Abstract
Progesterone (P) powerfully inhibits gonadotropin-releasing hormone (GnRH) secretion in ewes, as in other species, but the neural mechanisms underlying this effect remain poorly understood. Using an estrogen (E)-free ovine model, we investigated the immediate GnRH and luteinizing hormone (LH) response to acute manipulations of circulating P concentrations and whether this response was mediated by the nuclear P receptor. Simultaneous hypophyseal portal and jugular blood samples were collected over 36 hr: 0–12 hr, in the presence of exogenous P (P treatment begun 8 days earlier); 12–24 hr, P implant removed; 24–36 hr, P implant reinserted. P removal caused a significant rapid increase in the GnRH pulse frequency, which was detectable within two pulses (175 min). P insertion suppressed the GnRH pulse frequency even faster: the effect detectable within one pulse (49 min). LH pulsatility was modulated identically. The next two experiments demonstrated that these effects of P are mediated by the nuclear P receptor since intracerebroventricularly infused P suppressed LH release but 3α-hydroxy-5α-pregnan-20-one, which operates through the type A γ-aminobutyric acid receptor, was without effect and pretreatment with the P-receptor antagonist RU486 blocked the ability of P to inhibit LH. Our final study showed that P exerts its acute suppression of GnRH through an E-dependent system because the effects of P on LH secretion, lost after long-term E deprivation, are restored after 2 weeks of E treatment. Thus we demonstrate that P acutely inhibits GnRH through an E-dependent nuclear P-receptor system.
Progesterone (P) is the dominant ovarian steroid present in the peripheral circulation during the mammalian reproductive cycle and serves a number of important regulatory roles. The luteal phase elevation in P inhibits pulsatile gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion (1–4) and prevents the occurrence of GnRH (5) and LH (6, 7) surges in response to fluctuations in peripheral estrogen (E) levels that accompany the waves of follicular growth occurring in the ovary (8). We have also recently demonstrated that the luteal phase elevation of P affects both the timing of the LH surge relative to E stimulation and the magnitude of the coincident GnRH surge (D.C.S., N.P.E., and A.C., unpublished results). Despite its obvious importance in regulating reproduction, little is known about how and where P acts to powerfully inhibit the neuroendocrine reproductive axis.
Our first study, using the hypophyseal portal cannulation approach, assessed directly the timing of the changes in GnRH secretion that follow both an abrupt decrease and an increase in circulating P concentrations. To avoid potential P–E interactions, short-term ovariectomized (OVX) ewes were used.
Since P modulates tonic LH secretion by affecting GnRH pulse frequency (1–4), it is held that P acts through neural targets and putative candidates include the opioidergic, noradrenergic, and γ-aminobutyric acid (GABA) systems (9, 10). The possible involvement of GABA is of particular interest because in humans (11), cattle (12, 13), and possibly monkeys (3), P can suppress LH release very rapidly, with the speed of the response suggesting that the inhibition may be through a nongenomic system. In this respect, P is rapidly metabolized in the brain into a number of neurosteroids, including 3α-hydroxy-5α-pregnan-20-one (3α,5αTHP) (14), which is a potent barbiturate-like modulator of the type A GABA (GABAA) receptor in the brain (15, 16). Furthermore, studies in rats suggest that 3α,5αTHP activates the GABAA receptor to influence LH release (17). In the next experiments, therefore, we determined whether the rapid effects of P on GnRH release were mediated through the GABAA or nuclear P receptor (PR). Accordingly, the effects on LH secretion of intracerebroventricularly (i.c.v.) P and 3α,5αTHP infusion and peripheral treatment with P and the PR antagonist RU486 were investigated.
Numerous studies have reported that nuclear PR expression is significantly up-regulated by prior E exposure (18–22) and the presence of E has been shown to enhance the ability of P to suppress LH secretion (23, 24). Since short-term OVX ewes were used in our first three studies and, although no ovarian E would have been circulating during these experiments, the E exposure prior to OVX may have been sufficient to induce the PRs needed for the rapid suppression of GnRH. Thus the final study, using long-term OVX ewes, established whether the suppressive effect of P on GnRH was truly independent of E.
MATERIALS AND METHODS
Animals.
Sexually mature ewes (Dorset Horn in experiment 1 and Ile-de-France in experiments 2–4) were OVX 1 month before experimentation, housed in rooms with a natural photo period, allowed free access to water, and fed daily with hay, straw, and corn. At the time of ovariectomy, a 10-mm Silastic 17β-estradiol implant was inserted s.c. Unless stated, chemicals used in these experiments were purchased from Sigma.
Experiment 1: GnRH and LH Changes After P Withdrawal and Insertion.
