Abstract
Oxidative damage has been proposed as an important factor in the progression of pathological and non-pathological age-related functional declines. Here, we examine functional deterioration in short-lived flies mutant for the mitochondrial antioxidant Manganese Superoxide Dismutase (Sod2). We find that the decline of several functional measures of aging occurs, in an accelerated fashion, in Sod2 mutants. Olfactory behavior, locomotor ability and cardiac performance were all seen to decline rapidly in Sod2 mutants. On average, functional declines in Sod2 mutants occur in a pattern similar to that seen in late-life Drosophila with a normal complement of Sod2. In longitudinal experiments, however, we find that functional failures occur in every possible sequence in Sod2 mutants. Significantly, failure of these functional measures is not irreversible, as spontaneous functional recovery was sometimes observed. These findings support a model where ROS-related damage strikes at multiple organ systems in parallel, rather than a “chain of dominos” model, in which primary organ failure contributes to the deterioration of further organ systems.
Keywords: Sod2, Functional senescence, Cardiac, ROS, Longitudinal
Introduction
Reactive oxygen species (ROS) have long provided an attractive mechanistic model for explaining pathological as well as nonpathological age-related deterioration in organ function (Huang and Manton 2004; Ballinger 2005; Chan 2006; Kregel and Zhang 2007). Although experiments in both vertebrates and invertebrates have demonstrated that metabolic rate and ROS production are not necessarily determinant of lifespan (Hulbert et al. 2004; Miwa et al. 2004; Andziak et al. 2006; Brys et al. 2007; Melvin et al. 2007; Rottenberg 2007), interventions that alter resistance to oxidative stress have been correlated with lifespan changes in yeast (Agarwal et al. 2005; Kharade et al. 2005), worms (Lee et al. 2003; Nakai et al. 2004; Morcos et al. 2008), flies (Sun et al. 2002; Curtis et al. 2007) and mice (Migliaccio et al. 1999). Additionally, oxidative damage has been implicated in progressive pathological changes including various forms of neurodegeneration in worms (Cao et al. 2005; Wu and Luo 2005; Boyd-Kimball et al. 2006), flies (Botella et al. 2004; Llorens et al. 2007) and mice (Zoghbi and Botas 2002).
One way in which resistance to oxidative stress has been manipulated in vivo is through altered expression of enzymes that scavenge ROS (Duttaroy et al. 2003; Sun et al. 2002; Curtis et al. 2007; Paul et al. 2007). An important family of such proteins is the superoxide dismutase (SOD) family. The activity of SOD proteins converts superoxide anions into hydrogen peroxide, following which catalase activity catalyzes the reaction converting peroxide into water (Fridovich 1995).
The SOD family is highly conserved, with most species having a cytoplasmic SOD (SOD1), a mitochondrial SOD (SOD2), and an extracellular SOD (SOD3) (Zelko et al. 2002; Landis and Tower 2005). Due to its role near the primary source of ROS in the mitochondria, SOD2 (aka MnSod) has been particularly well studied.
Mice homozygous for null mutations in SOD2 display cardiac defects, neurodegeneration, mitochondrial dysfunction and reduced lifespan (Li et al. 1995; Lebovitz et al. 1996). Heterozygous SOD2 mutant mice display an increased rate of DNA damage accumulation and cancers (Van Remmen et al. 2003), as well as a higher frequency of apoptosis when subjected to oxidative stress (Van Remmen et al. 2001). However, these phenotypes do not result in a significant alteration of lifespan in heterozygotes (Van Remmen et al. 2003).
Flies carrying null mutations in Drosophila Sod2 develop to adulthood. However, such flies are highly sensitive to oxidative stress and exhibit precocious neurodegeneration and DNA strand breakage (Paul et al. 2007) and have a maximum lifespan of only 36 h (Duttaroy et al. 2003). Heterozygous null mutants show a significant increase in mortality trajectory rather than initial baseline mortality, consistent with an increase in the adult “rate of demographic aging” in these flies (Paul et al. 2007). Altered Sod2 function in flies also impacts age-related behavioral declines. Flies with partial loss of function in Sod2 have accelerated age-related declines in locomotor ability (Bhandari et al. 2007) and olfactory behavior (Paul et al. 2007).
A key tissue where the effects of ROS-related damage have not been investigated in invertebrate model systems is cardiac muscle tissue. In various SOD2 knockout mouse models, loss of SOD2 has been shown to lead to enlarged, dilated hearts (Lebovitz et al. 1996) and severe cardiomyopathy (Li et al. 1995). In other vertebrate models, the presence of high levels of ROS has been associated with pressure-induced cardiac hypertrophy (Aikawa et al. 2001; Date et al. 2002; Pimentel et al. 2006; Adiga and Nair 2008) and with sensitivity to ischemic stress (Asimakis et al. 2002). These phenotypes are, at least in part, dependent on SOD2 activity levels, as treatment with a SOD2 mimetic in vivo (Ding et al. 2007) or expression of SOD2 in cultured cardiomyocytes (Adiga and Nair 2008) can alleviate these effects. The extent to which functional declines in models of increased ROS levels relate to changes seen during normal aging in any organism is still a controversial one, and one of great importance. The answer to this question will shed light on whether high-ROS models are best suited to model progressive disease states, late-life physiological failures, or as accelerated models of normal aging.
