Abstract
Relapse is common in substance use disorders (SUDs), even among treated individuals. The goal of this article was to systematically review the existing evidence on mindfulness meditation-based interventions (MM) for SUDs.
The comprehensive search for and review of literature found over 2,000 abstracts and resulted in 25 eligible manuscripts (22 published, 3 unpublished: 8 RCTs, 7 controlled non-randomized, 6 non-controlled prospective, 2 qualitative studies, 1 case report). When appropriate, methodological quality, absolute risk reduction, number needed to treat, and effect size (ES) were assessed.
Overall, although preliminary evidence suggests MM efficacy and safety, conclusive data for MM as a treatment of SUDs are lacking. Significant methodological limitations exist in most studies. Further, it is unclear which persons with SUDs might benefit most from MM. Future trials must be of sufficient sample size to answer a specific clinical question and should target both assessment of effect size and mechanisms of action.
Keywords: mindfulness, meditation, addiction, relapse prevention, substance abuse
INTRODUCTION
According to the United Nations Office on Drugs and Crime,(1) approximately 200 million people worldwide are current drug users. In the U.S., an estimated 22.6 million were diagnosed with substance dependence or abuse in 2006.(2) The cost of drug abuse worldwide, in terms of crime, loss of work and health care costs, was estimated at 180.9 billion USD in 2002.(3) The human suffering related to substance use disorders is immeasurable.
Substance use disorders (SUDs) have been described as "chronic relapsing conditions," with rates of relapse exceeding 60% and being relatively consistent across substances of abuse.(4–6) A range of treatments have been developed to target relapse. Among behavioral interventions, cognitive behavioral therapy (CBT), including relapse prevention,(7) has received considerable support. However, in spite of best "standard of care" therapy, relapse rates continue to be high, highlighting the need for development of new treatment modalities to better assist individuals in their recovery.
The theoretical framework for mindfulness meditation suggests that it may be a promising approach to treating addictive disorders.(8,9) Mindfulness has been defined as the intentional, accepting and non-judgmental focus of one's attention on the emotions, thoughts and sensations occurring in the present moment.(10) Such a purposeful control of attention can be learned through training in techniques such as meditation.(11) The "observe and accept" approach, characteristic of meditation, refers to being fully present and attentive to current experience but not being pre-occupied by it. Thus, meditation can become a mental position for being able to separate a given experience from an associated emotion,(12) and can facilitate a skillful or mindful response to a given situation.(8) Meditation is often contrasted with everyday, habitual mental functioning or being on "auto-pilot." As such, meditation may be a valuable technique for SUD-affected persons, whose condition is often associated with unwanted thoughts, emotions and sensations (e.g. craving), the tendency to be on "auto-pilot", and pre-occupation with the “next fix”, rather than "being in the present moment." Meditation may also be a component of maintaining lifestyle balance, with meditation-acquired skills complementing and enhancing CBT effects for SUDs.(7,8,13,14)
Traditionally, meditative techniques have been taught and practiced through formal and in-formal meditation centers. More recently, meditation has also become a component of many therapeutic programs; in 1997, over 240 meditation programs were a part of U.S. health care systems,(15) and the basics of meditation are taught in many U.S. medical schools. Mindfulness Based Stress Reduction (MBSR) (10) is the most frequently cited method of mindfulness training in the medical context.(16) Based on the MBSR model, other therapies, combining both mindfulness and CBT elements have been developed, including the Mindfulness Based Cognitive Therapy (MBCT) for relapse related to recurrent depression (17) and Mindfulness Based Relapse Prevention (MBRP) for relapse related to SUDs.(9,18) Mindfulness is also a central part of Dialectical Behavior Therapy (DBT) for individuals with borderline personality disorder,(19) Acceptance and Commitment Therapy (ACT) (20) for individuals with a variety of mental health problems, and Spiritual Self-Schema (3-S) therapy designed for clients with SUDs.(21)
While the use of mindfulness meditation as a therapy for SUDs is not new and has been associated with anecdotal clinical success,(14) until recently there has been a paucity of research to support its empirical efficacy. The growing interest in complementary and alternative medicine (CAM), especially mind-body therapies,(15,22) has brought a surge of interest in research evaluating the effects of meditation in a range of clinical contexts, including addictive problems. The current evidence on the clinical applications of mindfulness meditation for SUDs has not been rigorously reviewed.