This study characterized the acute changes in GnRH and LH release after P manipulations. Eight breeding season ewes were prepared for portal blood collection (25). Four days later, the 10-mm 17β-estradiol implant was removed and two P-releasing implants (termed CIDRs; InterAg, Hamilton, New Zealand) were inserted intravaginally. After 8 days, 10-min integrated portal and jugular blood samples were collected and processed for GnRH and LH concentrations as described (25). Samples were collected for 36 hr with CIDRs removed after 12 hr and two new CIDRs were inserted at 24 hr. This created three treatment periods: chronic P treatment (period 1, 0–12 hr), acute P withdrawal (period 2, 12–24 hr), and acute P treatment (period 3, 24–36 hr). In addition to LH, prolactin and cortisol were measured in jugular blood samples to confirm the specificity of any effect of P on GnRH secretion.
Since the inhibitory response to P insertion during period 3 was the most dramatic in this experiment, experiments 2–4 concentrated on the changes in gonadotropin secretion occurring at this time. The temporal sequence of these experiments is always described relative to the time P was removed at the end of period 1. In addition, since experiment 1 validated the use of LH as an index of GnRH release, other experiments only analyzed jugular LH levels.
Experiment 2: i.c.v. Infusion of P and 3α,5αTHP.
This study investigated whether the P-induced suppression of GnRH secretion observed during period 3 of experiment 1 was transduced by the P metabolite 3α,5αTHP. Ewes were prepared for third ventricular access as described (26) and received the same steroidal pretreatment used in experiment 1. Steroids for i.c.v. infusion were mixed with 2-hydroxypropyl-β-cyclodextrin, 1:16 (wt/wt) in Ringer’s lactate (Bruneau, Paris, France) to assist solubility.
Six hours after the P implant removal, the steroid vehicle was infused for 6 hr i.c.v. (2.2 μl/min) by using a constant-rate infusion pump (Syringe Driver Type MS 16A, Graseby Medical, France). Either P (n = 6) or 3α,5αTHP (n = 6) was then infused at a rate of 2.2 μg/min for a further 6 hr. The experiment was repeated 8 months later with a 10-fold lower, 0.22 μg/min, rate of infusion for P (n = 4) and 3α,5αTHP (n = 6). Jugular blood samples (15 min) were collected for LH estimation, commencing 3 hr after the start of vehicle infusion.
Experiment 3: Influence of RU486 on P Suppression.
In this study we investigated whether the P-induced suppression observed during period 3 of experiment 1 was transduced by the nuclear PR. Ewes (n = 18) received the same steroidal pretreatment used in experiment 1 and 9 hr after P implant removal; vehicle (20 ml of 10% alcohol in peanut oil) was injected i.m. into 6 ewes (control ewes) and RU486 (200 mg in vehicle) was injected into the remaining 12 ewes. P implants were inserted 3 hr later into the control and six RU486-treated ewes. The remaining RU486-treated ewes received no P implants. Jugular blood samples (15 min), starting 6 hr after P removal, were collected.
Experiment 4: Influence of E on P Suppression.
To determine the effects of E on the rapid inhibitory effects of P, 10 long-term (at least 4 months) OVX ewes were allocated to two groups (n = 5 ewes per group). One group was treated with s.c. 10-mm Silastic 17β-estradiol implants for 2 weeks, the implants were removed, and P implants were inserted in both groups for 8 days. As before, new CIDRs were inserted 12 hr after P removal. LH was monitored in 15-min jugular blood samples collected starting 9 hr after P removal and continuing for 6 hr after the new CIDRs were inserted.
Radioimmunoassay (RIA).
Portal plasma samples were assayed for GnRH after extraction by a well-described RIA method (27). All samples from individual ewes were measured in duplicate in the same assay and the intraassay coefficient of variation averaged 8% (eight assays). For these assays, the average sensitivity (two standard deviations from the buffer control) was 0.25 pg per tube (0.83 pg/ml).
Blood samples were assayed for LH in duplicate 100-μl aliquots of plasma as described (28), and all samples from an individual ewe were tested in a single assay. The intraassay coefficient of variation averaged 7.6% and sensitivity was 0.15 ng/ml (four assays) of standard 1051-CY-LH.
P concentrations were estimated in a single RIA (29) in hourly samples, except at the time of the P implant changes, when 30-min samples were assayed. The intraassay coefficient of variation was 10% and the sensitivity was 0.05 ng/ml.
In experiment 1, cortisol and prolactin were assayed in 30-min samples starting from the second sample (i.e., after 20 min). Concentrations of these hormones were determined in a single RIA for the respective hormones. Cortisol and prolactin concentrations were determined as described with RIAs (30, 31). The intraassay coefficients of variation were 8% and 6% and sensitivities were 0.2 ng/ml and 1.0 ng/ml for cortisol and prolactin, respectively.
Analysis.
LH and GnRH pulses were detected as described (26) by using the munro software (32). The amplitude (peak minus preceding nadir) was calculated for each pulse, and the pulse frequency and mean level were estimated for each period of P treatment.
