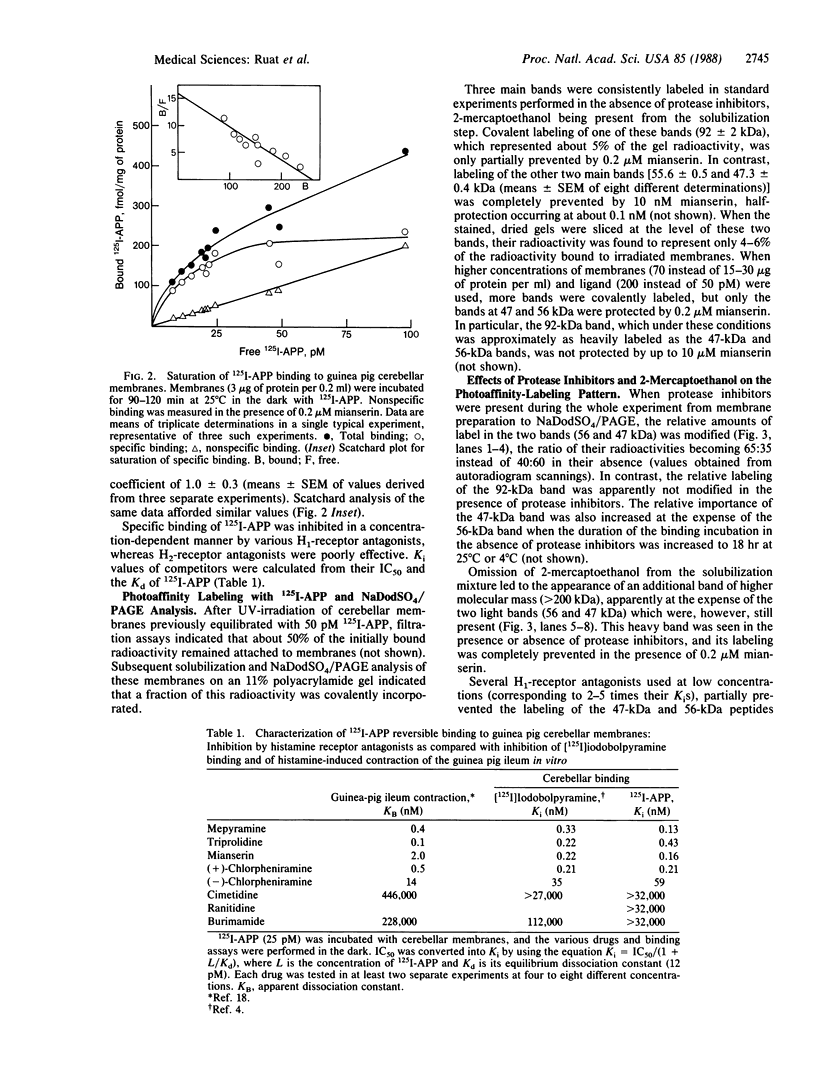
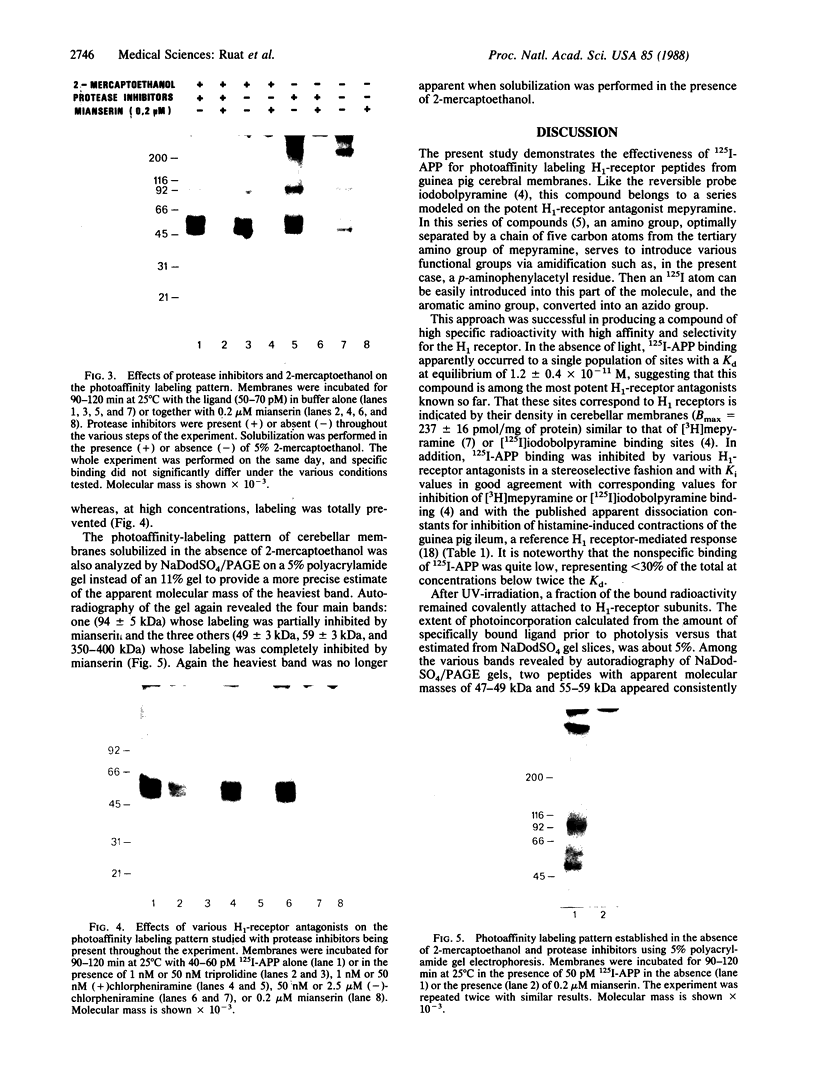
Abstract
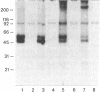
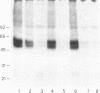
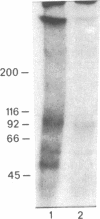
Aminophenpyramine--i.e., N-(5-[2-(4-aminophenyl)ethanamidopentyl])-N'-(4-methoxybenzyl)-N-m ethyl-N'- (2-pyridinyl)-1,2-ethanediamine, a derivative of mepyramine (pyrilamine), a typical antagonist of histamine at its H1 receptor--was synthesized and converted into [125I)iodoazidophenpyramine, a potential photoaffinity probe for the H1 receptor. In the dark, reversible binding of this probe to cerebellar membranes occurred with a Kd of 1.2 x 10(-11) M and a Bmax of 240 fmol/mg of protein and was inhibited by various H1-receptor antagonists with the expected potencies. These features establish the compound as one of the most potent H1-receptor antagonists known so far. Upon UV irradiation, 5% of the bound radioactivity was covalently incorporated into cerebellar membrane polypeptides as shown by standard NaDodSO4/PAGE. Two bands of 47 and 56 kDa were consistently labeled, labeling being prevented by various H1-receptor antagonists with the expected potencies and stereoselectivity. In the presence of protease inhibitors, labeling of the 56-kDa peptide increased at the expense of the 47-kDa peptide, suggesting that the latter was produced by hydrolysis of the former under the action of membrane proteases. In the absence of 2-mercaptoethanol, a band of 350-400 kDa appeared, apparently at the expense of the lighter bands, suggesting that the latter might be linked by one or more disulfide bridges to a higher molecular mass complex. We propose that at least part of the ligand binding domain of the histamine H1 receptor resides within a subunit of apparent molecular mass 56,000.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang R. S., Synder S. H. Histamine H1-receptor binding sites in guinea pig brain membranes: regulation of agonist interactions by guanine nucleotides and cations. J Neurochem. 1980 Apr;34(4):916–922. doi: 10.1111/j.1471-4159.1980.tb09666.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. 1,4-Dithiothreitol-induced alteration in histamine H1-agonist binding in guinea-pig cerebellum and cerebral cortex. Eur J Pharmacol. 1986 Sep 23;129(1-2):25–31. doi: 10.1016/0014-2999(86)90332-8. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Young J. M., Marrian D. H. Specific binding of 3H-mepyramine to histamine H1 receptors in intestinal smooth muscle. Nature. 1977 Nov 24;270(5635):361–363. doi: 10.1038/270361a0. [DOI] [PubMed] [Google Scholar]
