Abstract
Salicylic acid (SA), which is central to defense mechanisms in plants and the principal metabolite of aspirin, occurs naturally in man with higher levels of SA and its urinary metabolite salicyluric acid (SU) in vegetarians overlapping with levels in patients on low-dose aspirin regimens. SA is widely distributed in animal blood. Fasting for major colorectal surgery did not cause disappearance of SA from plasma, even in patients following total proctocolectomy. A 13C6 benzoic acid load ingested by six volunteers led, between 8 and 16 h, to a median 33.9% labeling of urinary salicyluric acid. The overall contribution of benzoic acid (and its salts) to the turnover of circulating SA thus requires further assessment. However, that SA appears to be, at least partially, an endogenous compound should lead to reassessment of its role in human (and animal) pathophysiology.
Keywords: Salicylic acid, aspirin, benzoic acid, synthesis, salicyluric acid
Introduction
In plants, salicylic acid (SA) is a ubiquitous secondary metabolite pivotally concerned in initiation of the response to a variety of physical, chemical, and biological insults (1). The potent analgesic and antipyretic properties of plant extracts, notably dried myrtle leaves and willow bark, had been known for many centuries before the isolation of salicylic acid, as the likely active principle, by Meyer in 1870 (2). Since synthesis of aspirin (acetylsalicylic acid) by Hoffman in 1897, investigation has focused on the properties of that compound—designed as a pro-drug. Aspirin is very rapidly hydrolyzed, having a plasma t1/2 of 20 min, to the deacetylated metabolite SA (2-hydroxybenzoic acid), which has a half-life of between 2 and 4 h (3). However, the demonstration that aspirin acts by serine side-chain acetylation of Cox-1 and Cox-2 isoforms, restricting substrate access to the active sites of these enzymes, has deflected attention from salicylic acid itself, although in vivo SA is, despite weak, reversible Cox-1 and absent Cox-2 inhibition, as effective as aspirin in suppressing inflammation (4). The inhibition of transcription of the Cox-2 gene by micromolar concentrations of SA (5, 6) is the likely explanation.
Previous work by our group has demonstrated and confirmed the presence of SA in serum (7) and urine (8), where its metabolite salicyluric acid (SU) predominates, from individuals who had not recently consumed aspirin. The measured levels were significantly higher in vegetarians (9, 10), overlapping with levels in patients on low-dose aspirin regimens, and our past publications have focused on a dietary origin (11) of this naturally occurring SA in man. However, confirmation of the contribution from fruit and vegetable consumption in the diet to circulating SA levels in man (12) has demonstrated that <20% of the variability in serum SA, measured by a sensitive specific method, may derive from that source. Some circulating SA in aspirin-naïve or -free individuals could possibly derive from another dietary source or be of nondietetic origin.
In plants, SA is synthesized through the shikimic acid pathway via isochorismate (13) or, probably predominantly, by the phenyl-propanoid route via cinnamic and benzoic acids (14). Benzoic acid is a natural constituent of plants, with high amounts being found in fruits and berries (15, 16). That hippuric acid, the main metabolite of benzoic acid, may be formed endogenously in man was demonstrated by formula diet feeding (17, 18). In the Sprague−Dawley rat a radiolabel experiment showed phenylalanine to be the likely precursor (17). Use of the generally regarded as safe (GRAS) sodium benzoate as a food preservative also contributes to human intake. With regard to levels of regular intake, the FDA estimate is of 0.9−34 mg/day as benzoic acid and 34−328 mg/day as sodium benzoate, although much higher levels of sodium benzoate—up to 5 g twice daily—have been used therapeutically in the treatment of acute hepatic encephalopathy. Thus, in this paper we have determined whether the addition of benzoic acid to a standardized diet produced any change in serum or urinary salicylates.
Materials and Methods
Blood samples, serum or plasma, were obtained from animals at the London Zoo or at the Department of Biological Services, University of Glasgow. Blood was collected according to the approved codes of practice of these institutions.