To determine whether P had an effect in experiment 1, we looked at the overall pulse interval during the three experimental periods by using ANOVA for repeated measures followed by Tukey’s post-hoc comparison. After the demonstration of an effect on both GnRH and LH after both P implant removal and insertion, we used two statistical methods to analyze the time course of the P-dependent effects on the secretion of these hormones. The pulse interval data were first divided into 3-hr blocks and compared by using ANOVA for repeated measures and Tukey’s post-hoc comparison. Further precision was obtained by using the interpulse interval for each P period to predict the occurrence of the next two pulses after the P transitions. The predicted and observed pulse times were then compared statistically by using Student’s t test for paired data. It should be noted, however, that despite the refined accuracy of this second method, it is inherently limited by the individual pulsatility in each ewe and by the time, relative to the preceding pulse, that the P implants were removed or inserted. Thus, effects will be detected sooner in ewes with shorter interpulse intervals and, if the first predicted pulse is near the time of P implant manipulation, P levels are unlikely to change sufficiently to have an effect on this pulse.
In experiments 2–4, data were analyzed in 3-hr blocks and statistically compared by two-factor repeated-measures ANOVA (between factor, treatment; within factor, time) and the least squares method for subsequent comparisons within each treatment.
RESULTS
Experiment 1: Portal GnRH and LH After P Withdrawal and Insertion.
At P implant removal, P levels fell significantly (P < 0.001) within 1 hr (period 1, 2.0 ± 0.2 ng/ml; period 2, 0.8 ± 0.1 ng/ml), and after P implant insertion, P levels increased significantly (P < 0.001) above preinsertion levels within 20 min (Fig. 1). The mean P level produced during this period 3 (2.8 ± 0.3 ng/ml) was significantly higher (P < 0.01) than the level during period 1, probably reflecting the use of new P implants during period 3 while implants had been in place for 8 days before period 1.
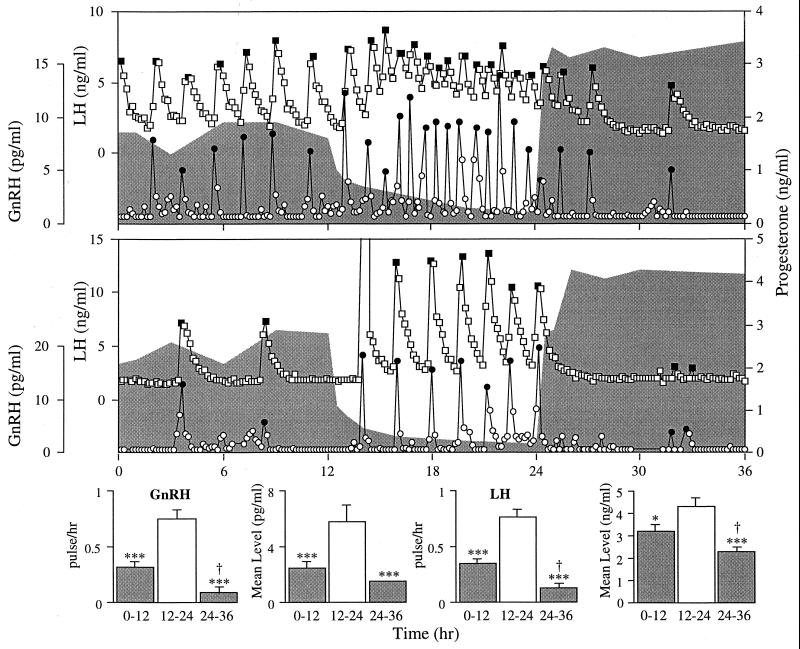
Figure 1.
(Upper) Circulating concentrations of portal GnRH and jugular LH and P for two representative ewes. Eight days before the start of this experiment, E was removed and two P implants were inserted. After removal of the implants (hour 12), P concentrations (shaded regions) fell steadily but rose rapidly after implants were replaced (hour 24). •, GnRH pulses; ■, LH pulses. (Lower) GnRH and LH pulse frequencies (mean ± SEM) and mean level over the three 12-hr periods for all 8 ewes. ∗, P < 0.05; ∗∗∗, P < 0.001 vs. 12- to 24-hr period; †, P < 0.05 vs. 0- to 12-hr period.
P dynamically altered the neurosecretory activity of the GnRH system (Fig. 1). In all ewes, the removal of P caused both GnRH and LH secretion to increase rapidly and P insertion caused an even more rapid reduction in pulsatility. Further statistical analysis indicated that the effect of P withdrawal was discernible by the time of the second predicted pulse (175 ± 18 min; range, 110–240 min). The effect of P insertion was detectable even earlier at the time of the first predicted pulse (49 ± 12 min; range, 10–110 min).
The changes in hormonal pulse frequency were reflected by changes in mean levels. After P removal, GnRH and LH increased significantly and levels were suppressed again after P insertion. There was no significant effect of P on the amplitude of GnRH (20.1 ± 5.8 vs. 30.4 ± 9.1 pg/ml) and LH (4.2 ± 0.4 vs. 4.4 ± 1.5 ng/ml) pulses between periods 1 and 2. Since few pulses occurred during period 3 (total = 9; no pulses in four ewes), these data were not analyzed statistically.
