Abstract
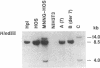
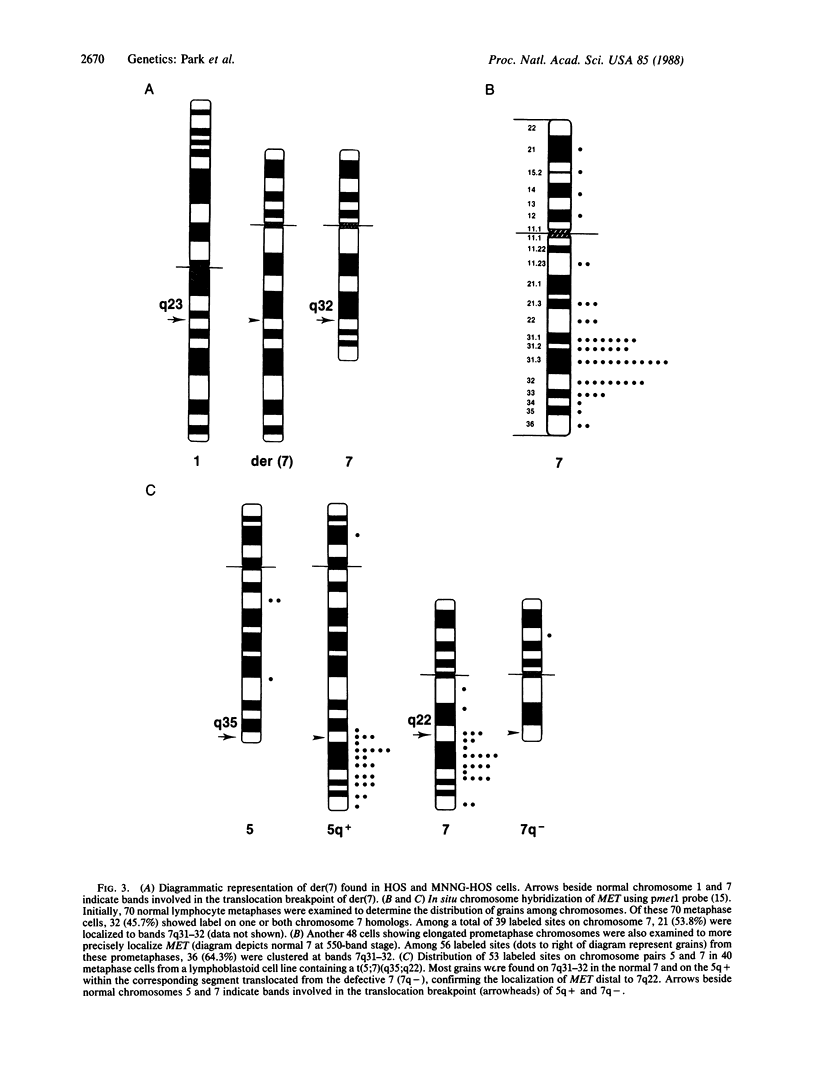
We have found that two alleles of the MET locus are rearranged in the human cell line MNNG-HOS. One allele is the previously characterized TPR-MET oncogene and the other is found on a der(7)t(1;7)(q23;q32) marker chromosome. These data and in situ chromosomal hybridization analysis would indicate that MET and, therefore, the cystic fibrosis locus are located at bands q31-q32 on human chromosome 7. Using somatic cell hybrids, we show that the chromosome containing the TPR-MET oncogene is grossly rearranged and contains both the upstream and downstream portions of the MET protooncogene locus. These results demonstrate that the TPR-MET oncogene rearrangement involving chromosomes 1 and 7 is either due to an insertion of TPR sequences into the MET locus or is more complex. We also show that the upstream MET protooncogene locus is deleted on der(7), while the downstream portion is retained. We cannot exclude that this is due to an interstitial chromosomal deletion or to a more complex rearrangement, but if MET maps at the breakpoint in der(7), then the 3' end of the MET transcription unit should be oriented towards the centromere. We also show that other DNA restriction fragment length polymorphism markers tightly linked with the inheritance of cystic fibrosis are deleted on der(7).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A., Bowcock A., Buchwald M., Cavalli-Sforza L., Farrall M., King M. C., Klinger K., Lalouel J. M., Lathrop G., Naylor S. Linkage of cystic fibrosis to two tightly linked DNA markers: joint report from a collaborative study. Am J Hum Genet. 1986 Dec;39(6):681–693. [PMC free article] [PubMed] [Google Scholar]
- Buckle V. J., Scambler P. J., Wainwright B. J. Localisation of a sequence, 7C22, showing close linkage to the cystic fibrosis locus. Cytogenet Cell Genet. 1987;44(1):41–42. doi: 10.1159/000132338. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Drumm M. L., Cole J. L., Lockwood W. K., Vande Woude G. F., Iannuzzi M. C. Construction of a general human chromosome jumping library, with application to cystic fibrosis. Science. 1987 Feb 27;235(4792):1046–1049. doi: 10.1126/science.2950591. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., Vande Woude G. F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984 Sep 6;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Dean M., Park M., Le Beau M. M., Robins T. S., Diaz M. O., Rowley J. D., Blair D. G., Vande Woude G. F. The human met oncogene is related to the tyrosine kinase oncogenes. 1985 Nov 28-Dec 4Nature. 318(6044):385–388. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- Dean M., Park M., Vande Woude G. F. Characterization of the rearranged tpr-met oncogene breakpoint. Mol Cell Biol. 1987 Feb;7(2):921–924. doi: 10.1128/mcb.7.2.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiberg H., Mohr J., Schmiegelow K., Nielsen L. S., Williamson R. Linkage relationships of paraoxonase (PON) with other markers: indication of PON-cystic fibrosis synteny. Clin Genet. 1985 Oct;28(4):265–271. doi: 10.1111/j.1399-0004.1985.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Estivill X., Farrall M., Scambler P. J., Bell G. M., Hawley K. M., Lench N. J., Bates G. P., Kruyer H. C., Frederick P. A., Stanier P. A candidate for the cystic fibrosis locus isolated by selection for methylation-free islands. 1987 Apr 30-May 6Nature. 326(6116):840–845. doi: 10.1038/326840a0. [DOI] [PubMed] [Google Scholar]
- Estivill X., Schmidtke J., Williamson R., Wainwright B. Chromosome assignment and restriction fragment length polymorphism analysis of the anonymous DNA probe B79a at 7q22 (HMG8 assignment D7S13). Hum Genet. 1986 Nov;74(3):320–322. doi: 10.1007/BF00282558. [DOI] [PubMed] [Google Scholar]