Six mice were treated with neomycin, 100 mg/kg/day, for 4 days prior to collection of blood to reduce as much as possible the bacterial content of their gastrointestinal tracts. Samples from germ-free Sprague−Dawley rats were obtained from Charles River Ltd., Margate, Kent, U.K.
All human studies were approved by the Dumfries and Galloway Health Board Ethics Committee. Serum salicylic acid (SA) and urinary SA and salicyluric acid (SU) levels were assayed according to the HPLC methods described previously (7, 8).
The absence of any SA from the milk diet used in the preliminary controlled diet study was confirmed by total SA assay as we have previously described (11).
A pilot study, to assess whether ingestion of benzoic acid might raise endogenous SA levels, utilized weighed preprepared meals (requiring only reheating) replicating exactly the same menu and fluid intake over each 4 days. Throughout, urine samples were collected every 12 h. Fasting blood samples were obtained daily and on the morning of day 5. Two individuals were studied over 4 days of diet standardization after being aspirin-free for 2 weeks; the benzoic acid doses used were 1 g/day on days 3 and 4 in one subject and 2 g/day on these days in the other.
Subsequently, a labeled study was undertaken over 3 days in six individuals (four males, two females, ages 30−62 years) using a standard protocol. On day 2 all six individuals received 1 g of uniformly ring-labeled 13C benzoic acid with each of their three main meals. Very minor gastric upset was the only side effect in an individual who preferred to take the benzoic acid unencapsulated.
For that experiment uniformly ring-labeled 13C benzoic acid (ring-13C 99% pure) was supplied by Cambridge Isotope Laboratories Inc. USA. Food and fluid intake over the 3 days of the study were standardized. Each individual completed a detailed diary of all oral intake on day 1 and replicated that diet as closely as possible over the next two days. Cumulative urine samples were collected every 8 h over the whole 72 h of the study. The blood sampling protocol followed as closely as possible was day 1, 5 × 2 h, and days 2 and 3, 8 × 2 h, the first sample on each day being taken fasting. The serum SA levels were analyzed as areas under the SA level time curves over 0−6 h only as missing values precluded 0−8 h analysis. Statistical analysis of the total urinary SA and SU results was by nonparametric Friedman two-way analysis of variance, using SPSS version 12.
Gas chromatography−mass spectrometry (GC-MS) of the day 2, 8−16 h, urine samples was then performed. Preliminary fractionation was as described previously (8); samples prepared without internal standard were chromatographed under the usual conditions (with the electrochemical detector inactive), and the fraction between 1 min prior to the retention time (tR) of SU and 1 min after the tR of SA was collected.
Trimethylsilyl derivatives were prepared by treating the dried extracts with a mixture of acetonitrile and N,O-bis(trimethylsilyl)trifluoroacetamide (50:50, v/v, 30 min, 60 °C).
GC-MS analysis was carried out on an Agilent 6890/5973 MSD system (30 m × 0.25 mm i.d. DB5MS column) using both scanning and selected ion monitoring methods. The main ion fragments of interest were for SA m/z 267 (unlabeled) and m/z 273 (labeled) and for SU m/z 324 (unlabeled) and m/z 330 (labeled).
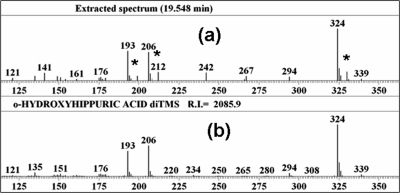
To obtain the mass spectra shown in part in Figure 1, aliquots of all of the second-day 8−16 h urine samples were initially pooled prior to derivatization. Data files were analyzed by the Automated Mass Spectral Deconvolution and Identification System (AMDIS; NIST, Gaithersburg, MD; http://chemdata.nist.gov/mass-spc/amdis/).
Figure 1.