Here, we take advantage of the extremely short lifespan and high levels of ROS in Sod2 null mutant flies to ask: do Sod2 null flies have even greater age-related declines in locomotor and olfactory behavior than partial loss of function mutants? Under conditions of increased ROS, do functions fail in a stereotyped order or do various functions fail stochastically? Additionally, we take advantage of the fact that insects are not dependent on cardiac function for oxygen exchange and, thus, can survive for extended periods without a functional heart to ask: what aspects of cardiac age-related functional decline in invertebrates are most sensitive to loss of ROS scavenging ability? Is systems failure as a result of low ROS-scavenging ability permanent, or can the animal regain functional homeostasis after it has been lost? Finally, we ask whether the pattern of functional failures seen in Sod2 mutant flies is similar to that seen in very late-life wild-type flies.
Materials and methods
Fly stocks and culture
Sod2−/− (Sod2n283/n283) and Sod2 revertants (generated by precise excision of the KG06854 insertion in the Sod2 locus) were generated previously (Paul et al. 2007). Sod2−/−; Sod2+ are Sod2 nulls harboring a rescue transgene with a wild-type copy of Sod2. y1w1 flies were obtained from the Bloomington Stock Center. Flies were raised at 25_C/50% relative humidity under a 12 h light/dark cycle on a 10% yeast, 10% sucrose and 2% agar diet. Additionally, propionic acid and tegosept were added to the food to limit bacterial growth and mold. In video-camera assays, flies were momentarily anesthetized with FlyNap to be mounted on the machine. All other experiments were performed without anesthesia.
Statistical methods
Odor avoidance behavior data were analyzed by two-way ANOVA for overall effects of age and genotype (with interaction between these factors) followed by Bonferroni’s multiple comparison tests for effects of genotype at individual ages (Prism, GraphPad Software, San Diego, CA, USA). Negative geotaxis behavioral data were analyzed by repeated measures of two-way ANOVA to evaluate overall effects of age and genotype (with interaction between these factors) followed by Bonferroni’s multiple comparison tests to assess differences at each age tested (Prism, GraphPad Software, San Diego, CA, USA). Heart parameters, such as frequency, velocity and contractile output change were analyzed by two-way ANOVA for overall effects of age and genotype (JMP, The Statistical Discovery Software, Cary, NC, USA). Contraction and relaxation velocity at one time point were analyzed using a Student’s t-test. Longitudinal measurements were analyzed by two-way ANOVA for effects of age and presence of function (JMP, The Statistical Discovery Software, Cary, NC, USA).
Odor avoidance behavior
Within 1 h after eclosion, adult flies were collected without anesthesia and housed in vials prior to behavioral testing. Odor avoidance was measured as described previously (Stoltzfus et al. 2003) with minor modifications. Groups of 15–25 flies were placed into a T-maze, allowed a 30 s recovery period, and then allowed to choose for 2 min between an arm of the maze containing air and an arm of the maze containing the aversive odorant 4-methylcyclohexanol (Sigma Chemical Company, St. Louis, MO, USA). Air flow in each arm was maintained at 750 ml/min throughout all experiments. Odor avoidance at each age was calculated as an index equal to the percentage of flies that moved into the arm without an odorant minus the percentage of flies that moved into the odor-containing arm (Stoltzfus et al. 2003). Groups of flies were tested only once in a cross-sectional design.
Negative geotaxis behavior
Adult flies were collected without anesthesia within 1 h of eclosion and then housed in fresh food vials until tested at different ages. Negative geotaxis (i.e. bang-induced climbing) was assessed in Rapid Iterative Negative Geotaxis (RING) assays on groups of 15–25 adult flies as described (Gargano et al. 2005) with minimal modifications. Flies were transferred to individual plastic tubes in a RING apparatus and rested for 1 min. Negative geotaxis was elicited by sharply rapping the RING apparatus (and therefore the flies) three times in rapid succession. The positions of the flies were captured in digital images taken 4 s after eliciting the behavior. Digital images of the flies were analyzed using Scion Image (NIH Image) to determine the distance climbed for each fly. The performance of flies in a single vial was calculated as the average of five consecutive trials (separated by 30 s) to generate a single datum. Five vials of 15–25 flies per vial were tested for each genotype. After each test, flies were returned to food vials and housed (aged) until the next RING test in a longitudinal design.
Longitudinal observations
Flies were age-matched within 1 h and housed individually until death. At 3 h intervals, flies were observed within the vial for at least 5 min to assess whether they were exhibiting spontaneous ambulation. Ambulation was scored in a binary yes/no fashion. Flies were then removed from the vial by gently grasping a wing with a dull forcep and placed on a dissecting microscope stage where they were observed for a minimum of 5 min to assess whether they exhibited a productive heartbeat. Where heartbeat was present, the heart rate was counted visually. Additionally, flies were gently prodded with a dull forcep to assess whether they responded with stereo-typed leg and antennal movements. Calculation of resting heart rate in this study was done not by video assay, but by visual hand counting, so as to avoid any harm coming to the flies under longitudinal study during immobilization.