Neither prolactin (period 1, 103.3 ± 10.2 ng/ml; period 2, 93.2 ± 7.3 ng/ml; period 3,110.6 ± 28.9 ng/ml) nor cortisol (period 1, 12.7 ± 1.8 ng/ml; period 2, 20.7 ± 4.7 ng/ml; period 3, 39.0 ± 13.2 ng/ml) were significantly affected by either manipulation of P levels. Cortisol was seen to increase in three ewes midway through period 2 but this increase did not appear to have any noticeable effect on either GnRH or LH release. This increase is probably due to stress resulting from blood loss during the course of the experiment and not to the P manipulations because no obvious relationship existed between the changing concentrations of these steroids.
Experiment 2: i.c.v. Infusion of P and 3α,5αTHP.
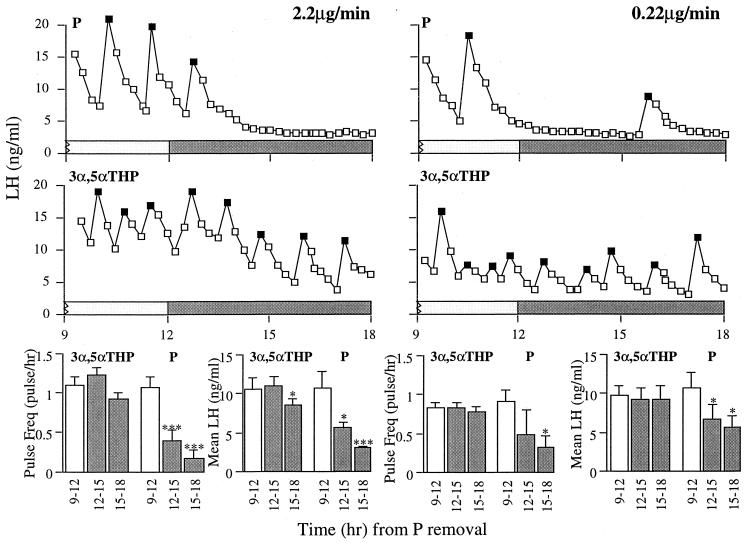
P infusions of both 2.2 μg/min and 0.22 μg/min i.c.v. significantly attenuated LH pulse frequency and mean concentration (Fig. 2). In contrast, neither dose of 3α,5αTHP affected LH pulse frequency. Although 3α,5αTHP infusion at 0.22 μg/min had no effect on mean LH levels, there was a decrease in basal LH during the infusion at 2.2 μg/min in three ewes (see Fig. 2), resulting in a slight but significant reduction in mean LH for the last 3 hr of the experiment.
Figure 2.
(Upper) Comparison of the effects of i.c.v. P and 3α,5αTHP infused at either 2.2 μg/min (Left) or 0.22 μg/min (Right) on LH pulsatility. P administered i.c.v. replicated the suppression of LH produced by intravaginal P implants. In contrast, i.c.v. administered 3α,5αTHP did not modify LH pulsatility. (Lower) LH pulse frequency (mean ± SEM) and mean LH concentration over the three 3-hr periods. LH pulses are noted by solid symbols. ∗, P < 0.05; ∗∗∗, P < 0.001.
Experiment 3: Influence of RU486 on P Suppression.
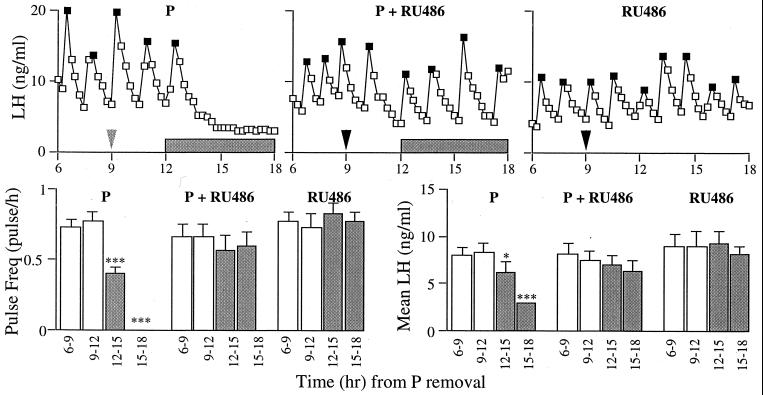
RU486 completely blocked the powerful inhibition of P on LH pulsatility (Fig. 3). As before, LH pulsatility and mean levels were suppressed by P in the control ewes, but when ewes were injected with RU486, this effect was blocked. RU486 alone had no effect on LH secretion.
Figure 3.
(top) Results from representative ewes demonstrating that RU486 blocks the ability of P to inhibit the pulsatile secretion of LH. (Upper Left) Positive control ewes received vehicle followed by intravaginal P implants. (Upper Middle) Experimental ewes were injected with RU486 and received intravaginal P implants. (Upper Right) To control for any effects of RU486 per se, a third group was injected with RU486 and did not receive P implants. (Lower Left) LH pulse frequency (mean ± SEM). (Lower Right) mean level over the four 3-hr periods (mean ± SEM). LH pulses are noted by solid symbols and the shaded bars mark the period that P is present. ∗, P < 0.05; ∗∗∗, P < 0.001.