Salicyluric acid in pooled urine sample: (a) partial mass spectrum obtained by gas chromatogrphaphy−mass spectrometry of a trimethylsilylated extract of pooled urine (isotopically labeled peaks are indicated by asterisks at m/z values 199, 212, and 330); (b) partial library mass spectrum of the di-trimethylsilyl derivative of standard salicyluric acid (o-hydroxyhippuric acid). Data were analyzed using the Automated Mass Spectral Deconvolution and Identification System (AMDIS; NIST, Gaithersburg, MD; http://chemdata.nist.gov/mass-sps/amdis).
Further aliquots from individual samples were then fractionated, derivatized, and analyzed by selected ion monitoring to give the individual results. The ratios quoted were calculated from the m/z ratios of 273/267 for 13C6 SA and 330/324 for 13C6 SU.
Results
As Table 1 shows, those species regarded as primarily carnivorous had blood SA levels comparable to those measured in herbivores. The highest levels we detected were in the range associated with aspirin use in man, as comparison with our appended published results (9, 11) shows, and only the crustacean samples examined did not contain SA.
Table 1. Concentration of Salicylic Acid (SA) in the Blood of a Variety of Animals.
| Results in Animals | ||||||||
|---|---|---|---|---|---|---|---|---|
| animal | phylogenetic class | [SA] (μmol/L) | animal | phylogenetic class | [SA] (μmol/L) | animal | phylogenetic class | [SA] (μmol/L) |
| burrowing owl | Aves | 9.854 | tiger | Mammalian | 0.661a | Chinese alligator | Reptilia | 0.156 |
| ne-ne | Aves | 5.609 | brown trout | Pisces | 0.538 | domestic cat | Mammalian | 0.144 |
| Indian rhinoceros | Mammalian | 4.700 | giraffe | Mammalian | 0.507 | pond heron | Aves | 0.136 |
| pygmy hippopotamus | Mammalian | 2.384 | donkey | Mammalian | 0.473 | gorilla | Mammalian | 0.125 |
| agouti | Mammalian | 2.116 | sacred ibis | Aves | 0.353 | red faced spider monkey | Mammalian | 0.080 |
| Asian elephant | Mammalian | 1.635 | goat | Mammalian | 0.310 | mouse | Mammalian | 0.078 |
| Burmese python | Reptilia | 1.362 | giant anteater | Mammalian | 0.293 | rat | Mammalian | 0.069 |
| rabbit | Mammalian | 1.129 | collared peccary | Reptilia | 0.237 | domestic cat (fed only meat) | Mammalian | 0.058 |
| piglet | Mammalian | 1.010 | African lion | Mammalian | 0.226a | chimpanzee | Mammalian | 0.033 |
| Arabian oryx | Mammalian | 0.777 | cow | Mammalian | 0.216 | European shore crab | Crustacea | <0.005 |
| sheep | Mammalian | 0.715 | Gelada baboon | Mammalian | 0.210 | prawn | Crustacea | <0.005 |
| Results in Man (9, 11) | ||||||||
|---|---|---|---|---|---|---|---|---|
| median (μmol/L) | range (μmol/L) | |||||||
| vegetarians, n = 37 | 0.110 | 0.04−2.47 | ||||||
| nonvegetarians, n = 39 | 0.070 | 0.02−2.00 | ||||||
| southern Indian villagers, n = 21 | 0.763 | 0.05−0.64 | ||||||
| 75 mg aspirin takers, n = 14 | 10.03 | 0.23−25.40 | ||||||
| limit of detection of the method = 0.005 μmol/L | ||||||||
Mean concentration in five animals.
To examine the possibility of a gastrointestinal bacterial source for SA, two small groups of germ-free mice were assayed for SA. The pooled serum from six mice treated with neomycin, 100 mg/kg/day, for 4 days before sacrifice had a slightly higher concentration of SA in serum (0.309 μmol/L) than the pooled serum sample from six untreated animals (0.268 μmol/L). Another germ-free animal model studied was the Sprague−Dawley rat delivered by caesarean section, reared in a sterile environment, and fed sterilized food. A group of eight such germ-free animals had a pooled serum SA level of 0.166 μmol/L, approximately 2.5 times greater than the pooled serum salicylic acid level of 0.069 μmol/L from a group of control animals. The net effect of minimizing or eliminating a contribution from colonic bacteria in these animal models was therefore enhancement of serum SA levels in the host animal.
