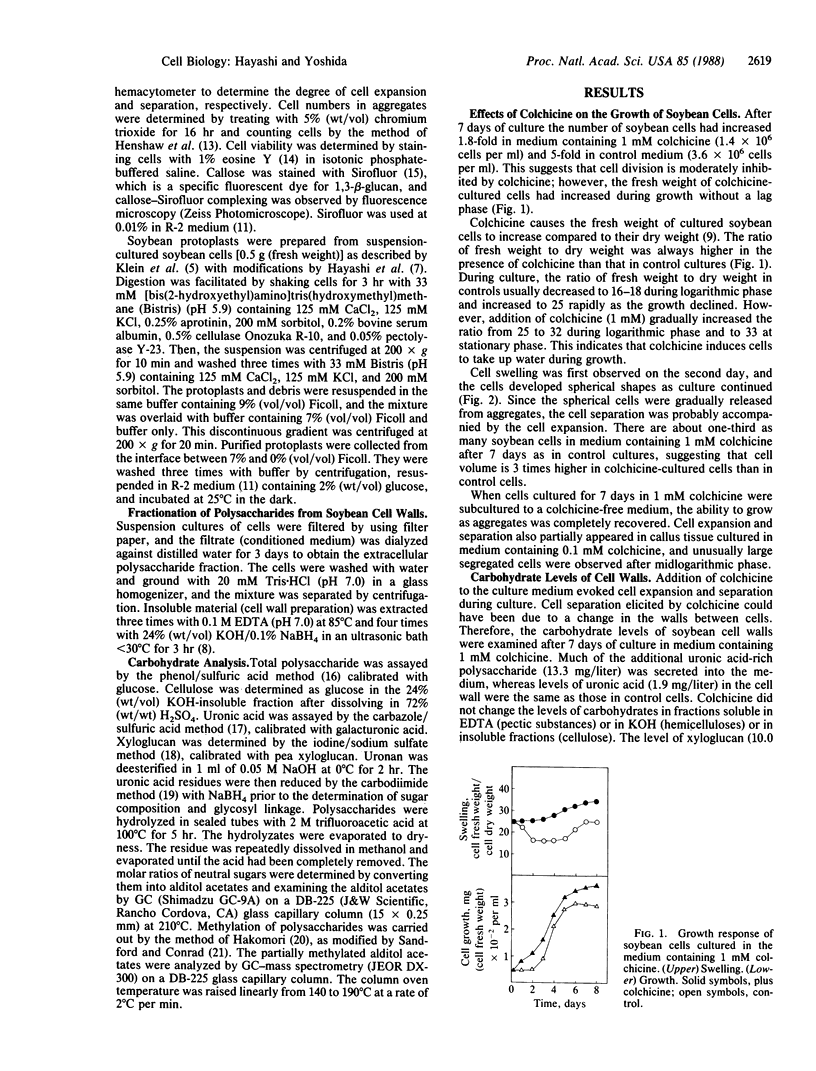
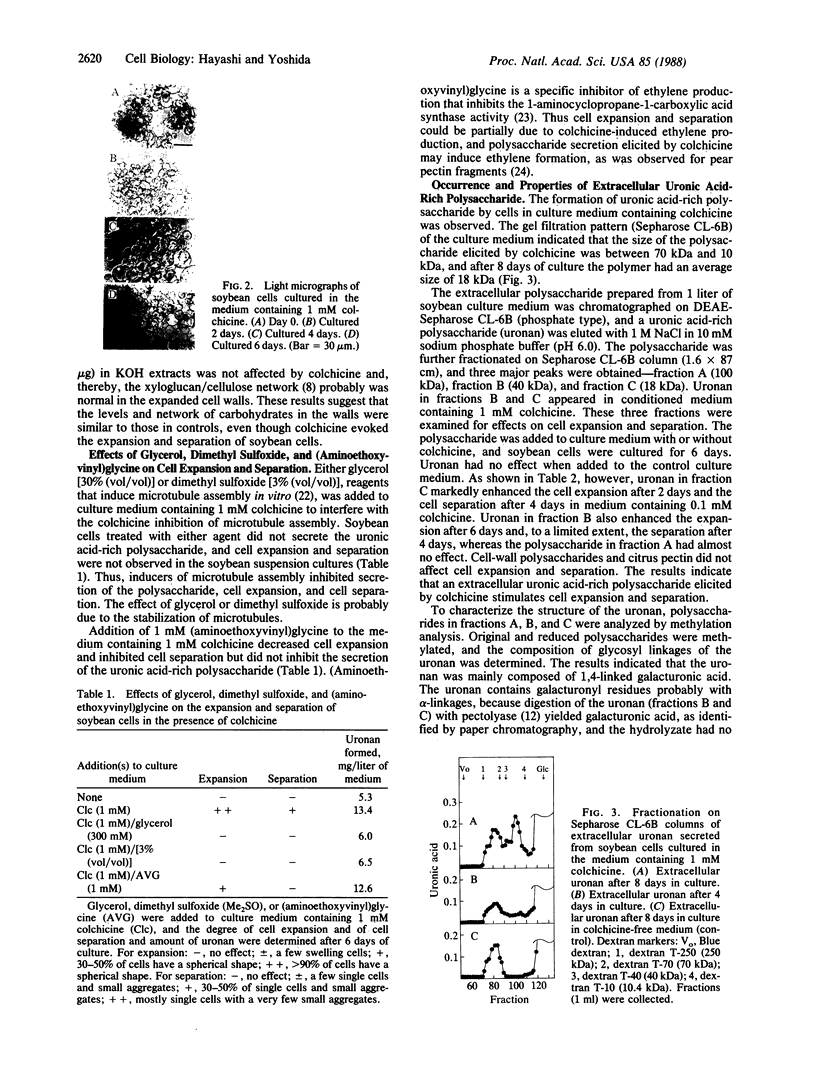
Single plant cells have been obtained without the preparation of protoplasts by culturing pieces of soybean callus tissue with colchicine. Cell expansion and separation were evoked by colchicine (1 mM) within a week of culture. Microscopic observation showed that cells took on a spherical shape in the presence of colchicine and then separated into single cells. Addition of colchicine to the culture medium did not affect the composition of cell wall polysaccharides, but a uronic acid-rich extracellular polysaccharide appeared during cell expansion and separation. Addition of microtubule stabilizers, glycerol (300 mM) or dimethyl sulfoxide [3% (vol/vol)], inhibited the secretion of the polysaccharide as well as cell expansion and separation. The extracellular polysaccharide elicited by colchicine was isolated by ion-exchange chromatography on DEAE-Sepharose and gel filtration on Sepharose CL-6B from the conditioned medium of colchicine-treated soybean cells. The purified 18-kDa polysaccharide immediately enhanced cell expansion and separation when added to soybean callus tissue cultured in medium containing colchicine, even at low concentrations (0.1 mM). The polysaccharide was composed of galacturonic acid and, after digestion with a pectinase preparation, had no effect on the cells. Methylation analysis suggests that the polysaccharide consists of ≈100 sequential α-1,4-galacturonic acids. The galacturonan increased the viability of separated cells cultured in medium containing colchicine, and the single cells obtained did not produce a wound-response callose. (Aminoethoxyvinyl)glycine, a specific inhibitor of ethylene production, extensively decreased the cell expansion and separation but did not inhibit the formation of the extracellular polysaccharide, suggesting that the polysaccharide may exert its effect by stimulating ethylene production.
