Abstract
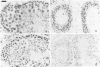
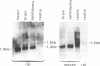
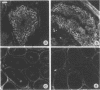
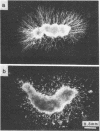
In situ hybridization using beta-nerve growth factor (NGF) DNA probes was used to demonstrate NGF mRNA in spermatocytes and early spermatids of adult mouse. NGF mRNA-containing cells were also identified in the epithelium of convoluted ducts in mouse corpus epididymidis. Blot-hybridization analysis of RNA prepared from mouse testis and epididymis as well as from rat epididymis confirmed the presence of a 1.3-kilobase (kb) NGF mRNA in these tissues. In the rat testis, however, only a 1.5-kb NGF mRNA was found, corresponding in size to a minor NGF mRNA detected in the rat brain, heart, and epididymis. By using affinity-purified anti-NGF antibodies, NGF-like immunoreactivity was observed in germ cells of rat and mouse testis and in the lumen of epididymis. Extracts of both mouse epididymis and testis stimulated fiber outgrowth in cultured sympathetic ganglia, and the effect was blocked by antibodies to mouse NGF. A two-site enzyme immunoassay showed the presence of 10 and 70 ng of NGF per g of tissue in the mouse testis and epididymis, respectively. Furthermore, RNA blot analysis showed the presence of mRNA for the NGF receptor in mouse testis. These results suggest a nonneurotrophic role for NGF in the male reproductive system, possibly in survival maturation and/or motility of spermatozoa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acott T. S., Hoskins D. D. Bovine sperm forward motility protein: binding to epididymal spermatozoa. Biol Reprod. 1981 Mar;24(2):234–240. doi: 10.1095/biolreprod24.2.234. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Bloch B., Milner R. J., Baird A., Gubler U., Reymond C., Bohlen P., le Guellec D., Bloom F. E. Detection of the messenger RNA coding for preproenkephalin A in bovine adrenal by in situ hybridization. Regul Pept. 1984 Jul;8(4):345–354. doi: 10.1016/0167-0115(84)90045-4. [DOI] [PubMed] [Google Scholar]
- Brandt H., Acott T. S., Johnson D. J., Hoskins D. D. Evidence for an epididymal origin of bovine sperm forward motility protein. Biol Reprod. 1978 Nov;19(4):830–835. doi: 10.1095/biolreprod19.4.830. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chapman C. A., Banks B. E., Carstairs J. R., Pearce F. L., Vernon C. A. The preparation of nerve growth factor from the prostate of the guinea-pig and isolation of immunogenically pure material from the mouse submandibular gland. FEBS Lett. 1979 Sep 15;105(2):341–344. doi: 10.1016/0014-5793(79)80644-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A. The level of nerve growth factor (NGF) as a function of innervation. A correlation radio-immunoassay and bioassay study of the rat iris. Exp Cell Res. 1983 Oct 15;148(2):311–317. doi: 10.1016/0014-4827(83)90155-6. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Selby M. J., Rutter W. J. Differential RNA splicing predicts two distinct nerve growth factor precursors. 1986 Feb 27-Mar 5Nature. 319(6056):784–787. doi: 10.1038/319784a0. [DOI] [PubMed] [Google Scholar]
- Gnahn H., Hefti F., Heumann R., Schwab M. E., Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Brain Res. 1983 Jul;285(1):45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Goedert M., Fine A., Hunt S. P., Ullrich A. Nerve growth factor mRNA in peripheral and central rat tissues and in the human central nervous system: lesion effects in the rat brain and levels in Alzheimer's disease. Brain Res. 1986 Jul;387(1):85–92. doi: 10.1016/0169-328x(86)90023-9. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Harper G. P., Barde Y. A., Burnstock G., Carstairs J. R., Dennison M. E., Suda K., Vernon C. A. Guinea pig prostate is a rich source of nerve growth factor. Nature. 1979 May 10;279(5709):160–162. doi: 10.1038/279160a0. [DOI] [PubMed] [Google Scholar]
- Harper G. P., Glanville R. W., Thoenen H. The purification of nerve growth factor from bovine seminal plasma. Biochemical characterization and partial amino acid sequence. J Biol Chem. 1982 Jul 25;257(14):8541–8548. [PubMed] [Google Scholar]
