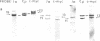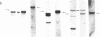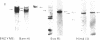Abstract
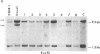
We show that endemic (eBL), sporadic (sBL), and acquired immunodeficiency syndrome-associated (AIDS-BL) forms of Burkitt lymphoma (BL) carrying t(8;14) chromosomal translocations display different breakpoints within the immunoglobulin heavy-chain locus (IGH) on chromosome 14. In sBL (7 out of 11) and AIDS-BL (5 out of 6), the breakpoints occurred within or near the IGH mu switch (S-mu) region on chromosome 14 and within the c-myc locus (MYC) on chromosome 8. In most eBL (13 out of 16) the breakpoints were mapped within or 5' to the IGH joining (JH region on chromosome 14 and outside the MYC locus on chromosome 8. Cloning and sequencing of the t(8;14) chromosomal junctions from two eBL cell lines and one eBL biopsy sample show that the recombinations do not involve IGH-specific recombination signals on chromosome 14 or homologous sequences on chromosome 8, suggesting that these events are not likely to be mediated by the same mechanisms or enzymes as in IGH rearrangements. In general, these data have implications for the timing of occurrence of chromosomal translocations during B-cell differentiation in different BL types.
Full text
PDF
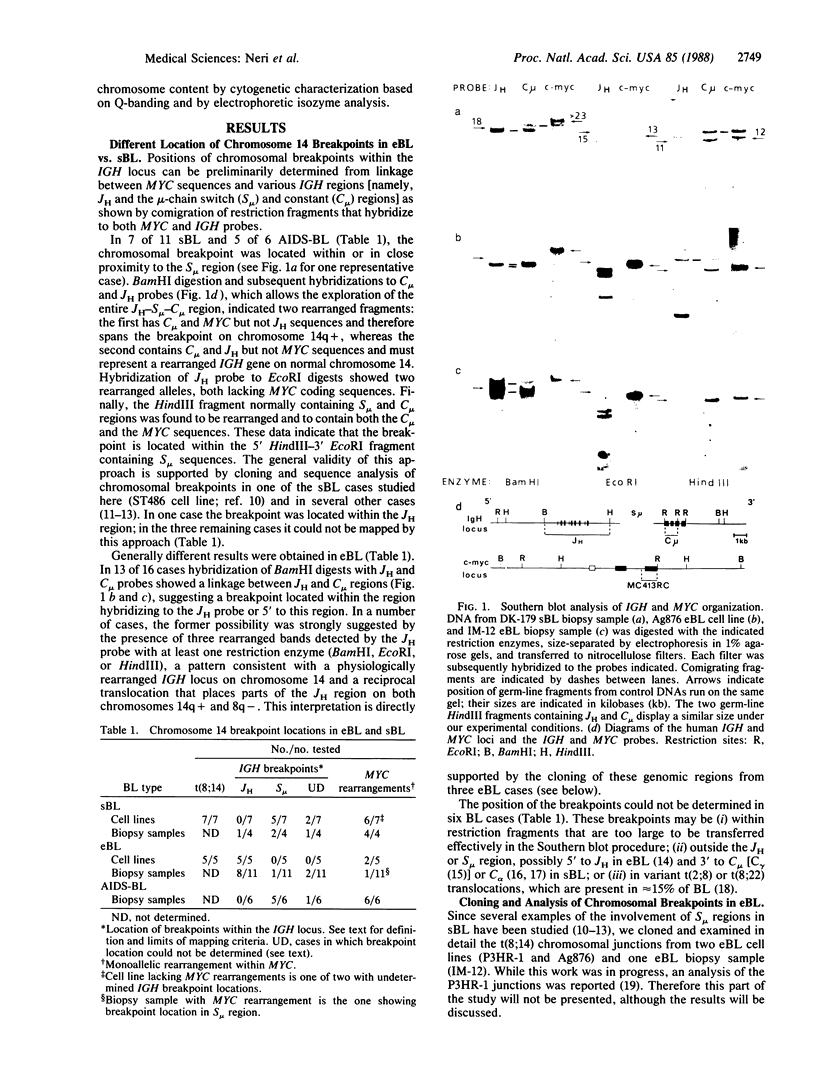
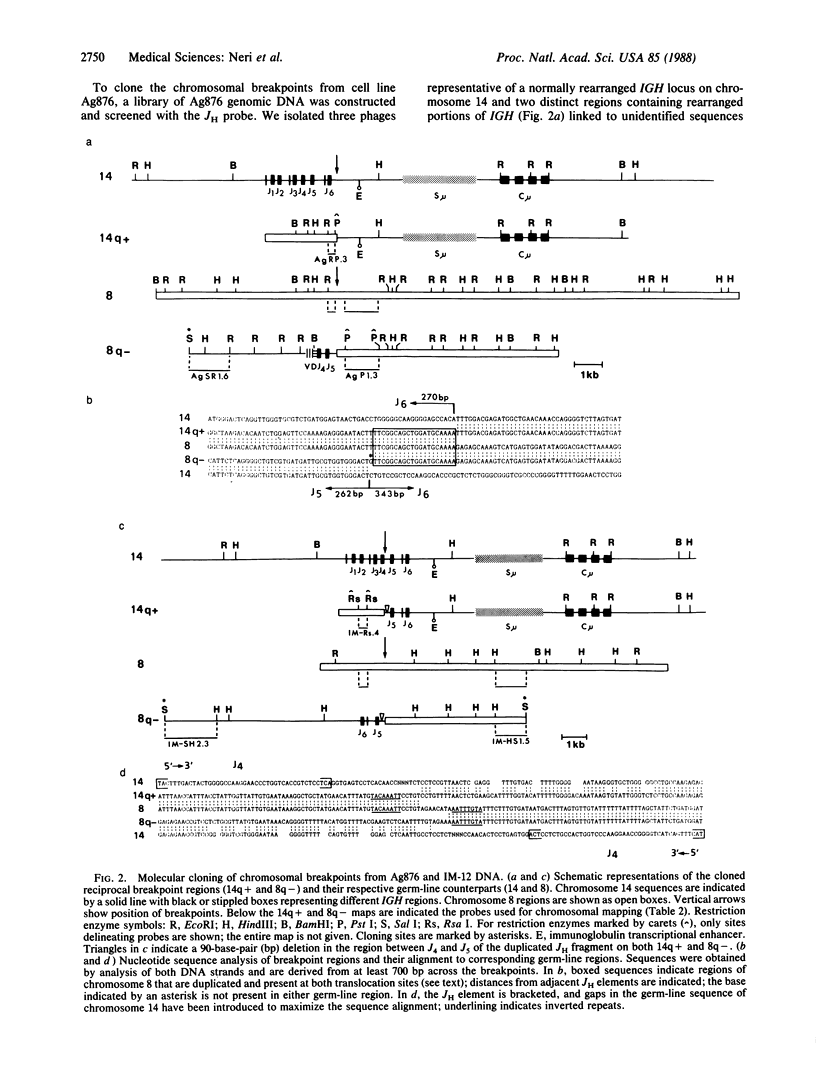


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Gerondakis S., Webb E., Corcoran L. M., Cory S. Cellular myc oncogene is altered by chromosome translocation to an immunoglobulin locus in murine plasmacytomas and is rearranged similarly in human Burkitt lymphomas. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1982–1986. doi: 10.1073/pnas.80.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhshi A., Wright J. J., Graninger W., Seto M., Owens J., Cossman J., Jensen J. P., Goldman P., Korsmeyer S. J. Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2396–2400. doi: 10.1073/pnas.84.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D., Magrath I. T., Maguire R., Janus C., Todd H. D., Parsons R. G. Immunoglobulin secretion by cell lines derived from African and American undifferentiated lymphomas of Burkitt's and non-Burkitt's type. J Immunol. 1982 Sep;129(3):1336–1342. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Underdahl J. P., Levene R. B., Ravera C. P., Morin M. J., Dombalagian M. J., Ricca G., Livingston D. M., Lynch D. C. Molecular cloning of the human gene for von Willebrand factor and identification of the transcription initiation site. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4393–4397. doi: 10.1073/pnas.84.13.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Nowell P. C. Molecular basis of human B cell neoplasia. Blood. 1985 Jan;65(1):1–7. [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Finan J., Nowell P. C., Croce C. M. Translocation of immunoglobulin VH genes in Burkitt lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5611–5615. doi: 10.1073/pnas.79.18.