The goal of this article was to systematically review and assess the existing evidence on the effects of mindfulness or mindfulness meditation based therapies for addictive disorders. Although there are various forms of meditation, it is not known whether these approaches have similar effects on the problems or disorders under consideration. This review focused specifically on mindfulness meditation, and the term "meditation", as used in this manuscript, refers exclusively to mindfulness meditation.
METHODS
Criteria for selection of studies
Inclusion criteria: 1) study intervention was mindfulness or mindfulness meditation-based (MM), 2) used as a therapy for substance use, misuse or related disorders; 3) the study was longitudinal, with pre- and post-intervention assessments; and 4) it involved human subjects. Exclusion criteria: 1) lack of a sufficient description of the study intervention to determine if it was rooted in mindfulness; 2) non-English; and 3) only interim results of an unpublished study were available. We anticipated that the number of eligible studies would be limited; therefore, we did not exclude studies based on design (experimental vs. non-experimental), methodological quality or specific intervention protocol. Both published and unpublished reports were eligible for inclusion.
Search strategy
The research librarian (RK) worked closely with the co-authors (AZ, KK) to refine search strategies. Comprehensive searches (Table 1) were conducted through Mar 9, 2008 of the following electronic databases: Cochrane Database of Systematic Reviews (since 1995), EMBASE (since 1993), PubMed (including PreMed and Old Med, since 1950), PsycINFO (since 1967), CINAHL (since 1982), and Allied and Complementary Medicine (since 1985). The National Institutes of Health (NIH) CRISP electronic database of relevant institutes (National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, National Institute of Mental Health, National Center for Complementary and Alternative Medicine) was searched from 1995 – Mar 9, 2008 with keywords: “meditation” OR “mindfulness”. The Scientific Research on The Transcendental Meditation® Program: Collected Papers (Volumes 1 to 4) were hand-searched. The reference lists of relevant articles were reviewed to identify potentially eligible studies. E-mail or phone contact was attempted with relevant author(s) or Principle Investigator(s) of included articles or abstracts when additional information was needed.
Table 1.
Electronic database search strategy.
| Step | Search Strategy |
|---|---|
| 1 | Meditation [tw] or relaxation [tw] or mindfulness [tw] or “breathing technique” [tw] or “breathing exercises” [tw] |
| 2 | Smoking [tw] or tobacco [tw] or caffeine [tw] or “substance-related disorder” [tw] or “drug abuse” [tw] or addiction [tw] or “drug dependence” [tw] or “drug habituation” [tw] or “drug usage” [tw] or “substance abuse” [tw] or “substance dependence” [tw] or “substance use” [tw] or alcohol [tw] or alcoholism [tw] or “street drugs” [tw] or cocaine [tw] or marijuana [tw] or marihuana [tw] or opioid [tw] or heroin [tw] or morphine [tw] or stimulant [tw] or ecstasy [tw] or nicotine [tw] |
| 3 | 1 and 2 |
Identification of eligible studies
The titles and abstracts of all identified studies were screened (RK and AZ, “initial screening”). Studies that clearly described using only Transcendental Meditation, Progressive Muscle Relaxation, Biofeedback or Autogenic Training as their interventions were excluded. The full-text of studies describing the use of meditation, mindfulness, relaxation, yoga, breath practices or other techniques that were compatible or potentially compatible with mindfulness meditation (as the primary or comparison interventions) were then reviewed (AZ, “secondary screening”). Practices that were included as compatible with MM are described in the following section. The secondary screening resulted in an exclusion of ineligible articles (AZ); articles that were considered potentially eligible were then additionally reviewed (“tertiary screening”) by 3 independent reviewers (NC, GAM, KK), experts in psychology, meditation and relapse prevention for SUDs.
Data extraction and study assessments
Data from all eligible articles were extracted, and the methodological quality of controlled and prospective case series studies was assessed for internal validity by two unblinded reviewers (AZ, DR). We used an adapted version of a scoring instrument suited for studies using behavioral interventions and developed for the systematic review of alcohol treatment trials.(23) This instrument (Table 2) was adapted for use in SUDs with input from Dr. William Miller, co-developer of the original scale (personal communication, Mar-Apr, 2008).