Throughout the preliminary controlled diet study, in a subject who had taken no aspirin during the previous 2 weeks, there was persistence of measurable serum SA throughout 3 days on a milk/water diet (which was confirmed by analysis to be free from SA). In that experiment urine excretion of SA + SU continued at a rate of around 2.1 μmol/24 h throughout the period of dietary restriction. Blood samples were assayed for SA at 12 h intervals, and the serum SA levels did not change significantly, not falling below 0.1 μmol/L (20 times the limit of detection of the assay) over the 72 h of the study. These data suggested that another source of SA was responsible for the persistence of serum SA and the steady rate of excretion of SA + SU throughout the time course of this experiment.
In six patients (Table 2) who had total colectomy or rectal excision following standard preoperative bowel preparation low-level serum SA (range = 0.012−0.0847 μmol/L) was detected and urinary SA + SU excretion persisted (median of individual lowest levels = 0.613 μmol/24 h, range = 0.184−7.607) in all subjects, for up to 5 days postoperatively, rising only on refeeding.
Table 2. Patient Profile.
| no. of obs | patient | gender | age (years) at time of diagnosis | diagnosis | procedure/operation | days prior to eatinga | serum SA (μmol/L) median (range) | lowest urine (SA + SU, μmol/24 h, median) |
|---|---|---|---|---|---|---|---|---|
| 15 | 1 | male | 39 | ulcerative colitis with toxic megacolon | total colectomy with ileostomy | 2 | 0.0624 (0.054−0.082) | 0.368 |
| 6 | 2 | female | 59 | second colon cancer with multiple polyps | panproctocolectomy | 3 | 0.037 (0.013−0.074) | 0.470 |
| 12 | 3 | female | 68 | carcinoma of rectum | abdomino-perineal resection | 5 | 0.045 (0.012−0.132) | 7.707 |
| 11 | 4 | male | 47 | carcinoma of colon | extended r. hemicolectomy | 2 | 0.074 (0.061−0.088) | 0.288 |
| 4 | 5 | female | 64 | carcinoma of rectum | low ant. resection of rectum | 2 | 0.34 (0.079−0.847) | 1.01 |
| 3 | 6 | male | 66 | carcinoma of rectum + second cancer in cecum | panproctocolectomy with ileostomy | 2 | 0.103 (0.063−0.174) | 1.84 |
Patients were either fasting or receiving parenteral nutrition with no salicylic acid content (confirmed by assay).
During the pilot benzoic acid study in two subjects there was little variation in serum SA levels, which were in the ranges of 0.44−0.53 and 0.023−0.047, respectively, with no downward trend over the course of the experiment. The rate of combined SA and SU excretion in urine, however, increased from median levels of 3.87 (range = 3.47−4.32) and 4.50 (range = 4.39−4.62) μmol/24 h over the first 3 days of the study to 5.10 and 7.04 μmol/24 h, respectively, on day 4. That increase was greater in the subject who had consumed 2 g of benzoic acid per day on days 3 and 4.
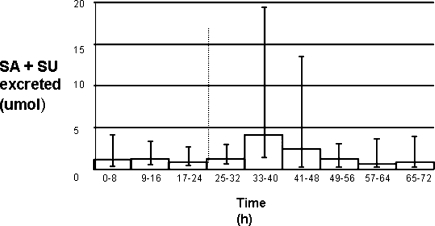
Further examination of the possible biosynthesis of SA in man required a labeled precursor study. That showed variability in total serum SA during the study, but in every individual the highest serum SA measured during the 3 days occurred during day 2—at a median time of 4 h (range = 2−8 h) after the first dose of benzoic acid. The areas under the SA level time curves confirmed the highest serum levels were reached on day 2, but that was not significant. The highest level (Figure 2, p = 0.052) of combined SA + SU urinary excretion was observed in the 8−16 h sample after the initial dose of benzoic acid.