- Harper G. P., Thoenen H. The distribution of nerve growth factor in the male sex organs of mammals. J Neurochem. 1980 Apr;34(4):893–903. doi: 10.1111/j.1471-4159.1980.tb09663.x. [DOI] [PubMed] [Google Scholar]
- Hefti F., Hartikka J., Eckenstein F., Gnahn H., Heumann R., Schwab M. Nerve growth factor increases choline acetyltransferase but not survival or fiber outgrowth of cultured fetal septal cholinergic neurons. Neuroscience. 1985 Jan;14(1):55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Scott J., Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 1984 Dec 20;3(13):3183–3189. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H. D., Unsicker K. The seminal vesicle of the bull: a new and very rich source of nerve growth factor. Eur J Biochem. 1982 Nov 15;128(2-3):421–426. [PubMed] [Google Scholar]
- Hoskins D. D., Acott T. S., Critchlow L., Vijayaraghavan S. Studies on the roles of cyclic AMP and calcium in the development of bovine sperm motility. J Submicrosc Cytol. 1983 Jan;15(1):21–27. [PubMed] [Google Scholar]
- Hoskins D. D., Brandt H., Acott T. S. Initiation of sperm motility in the mammalian epididymis. Fed Proc. 1978 Sep;37(11):2534–2542. [PubMed] [Google Scholar]
- Kann M. L., Serres C. Development and initiation of sperm motility in the hamster epididymis. Reprod Nutr Dev. 1980;20(6):1739–1749. doi: 10.1051/rnd:19801001. [DOI] [PubMed] [Google Scholar]
- Korsching S., Auburger G., Heumann R., Scott J., Thoenen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985 Jun;4(6):1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large T. H., Bodary S. C., Clegg D. O., Weskamp G., Otten U., Reichardt L. F. Nerve growth factor gene expression in the developing rat brain. Science. 1986 Oct 17;234(4774):352–355. doi: 10.1126/science.3764415. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Lärkfors L., Ebendal T. Highly sensitive enzyme immunoassays for beta-nerve growth factor. J Immunol Methods. 1987 Feb 26;97(1):41–47. doi: 10.1016/0022-1759(87)90103-7. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Rutkowski J. L., Tennekoon G. I., Buchanan K., Johnston M. V. Choline acetyltransferase activity in striatum of neonatal rats increased by nerve growth factor. Science. 1985 Jul 19;229(4710):284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- Olson L., Ayer-LeLievre C., Ebendal T., Seiger A. Nerve growth factor-like immunoreactivities in rodent salivary glands and testis. Cell Tissue Res. 1987 May;248(2):275–286. doi: 10.1007/BF00218194. [DOI] [PubMed] [Google Scholar]
- Radeke M. J., Misko T. P., Hsu C., Herzenberg L. A., Shooter E. M. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987 Feb 12;325(6105):593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Bradshaw R. A. Isolation and partial amino acid sequence analysis of nerve growth factor from the guinea pig prostate. J Neurosci Res. 1981;6(4):451–464. doi: 10.1002/jnr.490060403. [DOI] [PubMed] [Google Scholar]
- Scott J., Selby M., Urdea M., Quiroga M., Bell G. I., Rutter W. J. Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983 Apr 7;302(5908):538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- Serres C., Kann M. L. Motility induction in hamster spermatozoa from caput epididymidis: effects of forward motility protein (FMP) and calmodulin inhibitor. Reprod Nutr Dev. 1984;24(1):81–94. doi: 10.1051/rnd:19840109. [DOI] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2714–2718. doi: 10.1073/pnas.83.8.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Berman C., Dull T. J. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983 Jun 30;303(5920):821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]
- Whittemore S. R., Ebendal T., Lärkfors L., Olson L., Seiger A., Strömberg I., Persson H. Development and regional expression of beta nerve growth factor messenger RNA and protein in the rat central nervous system. Proc Natl Acad Sci U S A. 1986 Feb;83(3):817–821. doi: 10.1073/pnas.83.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]