Video-based cardiac performance assays
Video-based assays were performed as described (Ocorr et al. 2007), except that films were taken of intact flies rather than partially dissected flies. Frequency was calculated by measuring the number of full beats divided by the length of the film in seconds. Contraction and relaxation velocity were calculated by time-stamping a moment at full systole and a moment at full diastole, then measuring the length of time from one to the other. For each fly, three measurements were taken, then averaged. Graphs represent averages from 3 to 6 separate flies of each genotype. Contractile volume change (fractional shortening) was computed by measuring the distance change in micrometers of the edge of the myocardial wall from maximal systole to maximal diastole. A minimum of three and maximum of six flies were averaged for each time point and genotype.
Results
Accelerated loss of behavioral function in Sod2−/− adults
Age-related impairments in olfactory and locomotor behavior is a normal facet of aging in adult Drosophila that occurs over the time-span of weeks (Grotewiel et al. 2005). Although partial loss of function in Sod2 does not impact the ability of young flies to perform these behaviors, reduced Sod2 function accelerates the normal age-related defects in olfactory (Paul et al. 2007) and locomotor behavior (Bhandari et al. 2007).
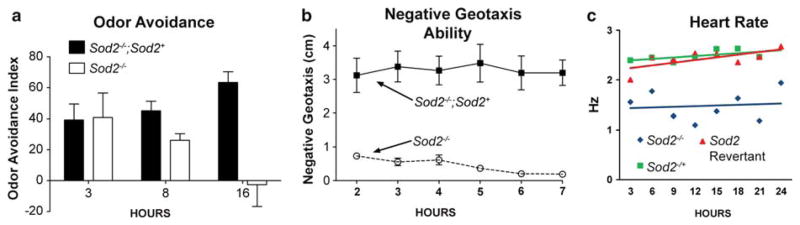
To determine the consequences of complete and therefore more severe loss of Sod2 on these age-related behavioral declines, we examined odor avoidance (an olfactory behavior) and negative geotaxis (a locomotor behavior) across age in Sod2 null flies (Sod2−/−) and control animals with two copies of a Sod2 wild-type transgene (Sod2−/−; Sod2+). Control flies exhibited robust odor avoidance (Fig. 1a) and negative geotaxis (Fig. 2b) at all ages evaluated as expected since these studies were performed over the course of only a few hours. Odor avoidance in Sod2−/− flies was indistinguishable from controls at 3 and 8 h of age, although there was a trend toward impairment at 8 h (Fig. 1a). By 16 ours of age, however, olfactory behavior in Sod2−/− flies had declined until it was no longer detectable in this assay (Fig. 1a). Negative geotaxis was also impacted in Sod2−/− flies. This locomotor behavior was substantially impaired in Sod2−/− animals by 2 h of age and thereafter declined to nearly undetectable levels by 7 h of age (Fig. 1b). Complete loss of Sod2 impairs both olfactory and locomotor behavior, but the resulting phenotypes are distinct for each behavior.
Fig. 1.
Sod2−/− flies show an accelerated decline of odor avoidance, negative geotaxis, and resting heart rate. a Odor avoidance in Sod2−/− flies (open bars) compared to Sod2−/− flies harboring the Sod2 wild type transgene (filled bars). b Negative geotaxis across age in Sod2−/− flies (open circles ) and Sod2−/−; Sod2+ flies (closed squares). c Heart rate across age of Sod2−/− flies (blue diamond) compared to both Sod2−/+ flies (green squares) and Sod2 revertant flies (red triangles)
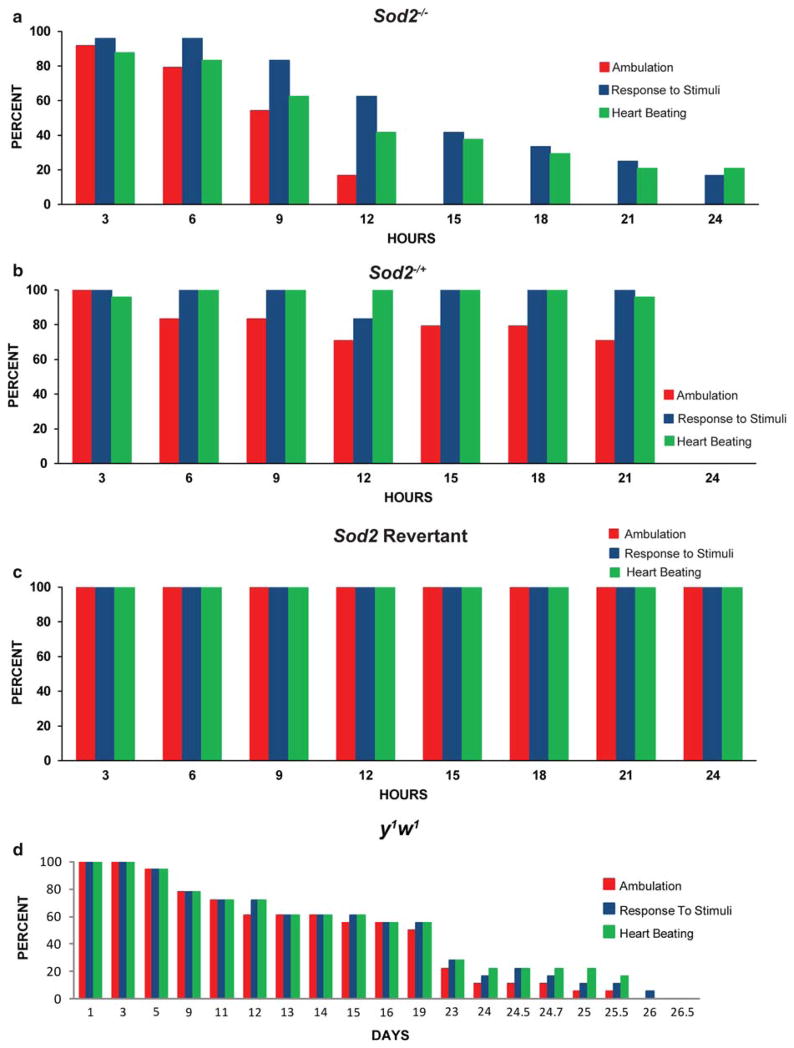
Fig. 2.