Table 2.
Scoring* of the prospective studies (range of points): Population Severity Rating (PSR); Clinical Benefit Score (CBS); Methodological Quality Score (MQS); and Cumulative Evidence Score (CES).
| PSR (range: 0 – 4) |
0 = insufficient information; 1 = non-clinical sample, mild or no problems; 2 = non-clinical sample of nicotine dependent smokers; or drug users or problem drinkers who did not seek treatment and did not have severe problems or dependence; 3 = clinical sample of drug users or problem drinkers who sought treatment for substance use related problems, but who did not have dependence; 4 = severely impaired clinical population in treatment for drug or alcohol dependence. |
| CBS (range: −2 / +2) |
+2 = controlled trials: MM was significantly better than ‘no Tx’ (MM > 0); or MM combined with other Tx was significantly better than that Tx alone (MM+A > A); non-controlled trials: significant improvement on primary outcome post- vs. pre-intervention. |
| +1= controlled trials: MM was significantly better than an alternative Tx (including SOC), or better than a briefer form of the same MM therapy, without control (MM > A);non-controlled trials: significant improvement post- vs. pre-intervention on secondary outcome only. | |
| −1 = controlled trials: MM outcomes were comparable to those of an alternative Tx (MM = A) or MM combined with other Tx (MM = MM+A), or briefer form of the same MM therapy, or mixed differences among active Tx arms, without control; non-controlled trials: no significant change in any outcomes post- vs. pre-intervention. | |
| −2 = controlled trials: MM was worse than a comparable other Tx of similar intensity without control (MM < A), or not better than a briefer dissimilar Tx without control, or MM combined with other Tx produced worse results than other Tx alone (MM+A < A), or MM is not better than no-Tx (MM ≤ 0); non-controlled trials: no improvement, but presence of worsening in the outcomes post- vs. pre-intervention. | |
| MQS (range: 0–17) non-controlled trials: maximum score = 12 |
|
| CES | CES score = CBS x MQS (range for an individual study: −34 / +34) |
| Negative (−) CES indicates studies showing a comparable or worse Tx outcome, positive (+) CES indicates studies showing a significant benefit of the MM therapy, compared to a comparison group in controlled trials, or baseline in uncontrolled trials. | |
MM: Mindfulness or Mindfulness Meditation based intervention; SOC: standard of care; Tx – treatment.
Adapted with permission from William Miller (personal communication, 2008) (23).
Using this instrument, the studies were assigned the following sub-scores (Table 3): a) Population Severity Rating (PSR), b) Methodological Quality Score (MQS), c) Clinical Benefit Score (CBS, called the Outcome Logic Score in the original scale (23)), and d) Cumulative Evidence Score (CES), where: CES = CBS x MQS. e) The Overall CES was also calculated for subgroups of studies (e.g., RCTs), as a sum of CES of individual studies.
Table 3.
Summary of the Cumulative Evidence Scores of the published included studies, grouped by study design, type of Mindfulness or Mindfulness Meditation intervention (MM) and subject population, sorted within groups by the mean Methodological Quality Score of the studies.