Keywords: single cells, galacturonan, wound response
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- Hahne G., Hoffmann F. Dimethyl sulfoxide can initiate cell divisions of arrested callus protoplasts by promoting cortical microtubule assembly. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5449–5453. doi: 10.1073/pnas.81.17.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Pea Xyloglucan and Cellulose : III. Metabolism during Lateral Expansion of Pea Epicotyl Cells. Plant Physiol. 1984 Nov;76(3):739–742. doi: 10.1104/pp.76.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Pea xyloglucan and cellulose : I. Macromolecular organization. Plant Physiol. 1984 Jul;75(3):596–604. doi: 10.1104/pp.75.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Polonenko D. R., Camirand A., Maclachlan G. Pea Xyloglucan and Cellulose : IV. Assembly of beta-Glucans by Pea Protoplasts. Plant Physiol. 1986 Sep;82(1):301–306. doi: 10.1104/pp.82.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes R. H., Burton P. R., Gaito J. M. Dimethyl sulfoxide-induced self-assembly of tubulin lacking associated proteins. J Biol Chem. 1977 Sep 10;252(17):6222–6228. [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Tong C. B., Labavitch J. M., Yang S. F. The induction of ethylene production from pear cell culture by cell wall fragments. Plant Physiol. 1986 Jul;81(3):929–930. doi: 10.1104/pp.81.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Proteinase inhibitor I accumulation in tomato suspension cultures : induction by plant and fungal cell wall fragments and an extracellular polysaccharide secreted into the medium. Plant Physiol. 1986 Jan;80(1):68–71. doi: 10.1104/pp.80.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]