Various measures of functionality decline at different rates in Sod2−/− flies. Percentage of flies observed to exhibit either spontaneous ambulation (red), response to gentle stimuli (blue), or spontaneous heartbeat (green) is charted for a Sod2−/−, b Sod2−/+, c Sod2 revertants or d y1w1. a, b and c are shown over a 24 h time period, whereas d are shown throughout a lifetime. In d, day 1 in the graph represents day 1 of the experiment, but day 64 of the age of the flies
Reduced heart rate in Sod2−/− adults
The Drosophila heart rate is known to gradually decrease with age (Paternostro et al. 2001; Wessells et al. 2004). To what extent this decrease is dependent on ineffective scavenging of ROS has not been examined. Sod2−/− flies were assessed for heart rate at 3 h intervals and compared to age-matched Sod2−/+ flies, as well as flies carrying a precise excision of the same transposable element used to generate the null deletion (Duttaroy et al. 2003). These flies are referred to hereafter as Sod2 revertants. At 3 h of age, Sod2−/− flies exhibit a 22% lower resting heart rate than controls. While both Sod2−/+ and Sod2 revertants retain a consistent resting heart rate throughout the first 24 h of adult life, Sod2−/− flies exhibit a much higher variability and remain, on average, much slower (Fig. 1c, genotype-by-age, P<0.0001).
Taken together, these results indicate that complete loss of Sod2 function causes very early defects in both climbing ability and resting heart rate, possibly due to developmental defects. These results are consistent with a reduction in both mobility and cardiac function as a result of Sod2 mutation and consequent increase in oxidative stress.
Longitudinal comparisons
Three measures of functional change that were possible to chart by non-invasive means: spontaneous ambulation, movement in response to stimuli and heartbeat, were tracked longitudinally and charted for all three indices over a 24 h period (Supplemental Table 1). Revertant flies displayed no reduction in spontaneous ambulation, heartbeat or movement in response to touch during the time-course (Fig. 2c). Approximately 20% of Sod2−/+ flies showed a rapid decline in ambulation within the first 8 h of life, while the others appeared similar to wild-type (Fig. 2b). No decline was observed in movement in response to touch, and rarely was any decline in heart rate observed in heterozygotes.
Sod2−/− flies, by contrast, declined in each of these measures in all individual cases (Fig. 2a, ambulation-by-age: P<0.0001, χ2 = 41.847, n = 24; response to stimuli-by-age: P<0.0001, χ2 = 49.997, n = 24; beating-by-age: P<0.0001, χ2 = 40.232, n = 24). On average, there is a clear trend for spontaneous ambulatory movement to begin to decline immediately within the first 3 h, then to be completely absent in most flies by 12 h. Some individuals had lost a detectable heartbeat within 6 h and the number of flies with functional, beating hearts declined at each time point thereafter. Meanwhile, there was a similar decline in the number of flies that responded to gentle stimulus with movement, starting at 9 h and continuing throughout the time course.
Thus, the trend of the population is for spontaneous movement to decline first, followed by heartbeat, then shortly thereafter by movement in response to stimulus. Importantly, however, this is not necessarily the case for a given individual. Some flies completely lose heart function at a time point when they still exhibit spontaneous walking movements. Others cease to exhibit reflexive movements in response to touch while their hearts are yet beating (Supplemental Table 2).