| Studies | Number of studies |
Methodological Quality Score, MQS (mean / 17) |
Population Severity Score, PSS (mean / 4) |
Cumulative Evidence Score, CES (sum of scores of the individual studies) |
% positive (% studies with +CES) |
|---|---|---|---|---|---|
| Total | 15* | 8.0 | 3.3 | ITT: +10 (6 studies) | ITT: 50% |
| PP: +143 (13 studies) | PP: 85% | ||||
| By design | |||||
| RCTs | 7 | 11.3 (range: 8–14) | 3.7 | ITT: +2 (4 studies) | ITT: 50% |
| PP: +93 (6 studies) | PP: 83% | ||||
| Controlled, non-randomized | 4* | 6.8 (range: 6–8) | 3 | ITT: −8 (1 study) | ITT: 0% |
| PP: +16 (3 studies) | PP: 67% | ||||
| Case series | 4 | 5.8 (range: 4–8) | 3.25 | ITT: +16 (1 study) | ITT: 100% |
| PP: +34 (4 studies) | PP: 100% | ||||
| By MM intervention | |||||
| DBT | 2 RCTs | 13.5 (13, 14) | 4 | ITT: +27 (2 studies) | ITT: 100% |
| PP: +13 (1 study) | PP: 100% | ||||
| ACT | 2 RCTs | 12.5 (12, 13) | 3 | ITT: −25 (2 studies) | ITT: 0% |
| PP: +50 (2 studies) | PP: 100% | ||||
| 3-S | 3 | 8.7 (range: 7–11) | 4 | ITT: − (0 studies) | ITT: - |
| (2 RCT, 1 controlled) | PP: +26 (3 studies) | PP: 67% | |||
| MBSR-based | 7 * | 6.4 (range: 4–8) | 3.3 | ITT: +8 (2 studies) | ITT: 50% |
| (1 RCT, 2 controlled, 4 case series) |
PP: +42 (6 studies) | PP: 83% | |||
| Vipassana | 1 controlled | 6 | 2 | ITT: − (0 studies) | ITT: - |
| PP: +12 (1 study) | PP: 100% | ||||
| By subject population | |||||
| SUDs, adults, outpatient settings | 7 | 10.3 (range: 7–14) | 4 | ITT: +15 (3 studies) | ITT: 67% |
| (5 RCTs, 1 controlled, 1 case series) |
PP: +69 (6 studies) | PP: 83% | |||
| SUDs, adults, tobacco dependence | 3* | 9 (6, 8, 13) | 2 | ITT: +3 (2 studies) | ITT: 50% |
| (1 RCT, 1 controlled, 1 case series) |
PP: +42 (2 studies) | PP: 100% | |||
| SUDs, adults, residential settings | 3 | 6.7 (4, 8, 8) | 4 | ITT: −8 (1 study) | ITT: 0% |
| (1 RCT, 1 controlled, 1 case series) |
PP: +12 (3 studies) | PP: 67% | |||
| Substance use, adults, jail | 1 controlled | 6 | 2 | ITT: − (0 studies) | ITT: - |
| PP: +12 (1 study) | PP: 100% | ||||
| Substance use, adolescents | 1 case series | 4 | 3 | ITT: − (0 studies) | ITT: − |
| PP: +8 (1 study) | PP: 100% | ||||
one non-randomized controlled study did not use statistical analysis to compare results
When possible, absolute risk reduction (ARR), number needed to treat (NNT) and effect size (ES) were calculated by the authors (AZ, DR) for the main substance-use related outcomes. The following formulas were used: a) ARR = absolute difference in outcome rates between the control and treatment groups; b) NTT = 1/ARR; and c) ES, Cohen’s d, was either converted from correlations (where r=0.37 corresponded to d=0.8, r=0.24 corresponded to d=0.5, and r=0.1 corresponded to d=0.2) or calculated from the mean values (M) and standard deviations (SDs); Cohen’s d for controlled trials (formula #1) was calculated as d = [M1 – M2] / SDpooled, where M1, M2 were the means in the two groups, and SDpooled = √ [(SD12 + SD22)/2]; Cohen’s d for uncontrolled pre-post trials (formula #2) was calculated as d = [mean of the pre-post difference] / [SD of this mean]). In cases where it was not possible to use formula #2, then formula #1 was used.