Figure 2.
Urinary SA + SU excreted throughout time course of 13C experiment: total SA + SU excreted during the 8 h urine collection periods. The columns represent the median value and the error bars the maximum and minimum values. The dotted line at 24 h shows the point at which the first dose of benzoic acid was introduced. One subject was omitted because of incomplete data.
To ascertain whether any of the 13C6 label in the benzoic acid administered could be detected in salicylates, we therefore focused on the day 2, 8−16 h urine.
No 13C6 was detected in the samples obtained prior to ingestion of labeled benzoic acid. In Figure 1 the marked peaks (∗) indicate fragments from the presence of the (six ringed) 13C-labeled SU derivative (m/z 330), 6 mass units higher than the unlabeled SU derivative (m/z 324). The 13C isotope was also confirmed in all six individual urine, day 2, 8−16 h samples and accounted, by selective ion monitoring, for 0.4−10.9% (median = 3.4%) in the SA derivative and 6.8−43.1% (median = 33.9%) in the SU derivative (Table 3). In addition, considerable amounts of the expected 13C6-labeled hippuric acid were found (data not shown).
Table 3. Isotopic Enrichment of Salicylic Acid (SA) and Salicyluric Acid (SU) in Individual Urinesa.
| sample | SA % 13C6m/z 273/267 | SU % 13C6m/z 330/324 |
|---|---|---|
| urine 1 | 0.06 | 31.29 |
| urine 2 | 3.31 | 43.07 |
| urine 3 | 0.41 | 6.82 |
| urine 4 | 10.91 | 36.53 |
| urine 5 | 3.50 | 23.96 |
| urine 6 | 5.45 | 36.98 |
| pooled urine (no 13C6) | 0.00 | 0.00 |
| standard soln SA | 0.00 | 0.00 |
| standard soln SU | 0.00 | 0.00 |
Urines 1−6 show the relevant m/z ratio values, for each individual, of 13C6-labeled urinary salicylic acid (SA) and salicyluric acid (SU) SA, unlabeled 267, labeled 273 SU, unlabeled 324, labeled 330. Also shown are results for pooled, unlabeled (day 1) urine and for standard solutions of SA and SU.
Discussion
There is now no doubt that salicylic acid and its salts are components of the human diet (11, 19−22). Although the bioavailability of dietary salicylates was estimated to be low (23), it is clear that serum SA levels in some aspirin-free vegetarian subjects and populations (9, 11) overlap with those of patients on low-dose aspirin regimens. One particularly revealing recent observation, in a study which confirmed that circulating SA levels related to fruit and vegetable consumption, was that <20% of the variability in serum SA derived from that source (12).
Results of SA levels in a large variety of animals showed that those species regarded as primarily carnivorous had levels comparable to those measured in herbivores. Some bacteria, notably mycobacterial, yersinia, and pseudomonas species, synthesize SA to enhance iron chelation, so there was a possibility that gut, particularly colonic, bacteria might be the source of SA not readily explicable by lack of dietary exposure. However, preliminary study of two germ-free animal models reported here showed the net effect of minimizing or eliminating a contribution from colonic bacteria was, in fact, an increase in serum SA levels.
It was these animal observations which led us to postulate that serum SA levels in aspirin-free individuals may be, at least partially, endogenous. That salicylate levels in serum and urine plateaued in a subject on a water/milk diet for 3 days should be considered in light of a SA half-life of 4 h, which would cause the serum SA level to fall to ∼0.004% of its initial level at the end of this time interval. The levels of serum SA in the fasting surgical patients study supported these milk diet observations. Persistence of low levels of serum SA and combined urinary SA + SU excretion in the two patients who had panproctocolectomy, and therefore no colonic or dietary source of SA, was considered to be particularly strong evidence for an endogenous source of this simple organic molecule.
The recourse to benzoic acid as a possible SA precursor in man was directed by the relevant plant biochemistry (14) and its prevalence in fruits and berries (15, 16). Benzoic acid may also considered to be formed in vivo (17, 18) and is well-known to be an acceptable diet additive.