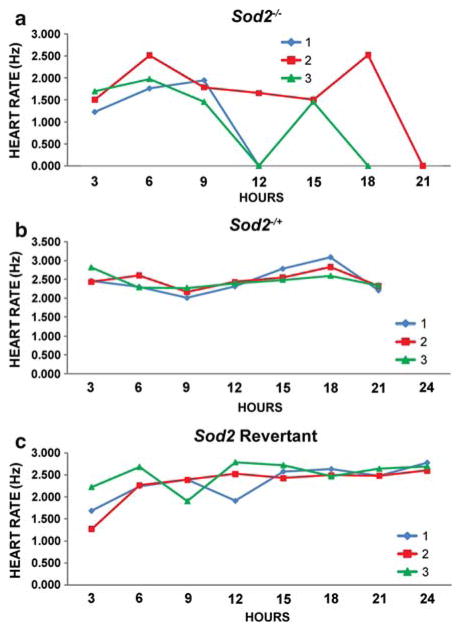
Strikingly, some individuals who appeared to have lost a functional heartbeat or response to stimulus completely at one time point were capable of recovering it at a later time point. Taking heartbeat as an example, both revertants and Sod2−/+ flies showed relatively consistent heart rates throughout the time course when graphed as individuals (Fig. 3b, c). Sod2−/− individuals, however, had wildly variant heartbeats over time, in several cases stopping completely, while recovering some degree of heart function later (Fig. 3a, Individual: P<0.0001, χ2 = 30.881).
Fig. 3.
Representative heart measurements reveal frequent individual differences in the temporal pattern of cardiac functional decline in a Sod2−/− flies. b Sod2−/+ flies and (c and d) Sod2 revertant flies show consistent heart rates across age with minimal individual variation
Cardiac dysfunction in Sod2−/− flies resembles that seen in aged wild-type flies
The Drosophila heart undergoes a suite of age-related changes during the aging process. Rhythmicity becomes gradually disorganized, with longer, more frequent pauses, punctuated by quicker bursts of rapid heartbeats, while both relaxation velocity and contractile volume gradually decrease (Ocorr et al. 2007; Taghli-Lamallem et al. 2008). A similar suite of changes occurs in an accelerated fashion in Sod2−/− flies.
According to measurements with non-invasive video-based techniques, Sod2 revertants demonstrate a normal heart beating (see Supplemental Movie 1) while Sod2−/− flies quickly exhibit rhythmic dysregulation, with long passages of non-productive movements, followed by passages of rapid heartbeats that display a reduced contractile volume change (see Supplemental Movie 2).
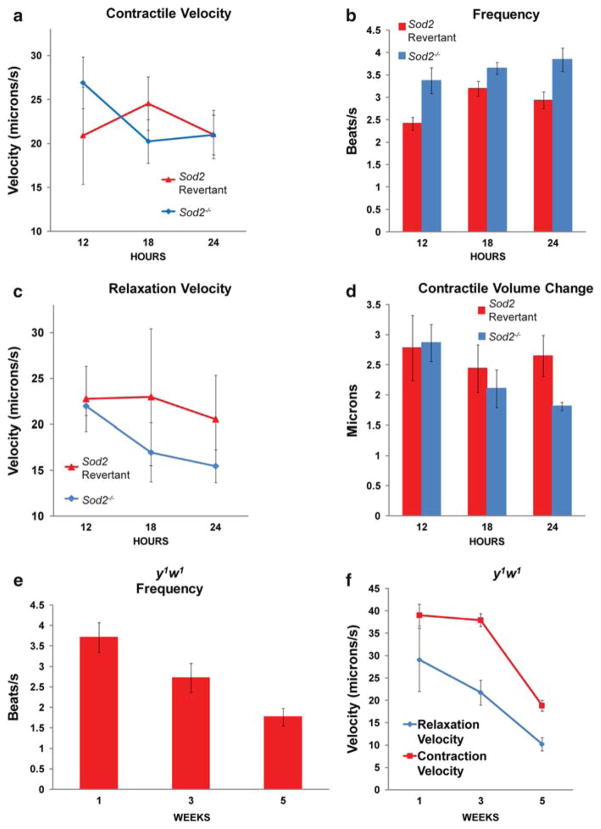
The contractile velocity of Sod2−/− hearts is not significantly different from that of revertant flies (Fig. 4a). However, the relaxation velocity of Sod2−/− hearts declines steadily (Fig. 4c, genotype-by-age: P = 0.0463), while the relaxation velocity of the revertants remains similar throughout a 24 h timecourse.
Fig. 4.
Relaxation velocity and contractile volume change decline in Sod2−/− flies. a Contractile velocity of Sod2−/− flies (blue) and Sod2 revertants (red).
b Frequency is shown of Sod2 revertants (red) and Sod2−/− flies (blue)
c Relaxation velocity of Sod2 revertants (red) and Sod2−/− flies (blue).
d Contractile volume output of Sod2 revertants (red) and Sod2−/− flies (blue).e y1w1 heart frequency is depicted across 5 weeks.
f Relaxation and contraction velocity of y1w1 is depicted across 5 weeks
When resting heart rate is measured over a minute or more, the total beats per minute of hearts from Sod2−/− flies is greatly reduced (Fig. 1c), due primarily to the frequent pauses, during which mutant hearts fail to initiate productive contractions (Supplemental Movie 2). As has been observed in aging wild-type hearts, however (Ocorr et al. 2007, Taghli-Lamallem et al. 2008), following long pauses, when heartbeat resumes, it tends to beat more rapidly. This is reflected in quantitative measurements of frequency of heartbeat over 15 s of activity. Under these conditions, mutant flies consistently show a higher heart rate across age (Fig. 4b).