Experiment 4: Influence of E on P Suppression.
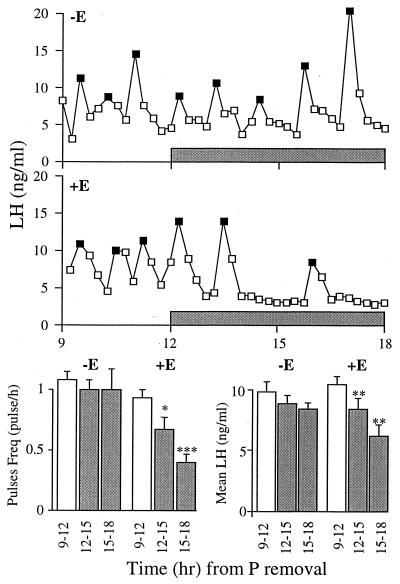
Ovarian E exposure is essential for P to suppress LH release (Fig. 4). In ewes that had been free of ovarian E for at least 4 months, P had no effect on LH pulse frequency or mean level. In contrast, when animals were exposed to a basal level of E for only 2 weeks, the ability of P to suppress LH was restored.
Figure 4.
In the absence of a 2-week E priming period before P insertion, P had no significant effect on LH pulsatility (Top), whereas after an E-priming period (Middle), the suppressive power of P was restored. (Bottom) LH pulse frequency and level over the three 3-hr periods (mean ± SEM). LH pulses are noted by solid symbols and shaded bars mark the period that P is present. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
DISCUSSION
Thus this study shows that P is able to suppress GnRH and LH pulse frequency by an acutely sensitive neural system. Importantly, we demonstrate that this inhibition is most likely mediated by the nuclear PR and not the GABAA receptor via the neurosteroid 3α,5αTHP. This study also shows that these rapid effects of P on GnRH secretion are dependent on prior E exposure.
Our study reports the dynamics of the changes in GnRH secretion after acute P manipulations. Gibson et al. (11) showed that by elevating circulating P levels during the midluteal phase of the human menstrual cycle, LH secretion was acutely suppressed. Similarly, recent studies in the bovine have also noted that the acute elevation or reduction in circulating P levels, respectively, suppresses or increases LH pulse frequency rapidly (12, 13). Our data concur with these studies and extend them by demonstrating that P acts on the GnRH system to exert its acute effect.
The specificity of the effects of P on LH pulse frequency rather than pulse amplitude in all four experiments suggest that this acute action is mediated through a neural and not a pituitary target. This inference is consistent with the changes in GnRH pulses observed in experiment 1 and with previous reports that chronic P treatment inhibited LH (1–3) and GnRH (4) pulse frequency. Similar inhibitory effects of P on tonic LH release have been observed in a number of species including rats (33, 34), cattle (24), rhesus monkeys (35), and humans (36). While this could reflect an overall inhibitory effect of P on hypothalamic neurosecretory function, the absence of any change in prolactin release in this study, after either P removal or addition, would argue that the observed effects of P are specific to the GnRH system.
Until recently it was thought that P influenced GnRH and LH secretion only through a classical genomic mechanism (37, 38). Subsequent studies, however, have suggested that there may also be a nongenomic component to the action of P, possibly through P metabolites acting through the GABAA receptor (17). In rats, GABA has an inhibitory effect on GnRH (39) and LH secretion (40, 41). GABA has also been implicated in LH inhibition in sheep (42) and specific manipulation of the GABAA, but not GABAB, receptor suppresses LH secretion (43–45). GABA may also mediate at least some of P’s inhibitory effects on LH secretion (10, 46). Our study suggests, however, that if the GABAergic system is involved in transducing P’s suppressive effects, then it is not through a 3α,5αTHP-mediated activation of the GABAA receptor. This conclusion is supported by (i) both P and 3α,5αTHP, which have nearly the same molecular weight, were used at the same concentration, (ii) the concentration of 3α,5αTHP could not have exceeded that of P, and (iii) when administered at the same dose, P is potent but 3α,5αTHP is totally inactive. In contrast, this study provides strong evidence that P acts through its own nuclear receptor to inhibit GnRH secretion: RU486 completely blocks the inhibitory effect of P on LH secretion. Although RU486 may exhibit mixed antagonist/agonist properties in other species (47, 48), our study shows that it is purely antagonistic of P actions in the ovine neuroendocrine axis and has no effect on its own.