Given the relatively high doses (1−2 g/day) used in the preliminary benzoic acid study, the increase in SA levels observed on the last day of that protocol might be thought to be a little disappointing. The findings led us to use a slightly higher benzoic acid dose for the labeled study. Even with that higher benzoic acid dose, the increased total SA and SU urinary excretion was not significant, although clearly very marked in some individuals. Whereas the renal excretion of SA depends strongly on pH and the presence of organic acids as well as urinary flow rate (3), these possible confounding factors should reduce SA plus SU excretion under the conditions of this small study. Moreover, in a study of SA metabolism (to SU) using the isolated perfused rat kidney, the addition of the competitive substrate benzoic acid produced a rapid formation and excretion of hippuric acid with corresponding inhibition of SU formation and excretion (24). It would not, therefore, be surprising if use of a larger dose of benzoic acid in a greater number of subjects should lead to significantly increased total salicylate excretion. However, it is likely that naturally occurring benzoic acid was contributing to the modest 20% variability in serum SA from fruits and vegetables in the diet (12), although the added sodium benzoate content of diets with considerable fruit and vegetable content would probably be low. Given the widespread use of sodium benzoate as an additive food preservative, further work on its contribution to serum SA levels may be justified. However, set against the nonsignificant increase of total SA and SU levels in the day 2 8−16 h urines, the extent of SU 13C6 labeling we determined in these samples might be considered to be surprising and may point to bioregulation of endogenous serum SA.
For more than a century the focus has predominantly been on the properties of aspirin, the pro-drug of SA. It is only recently that the in vivo efficacy of SA as an anti-inflammatory has been adequately explained—in terms of inhibition of Cox-2 gene transcription. Given the lability of its acetyl ester (aspirin) within the narrow pH range of 2−3, other in vitro Cox-independent effects, such as mediation of apoptosis (25) and inhibition of angiogenesis (26), may also be attributable to SA rather than its pro-drug. Other diverse effects of SA continue to be reported, such as the surprising protective effects of lower dose SA in a mouse heart model of myocardial ischemia (27). The mechanism by which aspirin (and SA in vitro) has antitumoral actions is unclear, but a recent observation that cell proliferation is dependent on mitochondrial Ca2+ uptake and inhibited by low-level SA may be relevant (28).
SA is widely found in the animal kingdom. We have adduced evidence for its biosynthesis from benzoic acid in man. These observations are, we suggest, appropriately assessed in light of the many effects of aspirin, its pro-drug. It is, we suspect, increasingly likely that SA is a biopharmaceutical with a central, broadly defensive, role in animals as in plants. This simple organic chemical is, we propose, likely to become increasingly recognized as an animal bioregulator, perhaps in a class of its own. Intriguingly, as a phytohormone it may have differential, concentration-dependent, effects on programmed cell death and surrounding tissue protection (29). Some of the tissue effects of SA already described (5, 6, 25−28) raise the probability of just such a hybrid function, perhaps at a critical point in apoptosis and/or inflammation, in animals as in plants.
If we are right that SA is not just an important phytochemical but also a key biopharmaceutical, its circulating level might well be subject to homeostatic control and not be solely influenced by dietary SA with or without benzoic acid intake.