This episodic, increased heart rate does not result in a maximized functional capacity. Indeed, the temporarily increased heart rate is gained at the expense of contractile productivity, as shown by the progressively reduced change in contractile volume in hearts from Sod2−/− flies (Fig. 4d, genotype-by-age: P = 0.0081). Note that this defect is likely to be exacerbated by the alterations in frequency and frequent pauses also observed in mutant hearts (Fig. 1c, Fig. 4b, Supplemental Movie 2).
In both aging wild-type flies (Wessells et al. 2004; Ocorr et al. 2007; Taghli-Lamallem et al. 2008) and in Sod2−/− flies (this study), this syndrome of rhythmic dysregulation, with long pauses followed by rapid, shallow heartbeats presumably results in a net reduction in circulatory volume and efficiency.
Functional assessment of very late-life wild-type flies
In order to assess the degree to which Sod2 mutant physiology mimics that seen as a consequence of normal aging, we performed similar longitudinal observations on y1w1 flies throughout life. No examples of failure to exhibit spontaneous ambulation, heartbeat or response to stimuli were observed prior to 64 days (Fig. 2d). After that time, the percentage of the cohort exhibiting failure in one or more of these categories steadily increased (Fig. 2d).
On a population basis, the pattern of observed functional failure was the same as that observed in Sod2 mutant flies, with spontaneous ambulation declining first, followed by heartbeat, with response to stimuli persisting the longest (Fig. 2a, d). However, far fewer wild-type flies exhibited any deviation from this pattern than did Sod2−/− flies. Indeed, only one example was found of a fly which exhibited functional failures in a different order (Supplemental Table 3).
To extend the comparison to qualitative aspects of cardiac function, we conducted video assays of cardiac function on y1w1 flies aged to 5 weeks, an age at which wild-type heart rate and cardiac stress resistance have already declined significantly (Wessells et al. 2004). Five-week-old y1w1 show a dramatic reduction in heart rate (2-way ANOVA, frequency-by-age: P = 0.0057), consistent with previously published wild-type data (Wessells et al. 2004), showing a heart rate of around 1.77 Hz. This is distinct from the phenotype seen in 24-hour-old Sod2−/− flies, in which there is little, if any, reduction in net heart rate (compare Fig. 4e with Fig. 1c). By contrast, both contraction velocity and relaxation velocity of five-week-old y1w1 flies was identical to that of 24-hour-old Sod2−/− flies (Student’s t-test: genotype effect, contraction velocity: P = 0.2118, relaxation velocity: P = 0.0008).
Discussion
Damage as a result of ROS has long been promoted as a potential mechanism of both normal and pathological aging (Harman 1956; Hekimi and Guarente 2003; Dugan and Quick 2005). ROS accumulation leads to accrual of damage to cellular macromolecules (Berlett and Stadtman 1997; Beal 2002; Choksi et al. 2004), and, eventually, contributes to loss of functionality in organ systems. Since different cell-types have different requirements for ATP generation, it is reasonable to expect that ROS will accumulate at different rates in different tissue-types, and that, as a consequence, different tissues may have a variable threshold of tolerance for accumulated ROS before a functional failure results.
The relationship between changes seen in models of high ROS accumulation and changes seen during normal aging is an important question. In the fly model for accelerated ROS accumulation used in this study, we observed changes in several cardiac functional parameters that qualitatively resemble changes seen during aging of wild-type flies (Wessells and Bodmer 2007). In particular, we observed a decline in relaxation velocity, with contraction velocity remaining relatively unaffected, a loss of control over rhythmicity, resulting in increasing occurrence of long pauses, followed by periodic bouts of rapid contractions with a lowered degree of contractile volume change. One potentially important difference between the two models is that the Sod2 mutant hearts appear to compensate for their decreased relaxation velocity by initiating additional contractions, resulting in both an increase in frequency and a corresponding decrease in output volume of each contraction. These changes are not seen in aging wild-type hearts (Wessells et al. 2004; Ocorr et al. 2007; Taghli-Lamallem et al. 2008). These results are consistent with a model in which accumulation of ROS is a contributing factor to some, but not all, functional changes during pathological aging and very late-life functional aberrations in the wild-type fly heart.
Interestingly, the functional failures observed in Sod2 mutant flies occur, on average, in the same order that systems failures occur in very late-life wild-type flies. Furthermore, we observed a similar pattern in two different genetic backgrounds, indicating that these effects were not peculiar to one background only. Nevertheless, there were important differences between Sod2 mutants and wild-type flies. Mutant flies with increased susceptibility to ROS do not appear to decline gradually, as if they were experiencing the same changes as wild-type flies in an accelerated timeframe. Rather, they exhibit almost immediate signs of aberrant function that would be characteristic of wild-type flies of very advanced ages. Arguably, Sod2 null flies are not so much an accelerated aging model, as a model for final pathologies associated with the last 2 days of the fruit fly lifespan. As such, Sod2 mutant flies may be a useful model for the examination of how age-related functional declines eventually result in lethal pathology.
Comparison of the results shown here from wild-type flies and flies with enhanced ROS production provides little evidence that ROS accumulation makes a significant contribution to a gradual age-related decline in functionality. Rather, they are more consistent with a threshold-based model, in which organ systems tolerate ROS accumulation up to a point, after which they are simply unable to perform their functions at any level. This has important implications for the use of high-ROS-sensitive model systems in gerontological studies.