Genomic mechanisms of action are generally characterized by their relatively prolonged time course, usually taking several hours or even days (38). The combined results of our studies would indicate that significant effects of P on GnRH secretion occur within 49 min and that the effects are mediated through a genomic action. There are striking parallels between the time course of the observed response to P in our study with those on dispersed rat pituitary cells in which P’s genomically mediated augmentative effect on GnRH-stimulated LH secretion was detectable within 45 min (49) or less (50). Indeed, the results from our study may well overestimate the time needed to see an effect of P at the neuronal level for two reasons. (i) It is unlikely that P acts directly on GnRH neurons. Although P binding in the ovine hypothalamus has been reported (51), neural PRs have not been localized in this species. However, immunocytochemical studies on other species have shown that most hypothalamic P-receptive neurons are also E-receptive (52, 53). Since ovine GnRH neurons do not have E receptors (54, 55), it is probable that these neurons will also be devoid of PRs. This suggests that an interneuronal system(s) must transmit P’s effects to the GnRH neurons. Although the noradrenergic and GABAergic systems are putative candidates (10), the strongest evidence implicates the opioidergic system (9, 10, 56–59). Indeed, anatomical studies in rats (60, 61) and monkeys (62) suggest a direct action of opiates on GnRH neurons, which could explain the rapid transduction of P’s effects. This interpretation should be tempered, however, by recent studies showing that, at least in rats, GnRH neurons do not express opioid μ, δ, or κ receptor mRNA (63). (ii) Since we have used GnRH pulsatility as an index of P inhibition, the likely response of its direct neural targets will be faster. Precise localization of P’s neural targets and electrophysiological recordings from these sites should further enhance our resolution.
Although our initial experiments suggested that P may be exerting its suppressive effects through an E-independent system, our final experiment demonstrated clearly that prior E exposure is essential. This finding is consistent with the hypothesis that nuclear PRs are up-regulated by E. Numerous studies on several mammalian species demonstrate that prior E exposure significantly increases the number of PR-expressing neurons (18, 19) and PR mRNA (20–22). This effect of E on PR gene expression appears to be direct since recent studies have reported E-response elements in its promoter region and selective site-directed mutations of these E-response elements attenuate the ability of E to induce PRs (64).
In summary, we have demonstrated unequivocally that the inhibition of LH pulse frequency by P occurs through an acute neural modulation of GnRH release. This modulation involves a direct effect of P on the nuclear PR, is E-dependent, and does not involve the GABAA receptor-affecting neurosteroid 3α,5αTHP.
Acknowledgments
We thank Dr. Elof Johansson, Director Center for Biomedical Research, Population Council (New York) for the gift of RU486 and Drs. B. Malpaux and P. Chemineau for constructive comments during the preparation of this paper. D.C.S. is a Wellcome Trust International Prize Travelling Research Fellow (046910/Z/96/Z/JMW/JPS/CG), and R.L.G was a Fogarty Senior International Fellow (FO6-TWO2219).
ABBREVIATIONS
- P
progesterone
- PR
progesterone receptor
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- i.c.v.
intracerebroventricular
- 3α
5αTHP, 3α-hydroxy-5α-pregnan-20-one
- E
estrogen
- OVX
ovariectomized
- GABA
γ-aminobutyric acid
- GABAA receptor
type A GABA receptor
- RIA
radioimmunoassay
References
- 1.Goodman R L, Karsch F J. Endocrinology. 1980;107:1286–1290. doi: 10.1210/endo-107-5-1286. [DOI] [PubMed] [Google Scholar]
- 2.Goodman R L, Bittman E L, Foster D L, Karsch F J. Endocrinology. 1981;10:1414–1417. doi: 10.1210/endo-109-5-1414. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne K T, Thalabard J C, Grosser P M, Wilson R C, Williams C L, Chen M D, Ladendorf D, Hotchkiss J, Knobil E. Endocrinology. 1991;129:1207–1214. doi: 10.1210/endo-129-3-1207. [DOI] [PubMed] [Google Scholar]