References
- Dangl J. Nature 1998, 394, 525–527. [DOI] [PubMed] [Google Scholar]
- Sneader W. Pharm. J. 1997, 259, 614–617. [Google Scholar]
- Needs C. J.; Brooks P. M. Clin. Pharmacokinet. 1985, 10, 164–177. [DOI] [PubMed] [Google Scholar]
- Higg G. A.; Salmon J. A.; Henderson B.; Vane J. R. Proc. Natl. Acad. Sci. U.S.A. 1987, 84, 1417–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-M.; Scansores-Garcia L.; Chen X.-M.; Matjevic-Aleksic N.; Min D.; Wu K. K. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 5292–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. K. Circulation 2000, 102, 2022–2023. [DOI] [PubMed] [Google Scholar]
- Paterson J. R.; Blacklock C.; Campbell G.; Wiles D.; Lawrence J. R. J. Clin. Pathol. 1998, 51, 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter G. J.; Lawrence J. R.; Graham A. B.; Wiles D.; Paterson J. R. Ann. Clin. Biochem. 2002, 39, 50–55. [DOI] [PubMed] [Google Scholar]
- Blacklock C. J.; Lawrence J. R.; Wiles D.; Malcolm E. A.; Gibson I. H.; Kelly C. J.; Paterson J. R. J Clin. Pathol. 2001, 54, 553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. R.; Peter R.; Baxter G. J.; Robson J.; Graham A. B.; Paterson J. R. J. Clin. Pathol 2003, 56, 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. R.; Srivastava R.; Baxter G. J.; Graham A. B.; Lawrence J. R. J. Agric. Food Chem. 2006, 54, 2891–2896. [DOI] [PubMed] [Google Scholar]
- Spadafranca A.; Bertoli S.; Fioriollo G.; Testolin G.; Battezzati A. Br. J. Nutr. 2007, 1–5. [DOI] [PubMed] [Google Scholar]
- El-Basyouni S.; Chen D.; Ibrahim R. K.; Neish A. C.; Towers G. H. N. Phytochemistry 1964, 3, 485–492. [Google Scholar]
- Yalpini M.; Leon J.; Lawton M. A.; Raskin I. Plant Physiol. 1993, 103, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y.; Wang C.; Zhan J. J. Agric. Food Chem. 2002, 50, 3789–3794. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Zuo Y. J. Agric. Food Chem. 2004, 52, 222–227. [DOI] [PubMed] [Google Scholar]
- Armstrong M. D.; Chao F.-C.; Parker V. J.; Wall P. E. Proc. Soc. Exp. Biol. Med. 1955, 90, 675–679. [DOI] [PubMed] [Google Scholar]
- Young D. S. Clin Chem 1970, 16, 681–686. [PubMed] [Google Scholar]
- Swain R.; Dutton S. P.; Truswell A. S. J. Am. Diet. Assoc 1985, 85, 950–960. [PubMed] [Google Scholar]
- Venema D. P.; Hollman P. C. H.; Janssen P. L. T. M. K. J. Agric. Food Chem. 1996, 44, 1762–1767. [Google Scholar]
- Variyar P. S.; Bandyopadhyay C. J. Spices Aromatic Crops 1995, 4, 129–134. [Google Scholar]
- Robertson G. I.; Kermode W. J. J. Sci. Food Agric. 1981, 833–836. [Google Scholar]
- Janssen P. L. T. M. K.; Hollman P. C. H.; Reichman E.; et al. Am. J. Clin. Nutr. 1996, 64, 743–747. [DOI] [PubMed] [Google Scholar]
- Bekersky I.; Colburn W. A.; Fishman L.; Kaplan S. A. Drug Metab. Dispos. 1980, 8, 319–324. [PubMed] [Google Scholar]
- Stark L. A.; Din F. V. M.; Zwacke R. M.; Dunlop M. G. FASEBJ 2001, 15, 1273–1276. [PubMed] [Google Scholar]
- Borthwick G. M.; Johnson A. S.; Partington M.; Burn J.; Wilson R.; Arthur H. M. FASEB J. 2006, 20, 2009–2016. [DOI] [PubMed] [Google Scholar]
- Farthing D.; Gehr L.; Karnes H. T.; Sica D.; Gehr T.; Larus T.; Farthing C.; Xi L. Biomarkers 2007, 12 (6), 623–634. [DOI] [PubMed] [Google Scholar]
- Nunez L.; Valero R.; Senovilla L.; Sanz-Blasco S.; Garcia-Sancho J.; Villalobos C. J. Physiol. 2006, 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M. E. Plant Mol. Biol. 2000, 44, 429–442. [DOI] [PubMed] [Google Scholar]