In addition to understanding the contribution of ROS accumulation to functional aging, another important question addressed here is how systems failures in aging animals relate to each other. Do tissue and organ functions deteriorate in a reproducible order, with the loss of each one putting further pressure on the functional decline of remaining organs, in a “chain of dominos”? Or, conversely, do various organ systems deteriorate in a more stochastic fashion, with each organ deteriorating at its own rate based on the individual life history of the animal?
These results are more consistent with a stochastic response of various tissues to the damage induced by oxidative damage. When viewed at the population level, on average, there is a clear tendency for organs to fail in a reproducible order. However, these tendencies do not have an absolute predictive value for individuals. We observed every possible permutation of the order of functional decline in individual flies, suggesting that, although differential sensitivity does appear to exist, individual environmental or activity differences are capable of altering the trend. Wild-type flies in very late-life showed a much lower degree of variation in the order of the observed functional declines than did mutant flies, however. This suggests that on the scale of a full lifespan, differential sensitivity of various organ systems plays a more robust role than in the highly accelerated disease model represented by the mutant flies.
The observation that each of the functions measured in this study can sometimes be recovered in mutant individuals after being lost for long periods of time is consistent with the idea that homeostatic mechanisms are continuously operating to replace ROS-induced damage to macromolecules. Although these results do support a threshold model, in which sufficient ROS accumulation makes an organ system unable to function, they also indicate that this threshold is a two-way phenomenon, and that endogenous mechanisms can bring ROS levels back down to a level consistent with organ function, if the animal can survive long enough for this to occur. The observation that individuals may indeed recover functionality of an organ such as the heart, even after complete organ failure has been observed, suggests the possibility that induction of protective measures against ROS-induced damage may have profound effects on functionality even late in life, after significant damage has already accrued by normal and/or pathological aging.
Given the qualitative similarities between the changes to cardiac function observed in Sod2−/− flies and those seen in aging wild-type flies, this mutant strain may be a highly useful model to test interventions that potentially reverse defects in cardiac function that occur as a result of ROS-induced systems failure.
Supplementary Material
Acknowledgments
This work was supported by grants from the American Heart Association and the Glenn Foundation For Biological Mechanisms of Aging to R.J.W. and by the American Federation of Aging Research and the National Institutes of Health to M.G.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10522-008-9210-2) contains supplementary material, which is available to authorized users.
Contributor Information
Nicole Piazza, Department of Internal Medicine, Institute of Gerontology, University of Michigan Medical School, 3013 BSRB, 109 Zina Pitcher Place, Ann Arbor, MI 48109, USA.
Michael Hayes, Department of Internal Medicine, Institute of Gerontology, University of Michigan Medical School, 3013 BSRB, 109 Zina Pitcher Place, Ann Arbor, MI 48109, USA.
Ian Martin, Department of Human and Molecular Genetics and Neuroscience Program, Virginia Commonwealth University, Richmond, VA 23298, USA.
Atanu Duttaroy, Biology Department, Howard University, NW, Washington, DC 20059, USA.
Mike Grotewiel, Department of Human and Molecular Genetics and Neuroscience Program, Virginia Commonwealth University, Richmond, VA 23298, USA.
Robert Wessells, Email: Wessells@med.umich.edu, Department of Internal Medicine, Institute of Gerontology, University of Michigan Medical School, 3013 BSRB, 109 Zina Pitcher Place, Ann Arbor, MI 48109, USA.
References
- Adiga IK, Nair RR. Multiple signaling pathways coordinately mediate reactive oxygen species dependent cardiomyocyte hypertrophy. Cell Biochem Funct. 2008;26:346–351. doi: 10.1002/cbf.1449. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma R. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa R, Nagai T, Tanaka M. Reactive oxygen species in mechanical stress-induced cardiac hypertrophy. Biochem Biophys Res Commun. 2001;289:901–907. doi: 10.1006/bbrc.2001.6068. [DOI] [PubMed] [Google Scholar]
- Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Asimakis GK, Lick S, Patterson C. Postischemic recovery of contractile function is impaired in SOD2(+/−) but not SOD1(+/−) mouse hearts. Circulation. 2002;105:981–986. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- Ballinger SW. Mitochondrial dysfunction in development and disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/S0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Jones MA, Martin I, Grotewiel MS. Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell. 2007;6:631–637. doi: 10.1111/j.1474-9726.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Botella JA, Ulschmid JK, Gruenewald C, Moehle C, Kretzschmar D, Becker K, Schneuwly S. The Drosophila carbonyl reductase sniffer prevents oxidative stress-induced neurodegeneration. Curr Biol. 2004;14:782–786. doi: 10.1016/j.cub.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM, Jr, Klein JB, Ferguson J, Link CD, Butterfield DA. Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Abeta (1–42): implications for Alzheimer’s disease. Neurobiol Aging. 2006;27:1239–1249. doi: 10.1016/j.neurobiolaging.2005. 07.001. [DOI] [PubMed] [Google Scholar]