- 4.Karsch F J, Cummins J T, Thomas G B, Clarke I J. Biol Reprod. 1987;36:1207–1218. doi: 10.1095/biolreprod36.5.1207. [DOI] [PubMed] [Google Scholar]
- 5.Kasa-Vubu J Z, Dahl G E, Evans N P, Thrun L A, Moenter S M, Padmanabhan V, Karsch F J. Endocrinology. 1992;131:208–212. doi: 10.1210/endo.131.1.1611998. [DOI] [PubMed] [Google Scholar]
- 6.Scaramuzzi R J, Tillson S A, Thorneycroft I H, Caldwell B V. Endocrinology. 1971;88:1184–1189. doi: 10.1210/endo-88-5-1184. [DOI] [PubMed] [Google Scholar]
- 7.Dierschke D J, Yamaji T, Karsch F J, Weick R F, Weiss G, Knobil E. Endocrinology. 1973;92:1496–1501. doi: 10.1210/endo-92-5-1496. [DOI] [PubMed] [Google Scholar]
- 8.Souza C J, Campbell B K, Baird D T. Biol Reprod. 1997;56:483–488. doi: 10.1095/biolreprod56.2.483. [DOI] [PubMed] [Google Scholar]
- 9.Brooks A N, Lamming G E, Lees P D, Haynes N B. J Reprod Fertil. 1986;76:693–708. doi: 10.1530/jrf.0.0760693. [DOI] [PubMed] [Google Scholar]
- 10.Robinson J E, Kendrick K M. J Neuroendocrinol. 1992;4:231–236. doi: 10.1111/j.1365-2826.1992.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 11.Gibson M, Nakajima S T, McAuliffe T L. Fertil Steril. 1991;55:522–528. [PubMed] [Google Scholar]
- 12.Bergfield E G M, Kojima F N, Cupp A S, Wehrman M E, Peters K E, Mariscal V, Sanchez T, Kinder J E. Biol Reprod. 1996;54:546–553. doi: 10.1095/biolreprod54.3.546. [DOI] [PubMed] [Google Scholar]
- 13.Burke C R, Macmillan K L, Boland M P. Anim Reprod Sci. 1996;45:13–28. doi: 10.1016/s0378-4320(96)01569-2. [DOI] [PubMed] [Google Scholar]
- 14.Baulieu E E. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 15.Majewska M, Harrison N, Schwartz R, Baker J, Paul S M. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 16.Smith S S, Gong Q H, Hsu F-C, Markowitz R S, Ffrench-Mullen J M H, Li X. Nature (London) 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 17.Brann D W, Putnam C D, Mahesh V B. Endocrinology. 1990;126:1854–1859. doi: 10.1210/endo-126-4-1854. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss D A, Seroogy K B, Millhorn D E. Endocrinology. 1991;128:2610–2617. doi: 10.1210/endo-128-5-2610. [DOI] [PubMed] [Google Scholar]
- 19.Bayliss D A, Millhorn D E. Brain Res Mol Brain Res. 1991;10:167–172. doi: 10.1016/0169-328x(91)90107-9. [DOI] [PubMed] [Google Scholar]
- 20.Lauber A H, Romano G J, Pfaff D W. Neuroendocrinology. 1991;53:608–613. doi: 10.1159/000125781. [DOI] [PubMed] [Google Scholar]
- 21.Camacho-Arroyo I, Pasapera A M, Cerbon M A. Neurosci Lett. 1996;214:25–28. [PubMed] [Google Scholar]
- 22.Shughrue P J, Lane M V, Merchenthaler I. Endocrinology. 1997;138:5476–5484. doi: 10.1210/endo.138.12.5595. [DOI] [PubMed] [Google Scholar]
- 23.Goodman R L, Legan S J, Ryan K D, Foster D L, Karsch F J. Biol Reprod. 1980;23:415–422. doi: 10.1095/biolreprod23.2.415. [DOI] [PubMed] [Google Scholar]
- 24.Stumpf T T, Roberson M S, Wolfe M W, Hamernik D L, Kittok R J, Kinder J E. Biol Reprod. 1993;49:1096–1101. doi: 10.1095/biolreprod49.5.1096. [DOI] [PubMed] [Google Scholar]
- 25.Caraty A, Locatelli A, Moenter S M, Karsch F J. Methods Neurosci. 1994;20:162–183. [Google Scholar]
- 26.Skinner D C, Malpaux B, Delaleu B, Caraty A. Endocrinology. 1995;136:3230–3237. doi: 10.1210/endo.136.8.7628356. [DOI] [PubMed] [Google Scholar]
- 27.Caraty A, Locatelli A, Schanbacher B D. C R Acad Sci [D] 1987;305:369–374. [PubMed] [Google Scholar]
- 28.Montgomery G W, Martin G B, Pelletier J. J Reprod Fertil. 1985;73:173–183. doi: 10.1530/jrf.0.0730173. [DOI] [PubMed] [Google Scholar]
- 29.Saumande J, Tamboura D, Chupin D. Theriogenology. 1985;23:719–731. doi: 10.1016/0093-691x(85)90147-5. [DOI] [PubMed] [Google Scholar]
- 30.Brunet A G, Sebastian A L. Anim Reprod Sci. 1991;26:251–268. [Google Scholar]
- 31.Kann G. C R Acad Sci [D] 1971;272:2808–2811. [PubMed] [Google Scholar]
- 32.Taylor P L. Munro. Hormone Pulse-Profile Analysis. Amsterdam: Elsevier; 1987. p. 16. [Google Scholar]
- 33.McCann S M. Am J Physiol. 1962;202:601–605. [Google Scholar]