- Korner M., Bouthenet M. L., Ganellin C. R., Garbarg M., Gros C., Ife R. J., Sales N., Schwartz J. C. [125I]Iodobolpyramine, a highly sensitive probe for histamine H1-receptors in guinea-pig brain. Eur J Pharmacol. 1986 Jan 21;120(2):151–160. doi: 10.1016/0014-2999(86)90535-2. [DOI] [PubMed] [Google Scholar]
- Kuno T., Kubo N., Tanaka C. Molecular size of histamine H-1 receptor determined by target size analysis. Biochem Biophys Res Commun. 1985 Jun 28;129(3):639–644. doi: 10.1016/0006-291x(85)91939-4. [DOI] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1977 Jan 25;490(1):27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- Toll L., Snyder S. H. Solubilization and Characterization of Histamine H1 receptors in brain. J Biol Chem. 1982 Nov 25;257(22):13593–13601. [PubMed] [Google Scholar]
- Tran V. T., Chang R. S., Snyder S. H. Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6290–6294. doi: 10.1073/pnas.75.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. P., Fukui H., Matsuoka H., Wada H. Determination of the molecular size of the hepatic H1-receptor by target size analysis. Biochem Biophys Res Commun. 1986 Jun 13;137(2):593–598. doi: 10.1016/0006-291x(86)91119-8. [DOI] [PubMed] [Google Scholar]
- Waud D. R., Parker R. B. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation. II. Competitive antagonists. J Pharmacol Exp Ther. 1971 Apr;177(1):13–24. [PubMed] [Google Scholar]
- Wouters W., Van Dun J., Leysen J. E., Laduron P. M. Differential photoaffinity labelling of serotonin-S2 receptors and histamine-H1 receptors using 7-azidoketanserin. FEBS Lett. 1985 Mar 25;182(2):291–296. doi: 10.1016/0014-5793(85)80318-5. [DOI] [PubMed] [Google Scholar]
- Wouters W., Van Dun J., Leysen J. E., Laduron P. M. Photoaffinity probes for serotonin and histamine receptors. Synthesis and characterization of two azide analogues of ketanserin. J Biol Chem. 1985 Jul 15;260(14):8423–8429. [PubMed] [Google Scholar]
- Yeramian E., Garbarg M., Schwartz J. C. N-ethylmaleimide-induced changes in agonist affinity for histamine H1-receptors in the guinea pig brain. Mol Pharmacol. 1985 Aug;28(2):155–162. [PubMed] [Google Scholar]