- Brys K, Vanfleteren JR, Braekman BP. Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol. 2007;42:845–851. doi: 10.1016/j.exger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta. 2004;1688:95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Curtis C, Landis GN, Folk D, Wehr NB, Hoe N, Waskar M, Abdueva D, Skvortsov D, Ford D, Luu A, Badrinath A, Levine RL, Bradley TJ, Tavaré S, Tower J. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:262. doi: 10.1186/gb-2007-8-12-r262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date MO, Morita T, Yamashita N. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–912. doi: 10.1016/S0735-1097 (01)01826-5. [DOI] [PubMed] [Google Scholar]
- Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Quick KL. Reactive oxygen species and aging: evolving questions. Sci Aging Knowl Environ. 2005;26:20. doi: 10.1126/sageke.2005.26.pe20. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics. 2003;165:2295–2299. doi: 10.1093/genetics/165.4.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005. 02.005. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- Huang H, Manton KG. The role of oxidative damage in mitochondria during aging: a review. Front Biosci. 2004;9:1100–1117. doi: 10.2741/1298. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp Gerontol. 2004;39:1137–1143. doi: 10.1016/j.exger.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Kharade SV, Mittal N, Das SP, Sinha P, Roy N. Mrg19 depletion increased S. cerevisiae lifespan by augmenting ROD defence. FEBS Lett. 2005;579:6809–6813. doi: 10.1016/j.febslet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev. 2005;126:365–379. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee RYN, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng 1295-376. [DOI] [PubMed] [Google Scholar]
- Llorens JV, Navarro JA, Martinez-Sebastian MJ, Baylies MK, Schneuwly JA, Botella Molto MD. Causative role of oxidative stress in a Drosophilamodel of Friedreich ataxia. FASEB J. 2007;21:333–344. doi: 10.1096/fj.05-5709com. [DOI] [PubMed] [Google Scholar]
- Melvin RG, Van Voorhies WA, Ballard JW. Working harder to stay alive: metabolic rate increases with age in Drosophilasimulans but does not correlate with life span. J Insect Physiol. 2007;53:1300–1306. doi: 10.1016/j.jinsphys. 2007.07.006. [DOI] [PubMed] [Google Scholar]
- Migliaccio M, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Miwa S, Riyahi K, Partridge L, Brand MD. Lack of correlation between mitochondrial reactive oxygen species production and life span in Drosophila. Ann N Y Acad Sci. 2004;1019:388–391. doi: 10.1196/annals.1297.069. [DOI] [PubMed] [Google Scholar]
- Morcos M, Du X, Pfisterer F, Hutter H, Sayed AA, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, Schlotterer A, Bozorgmehr F, El Baki RA, Stern D, Moehrlen F, Ibrahim Y, Oikonomou D, Hamann A, Becker C, Zeier M, Schwenger V, Miftari N, Humpert P, Hammes HP, Buechler M, Bierhaus A, Brownlee M, Nawroth PP. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–269. doi: 10.1111/j.1474-9726. 2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Nakai D, Shimizu T, Nojiri H, Uchiyama S, Koike H, Takahashi M, Hirokawa K, Shirasawa T. coq7/clk-1 regulates mitochondrial respiration and the generation of reactive oxygen species via coenzyme Q. Aging Cell. 2004;3:273–281. doi: 10.1111/j.1474-9728.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, Bodmer R. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophilathat mimic the effects of aging. Proc Natl Acad Sci USA. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternostro G, Vignola C, Bartsch DU, Omens JH, McCulloch AD, Reed JC. Age-associated cardiac dysfunction in Drosophila melanogaster. Circ Res. 2001;88:1053–1058. doi: 10.1161/hh1001.090857. [DOI] [PubMed] [Google Scholar]
- Paul A, Belton A, Nag S, Martin I, Grotewiel MS, Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128:706–716. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel DR, Adachi T, Ido Y, Heilbeck T, Jiang B, Lee Y, Melendez JA, Cohen RA, Colucci WS. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygenspecies-dependent Ras S-glutathiolation. J Mol Cell Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc. 2006.05.009. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Coevolution of exceptional longevity, exceptionally high metabolic rates, and mitochondrial DNA-coded proteins in mammals. Exp Gerontol. 2007;42:364–373. doi: 10.1016/j.exger.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Stoltzfus JR, Horton WJ, Grotewiel MS. Odor-guided behavior in Drosophila requires calreticulin. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:471–483. doi: 10.1007/s00359-003-0425-z. [DOI] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghli-Lamallem O, Akasaka T, Hogg G, Nudel U, Yaffe D, Chamberlain JS, Ocorr K, Bodmer R. Dystrophin deficiency in Drosophilareduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell. 2008;7:237–249. doi: 10.1111/j.1474-9726.2008.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richardson A. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:1422–1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Bodmer R. Cardiac aging. Semin Cell Dev Biol. 2007;18:111–116. doi: 10.1016/j.semcdb.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Wu Y, Luo Y. Transgenic C. elegans as a model in Alzheimer’s research. Curr Alzheimer Res. 2005;2:37–45. doi: 10.2174/1567205052772768. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Botas J. Mouse and fly models of neurodegeneration. Trends Genet. 2002;18:463–471. doi: 10.1016/S0168-9525(02)02729-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.