Abstract
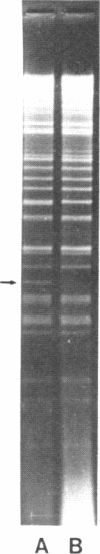
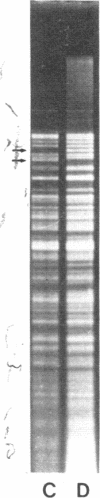
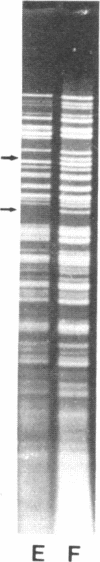
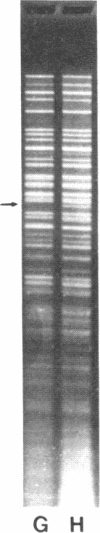
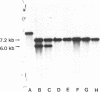
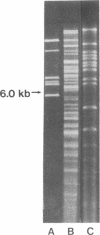
Restoration of pollen fertility to cytoplasmic male sterile (CMS) Phaseolus vulgaris by a nuclear restorer gene provides a system for studying nuclear-cytoplasmic interactions. Introduction of a nuclear restorer gene to this CMS line of P. vulgaris (CMS-Sprite) results in a mitochondrial genome rearrangement similar to that observed upon spontaneous cytoplasmic reversion to fertility. Three spontaneous heritable cytoplasmic revertants were derived from CMS-Sprite. Five fully fertile restored lines were also produced by using restorer line R-351 (BC3F3 populations). Comparison of the mitochondrial DNA restriction patterns of CMS-Sprite, the three fertile revertants, and the five restored lines revealed loss of a 6.0-kilobase (kb) Pst I fragment in all restored and revertant lines. Southern hybridizations with a 1.3-kb BamHI clone, internal to the 6.0-kb Pst I fragment, as a probe revealed two configurations of 6.0-kb homologous sequences in the sterile cytoplasm; one of the configurations was lost upon reversion or restoration. Mitochondrial DNA rearrangement has thus been observed upon restoration by a nuclear restorer gene in this CMS system.
Keywords: common bean, cytoplasmic-nuclear interaction
Full text
PDF
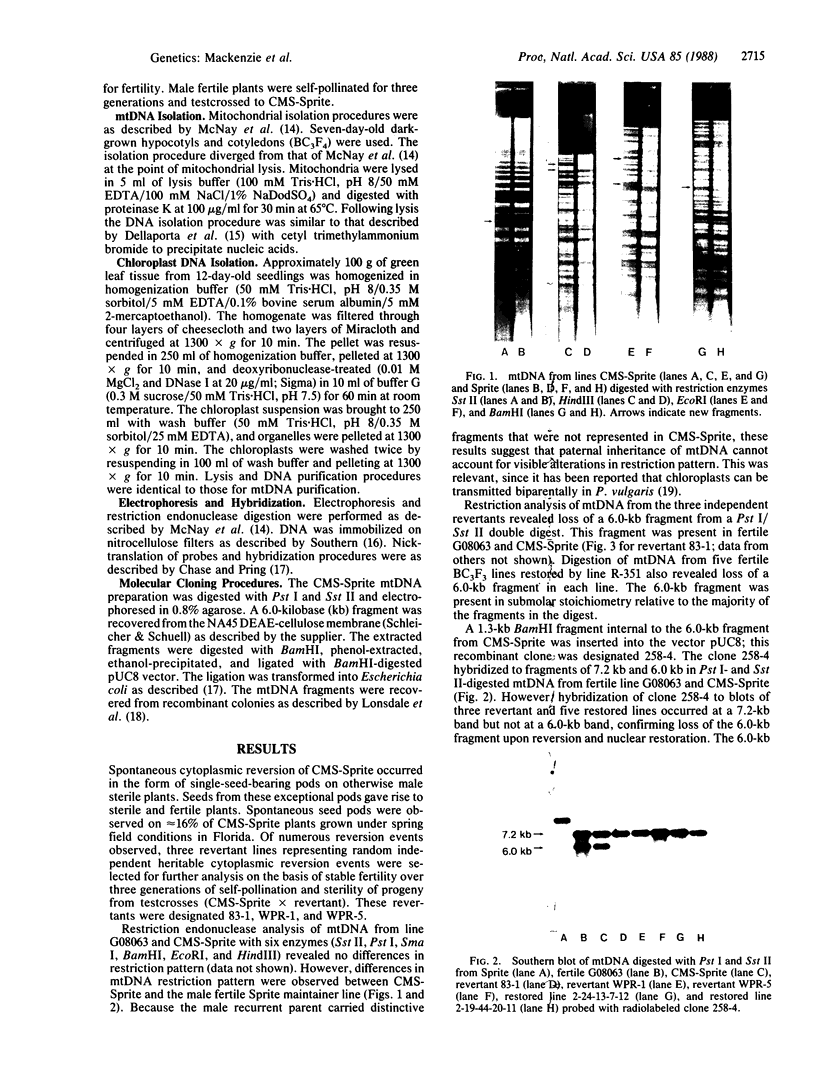


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3841–3845. doi: 10.1073/pnas.75.8.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galun E., Arzee-Gonen P., Fluhr R., Edelman M., Aviv D. Cytoplasmic hybridization in Nicotiana: mitochondrial DNA analysis in progenies resulting from fusion between protoplasts having different organelle constitutions. Mol Gen Genet. 1982;186(1):50–56. doi: 10.1007/BF00422911. [DOI] [PubMed] [Google Scholar]
- Grill L. K., Garger S. J. Identification and characterization of double-stranded RNA associated with cytoplasmic male sterility in Vicia faba. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7043–7046. doi: 10.1073/pnas.78.11.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. J., Coe E. H. Mitochondrial DNA changes in abnormal growth (nonchromosomal stripe) mutants of maize. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7363–7366. doi: 10.1073/pnas.83.19.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M. M. Genic Induction of an Inherited Cytoplasmic Difference. Proc Natl Acad Sci U S A. 1943 Dec;29(11):327–329. doi: 10.1073/pnas.29.11.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl C. L., Pring D. R., Lonsdale D. M. Mitochondrial DNA rearrangements associated with fertile revertants of S-type male-sterile maize. Cell. 1985 Nov;43(1):361–368. doi: 10.1016/0092-8674(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Small I. D., Isaac P. G., Leaver C. J. Stoichiometric differences in DNA molecules containing the atpA gene suggest mechanisms for the generation of mitochondrial genome diversity in maize. EMBO J. 1987 Apr;6(4):865–869. doi: 10.1002/j.1460-2075.1987.tb04832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Walbot V., Coe E. H. Nuclear gene iojap conditions a programmed change to ribosome-less plastids in Zea mays. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2760–2764. doi: 10.1073/pnas.76.6.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. P., Pring D. R., Gengenbach B. G. Mutation to male fertility and toxin insensitivity in Texas (T)-cytoplasm maize is associated with a frameshift in a mitochondrial open reading frame. Proc Natl Acad Sci U S A. 1987 May;84(9):2858–2862. doi: 10.1073/pnas.84.9.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]