- 34.Goodman R L. Endocrinology. 1978;102:142–150. doi: 10.1210/endo-102-1-142. [DOI] [PubMed] [Google Scholar]
- 35.van Vugt D A, Lam N Y, Ferin M. Endocrinology. 1984;115:1095–1101. doi: 10.1210/endo-115-3-1095. [DOI] [PubMed] [Google Scholar]
- 36.Soules M R, Steiner R A, Clifton D K, Cohen N L, Aksel S, Bremner W J. J Clin Endocrinol Metab. 1984;58:378–383. doi: 10.1210/jcem-58-2-378. [DOI] [PubMed] [Google Scholar]
- 37.Brann D W, Hendry L B, Mahesh V B. J Steroid Biochem Mol Biol. 1995;52:113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- 38.Wiebe J P. Recent Prog Horm Res. 1997;52:71–101. [PubMed] [Google Scholar]
- 39.Seilicovich A, Duvilanski B H, Pisera D, Theas S, Gimeno M, Rettori V, McCann S M. Proc Natl Acad Sci USA. 1995;92:3421–3424. doi: 10.1073/pnas.92.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbison A E, Chapman C, Dyer R G. Exp Brain Res. 1991;87:345–352. doi: 10.1007/BF00231851. [DOI] [PubMed] [Google Scholar]
- 41.Seltzer A M, Donosa A O. Neuroendocrinology. 1992;55:28–34. doi: 10.1159/000126093. [DOI] [PubMed] [Google Scholar]
- 42.Gallegos-Sanchez J, Picard S, Delaleu B, Malpaux B, Thiéry J C. J Endocrinol. 1996;151:19–28. doi: 10.1677/joe.0.1510019. [DOI] [PubMed] [Google Scholar]
- 43.Scott C J, Clarke I J. Endocrinology. 1993;133:2904–2912. doi: 10.1210/endo.133.6.8243318. [DOI] [PubMed] [Google Scholar]
- 44.Scott C J, Clarke I J. Endocrinology. 1993;132:1789–1796. doi: 10.1210/endo.132.4.8384997. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira S A, Scott C J, Kuehl D E, Jackson G L. Endocrinology. 1996;137:3453–3460. doi: 10.1210/endo.137.8.8754774. [DOI] [PubMed] [Google Scholar]
- 46.Unda R, Brann D W, Mahesh V B. Neuroendocrinology. 1995;62:562–570. doi: 10.1159/000127064. [DOI] [PubMed] [Google Scholar]
- 47.Pleim E T, Lipetz J, Steele T L, Barfield R J. Horm Behav. 1993;27:488–498. doi: 10.1006/hbeh.1993.1035. [DOI] [PubMed] [Google Scholar]
- 48.Skafar D F. Biochemistry. 1991;30:10829–10832. doi: 10.1021/bi00109a003. [DOI] [PubMed] [Google Scholar]
- 49.Turgeon J L, Waring D W. Endocrinology. 1990;127:773–780. doi: 10.1210/endo-127-2-773. [DOI] [PubMed] [Google Scholar]
- 50.Turgeon J L, Waring D W. Endocrinology. 1991;129:3234–3239. doi: 10.1210/endo-129-6-3234. [DOI] [PubMed] [Google Scholar]
- 51.Bittman E L, Blaustein J D. Am J Physiol. 1990;258:R135–R142. doi: 10.1152/ajpregu.1990.258.1.R135. [DOI] [PubMed] [Google Scholar]
- 52.Blaustein J D, Turcotte J C. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- 53.Warembourg M, Jolivet A, Milgrom E. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- 54.Herbison A E, Robinson J E, Skinner D C. Neuroendocrinology. 1993;57:751–759. doi: 10.1159/000126433. [DOI] [PubMed] [Google Scholar]
- 55.Lehman M N, Ebling F J P, Moenter S M, Karsch F J. Endocrinology. 1993;133:876–886. doi: 10.1210/endo.133.2.8344223. [DOI] [PubMed] [Google Scholar]
- 56.Whisnat C S, Curto K, Goodman R L. Neuroendocrinology. 1992;56:812–821. doi: 10.1159/000126311. [DOI] [PubMed] [Google Scholar]
- 57.Broad K D, Kendrick K M, Sirinathsinghji D J S, Keverne E B. J Neuroendocrinol. 1993;5:711–719. doi: 10.1111/j.1365-2826.1993.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 58.Simerly R B, Young B J, Carr A M. Brain Res Mol Brain Res. 1996;40:275–284. doi: 10.1016/0169-328x(96)00057-5. [DOI] [PubMed] [Google Scholar]
- 59.Pau K Y F, Berria M, Hess D L, Spies H G. Biol Reprod. 1996;55:478–484. doi: 10.1095/biolreprod55.2.478. [DOI] [PubMed] [Google Scholar]
- 60.Leranth C, MacLusky N J, Shanabrough M, Naftolin F. Brain Res. 1988;449:167–176. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]
- 61.Chen W P, Witkin J W, Silverman A J. J Comp Neurol. 1989;286:85–95. doi: 10.1002/cne.902860106. [DOI] [PubMed] [Google Scholar]
- 62.Thind K K, Goldsmith P C. Neuroendocrinology. 1988;47:203–216. doi: 10.1159/000124914. [DOI] [PubMed] [Google Scholar]
- 63.Sanella M I, Petersen S L. Endocrinology. 1997;138:1667–1672. doi: 10.1210/endo.138.4.5091. [DOI] [PubMed] [Google Scholar]
- 64.Kraus W L, Montano M M, Katzenellenbogen B S. Mol Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]