- Gonzatti-Haces M., Seth A., Park M., Copeland T., Oroszlan S., Vande Woude G. F. Characterization of the TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):21–25. doi: 10.1073/pnas.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Isobe M., Erikson J., Emanuel B. S., Nowell P. C., Croce C. M. Location of gene for beta subunit of human T-cell receptor at band 7q35, a region prone to rearrangements in T cells. Science. 1985 May 3;228(4699):580–582. doi: 10.1126/science.3983641. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Gardner M. B., Greene A. E., Bradt C., Nichols W. W., Landing B. H. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971 Feb;27(2):397–402. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Michiels F., Burmeister M., Lehrach H. Derivation of clones close to met by preparative field inversion gel electrophoresis. Science. 1987 Jun 5;236(4806):1305–1308. doi: 10.1126/science.3035716. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O'Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986 Jun 20;45(6):895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Kaul K., Braun M. J., Gonda M. A., Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Gonzatti-Haces M., Dean M., Blair D. G., Testa J. R., Bennett D. D., Copeland T., Oroszlan S., Vande Woude G. The met oncogene: a new member of the tyrosine kinase family and a marker for cystic fibrosis. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):967–975. doi: 10.1101/sqb.1986.051.01.110. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Park D. K., Arnstein P., Huebner R. J., Weisburger E. K., Nelson-Rees W. A. Transformation of human cells in culture by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1975 Aug 28;256(5520):751–753. doi: 10.1038/256751a0. [DOI] [PubMed] [Google Scholar]
- Scambler P. J., Law H. Y., Williamson R., Cooper C. S. Chromosome mediated gene transfer of six DNA markers linked to the cystic fibrosis locus on human chromosome seven. Nucleic Acids Res. 1986 Sep 25;14(18):7159–7174. doi: 10.1093/nar/14.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler P. J., Wainwright B. J., Watson E., Bates G., Bell G., Williamson R., Farrall M. Isolation of a further anonymous informative DNA sequence from chromosome seven closely linked to cystic fibrosis. Nucleic Acids Res. 1986 Mar 11;14(5):1951–1956. doi: 10.1093/nar/14.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui L. C., Buchwald M., Barker D., Braman J. C., Knowlton R., Schumm J. W., Eiberg H., Mohr J., Kennedy D., Plavsic N. Cystic fibrosis locus defined by a genetically linked polymorphic DNA marker. Science. 1985 Nov 29;230(4729):1054–1057. doi: 10.1126/science.2997931. [DOI] [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Schmidtke J., Watson E. A., Law H. Y., Farrall M., Cooke H. J., Eiberg H., Williamson R. Localization of cystic fibrosis locus to human chromosome 7cen-q22. 1985 Nov 28-Dec 4Nature. 318(6044):384–385. doi: 10.1038/318384a0. [DOI] [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Williamson R. Regional localization of three probes closely linked to the cystic fibrosis locus by deletion analysis. Cytogenet Cell Genet. 1987;44(2-3):101–102. doi: 10.1159/000132352. [DOI] [PubMed] [Google Scholar]
- White R., Leppert M., O'Connell P., Nakamura Y., Woodward S., Hoff M., Herbst J., Dean M., Vande Woude G., Lathrop G. M. Further linkage data on cystic fibrosis: the Utah Study. Am J Hum Genet. 1986 Dec;39(6):694–698. [PMC free article] [PubMed] [Google Scholar]
- White R., Woodward S., Leppert M., O'Connell P., Hoff M., Herbst J., Lalouel J. M., Dean M., Vande Woude G. A closely linked genetic marker for cystic fibrosis. 1985 Nov 28-Dec 4Nature. 318(6044):382–384. doi: 10.1038/318382a0. [DOI] [PubMed] [Google Scholar]
- Zengerling S., Tsui L. C., Grzeschik K. H., Olek K., Riordan J. R., Buchwald M. Mapping of DNA markers linked to the cystic fibrosis locus on the long arm of chromosome 7. Am J Hum Genet. 1987 Mar;40(3):228–236. [PMC free article] [PubMed] [Google Scholar]