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. J., Kawai Y., Wagner D. D., Ginsburg D., Bonthron D., Ohlsson-Wilhelm B. M., Chavin S. I., Abraham G. N., Handin R. I., Orkin S. H. Propolypeptide of von Willebrand factor circulates in blood and is identical to von Willebrand antigen II. Science. 1986 May 23;232(4753):995–998. doi: 10.1126/science.3486471. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Psallidopoulos M. C., Papas T. S., Dalla-Favera R. Identification of reciprocal translocation sites within the c-myc oncogene and immunoglobulin mu locus in a Burkitt lymphoma. Nature. 1983 Dec 22;306(5945):799–803. doi: 10.1038/306799a0. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Haluska F. G., Finver S., Tsujimoto Y., Croce C. M. The t(8; 14) chromosomal translocation occurring in B-cell malignancies results from mistakes in V-D-J joining. Nature. 1986 Nov 13;324(6093):158–161. doi: 10.1038/324158a0. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Rabbitts T. H. Translocation joins c-myc and immunoglobulin gamma 1 genes in a Burkitt lymphoma revealing a third exon in the c-myc oncogene. Nature. 1983 Jul 14;304(5922):135–139. doi: 10.1038/304135a0. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Gillies S. D., Saito H., Wood C., Wiman K., Hayward W. S., Tonegawa S. Activation of a translocated human c-myc gene by an enhancer in the immunoglobulin heavy-chain locus. 1984 Jan 26-Feb 1Nature. 307(5949):334–340. doi: 10.1038/307334a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K. W., Zehnbauer B. A., Brodeur G. M., Seeger R. C., Trent J. M., Meltzer P. S., Vogelstein B. Amplification units containing human N-myc and c-myc genes. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1031–1035. doi: 10.1073/pnas.83.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Lian E. C., Deykin D. In vivo dissociation of factor VII (AHF) activity and factor VII-related antigen in von Willebrand's disease. Am J Hematol. 1976;1(1):71–78. doi: 10.1002/ajh.2830010108. [DOI] [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Collins C. J., Brown M., Morin M. J., Ling E. H., Livingston D. M. Molecular cloning of cDNA for human von Willebrand factor: authentication by a new method. Cell. 1985 May;41(1):49–56. doi: 10.1016/0092-8674(85)90060-1. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Moulding C., Rapoport A., Goldman P., Battey J., Lenoir G. M., Leder P. Structural analysis of both products of a reciprocal translocation between c-myc and immunoglobulin loci in Burkitt lymphoma. Nucleic Acids Res. 1985 Mar 25;13(6):2141–2152. doi: 10.1093/nar/13.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo K. Y., Vergnaud G., Johnsson C., Lucotte G., Weissenbach J. A DNA probe detecting multiple haplotypes of the human Y chromosome. Am J Hum Genet. 1986 Apr;38(4):407–418. [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Magrath I., Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986 May;83(9):2984–2988. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschle C., Mavilio F., Sposi N. M., Giampaolo A., Caré A., Bottero L., Bruno M., Mastroberardino G., Gastaldi R., Testa M. G. Translocation and rearrangement of c-myc into immunoglobulin alpha heavy chain locus in primary cells from acute lymphocytic leukemia. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5514–5518. doi: 10.1073/pnas.81.17.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rocchi M., Roncuzzi L., Santamaria R., Archidiacono N., Dente L., Romeo G. Mapping through somatic cell hybrids and cDNA probes of protein C to chromosome 2, factor X to chromosome 13, and alpha 1-acid glycoprotein to chromosome 9. Hum Genet. 1986 Sep;74(1):30–33. doi: 10.1007/BF00278781. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Kan Y. W. A simple sensitive prenatal test for hydrops fetalis caused by alpha-thalassaemia. Lancet. 1985 Jan 12;1(8420):75–77. doi: 10.1016/s0140-6736(85)91967-1. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Mannucci P. M., Jeffcoate S. L., Ingram G. I. Immunoradiometric assay of factor VIII related antigen, with observations in 32 patients with von Willebrand's disease. Br J Haematol. 1976 Jun;33(2):221–232. doi: 10.1111/j.1365-2141.1976.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Chehab F. F., Mannucci P. M., Federici A. B., Sadler J. E. Gene deletions correlate with the development of alloantibodies in von Willebrand disease. J Clin Invest. 1987 May;79(5):1459–1465. doi: 10.1172/JCI112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Titani K., Sadler J. E. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986 Jun 3;25(11):3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Showe L. C., Ballantine M., Nishikura K., Erikson J., Kaji H., Croce C. M. Cloning and sequencing of a c-myc oncogene in a Burkitt's lymphoma cell line that is translocated to a germ line alpha switch region. Mol Cell Biol. 1985 Mar;5(3):501–509. doi: 10.1128/mcb.5.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton L. W., Yang J. Q., Eckhardt L. A., Harris L. J., Birshtein B. K., Marcu K. B. Products of a reciprocal chromosome translocation involving the c-myc gene in a murine plasmacytoma. Proc Natl Acad Sci U S A. 1984 Feb;81(3):829–833. doi: 10.1073/pnas.81.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986 Aug;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., de Vries C. J., Distel B., van Zonneveld A. J., van Kessel A. G., van Mourik J. A., Pannekoek H. Construction of cDNA coding for human von Willebrand factor using antibody probes for colony-screening and mapping of the chromosomal gene. Nucleic Acids Res. 1985 Jul 11;13(13):4699–4717. doi: 10.1093/nar/13.13.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wiman K. G., Clarkson B., Hayday A. C., Saito H., Tonegawa S., Hayward W. S. Activation of a translocated c-myc gene: role of structural alterations in the upstream region. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6798–6802. doi: 10.1073/pnas.81.21.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]