RESULTS
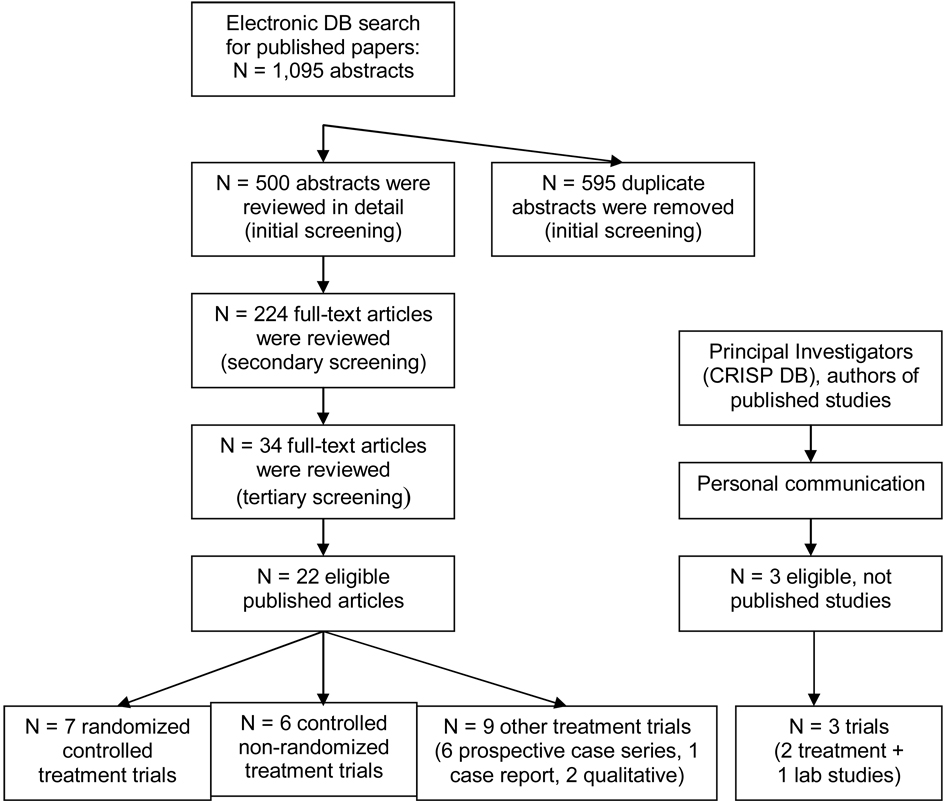
1. Literature search results (Figure 1)
Figure 1.
Results of the literature search (DB – database).
The search identified 1,095 abstracts of published articles. After removing 595 duplicates (RK), 500 abstracts were reviewed (AZ, initial screening). Of those, 276 were excluded. The full-texts of 224 articles were then reviewed (AZ, secondary screening), and 34 articles were submitted for a tertiary screening. Twelve of the 34 articles (24–35) did not meet the eligibility criteria and were excluded. Of the excluded studies, two deserve an additional comment.(24,34) Both studies used the body scan technique as a study intervention. Although the body scan was derived from a mindfulness meditation-based program, in isolation, this technique did not meet the criteria for mindfulness or mindfulness meditation because MM-based body scan involves not only instructions on paying attention to the various parts of the body, but also encourages focused awareness, without attempt to change or manipulate body sensations, thus promoting non-judgmental and compassionate acceptance of whatever is occurring in the body at any given moment.
The 22 published articles included 7 RCTs,(36–42) 6 controlled non-randomized trials,(43–48) 6 prospective case series,(49–53) 1 case report (54) and 2 qualitative studies.(55,56) Seventeen of these reports were based on separate clinical trials and 5 on secondary database analyses.(45,48,52,55,57) The CRISP database search found 324 “hits,” resulting in 9 additional relevant abstracts. Through personal communication, 2 ready-for-submission, but unpublished articles (58,59) and 1 PhD dissertation (60) were additionally identified as eligible, resulting in a total of 25 included studies. Of note, after completion of this manuscript, results of one of the "unpublished studies" (58) have been published.(61)
The heterogeneity of the included studies and interrater agreement on methodological quality scoring were not formally assessed. The wide variety of conditions treated, treatment protocols and outcome measures used was apparent on inspection, and made the pooling of data impossible. Disagreements between the reviewers were resolved by consensus.
2. Methods of the included published studies (Table 3 – Table 5)
Table 5.
Published controlled non-randomized trials, case series, case report and qualitative studies of mindfulness or mindfulness meditation based interventions (MM) used for the treatment of substance use, misuse or disorders: methods and results. Results from the final follow-up are reported, unless stated otherwise.
| Study | Indication | Subjects | Intervention | Outcome measures | Results | Methodological quality/ comments |
|---|---|---|---|---|---|---|
| Controlled non-randomized trials | ||||||
|
Study 1. Margolin et al. 2007 (47) |
Substance use (as a part of HIV risk behavior assessment) in HIV-positive, opiate dependent, methadone maintenance out-patients. |
38 (19 F); mean age 45.3 (33–57 yrs); PSR 4; 55% cocaine use disorder; 45% have continued using drugs; 71% were prescribed an HIV Tx. |
Per subject choice: • MM (N=21), SOC + 3-S, 12 wks, therapist-led individual (60 min/wk) & group (60 min/wk) sessions; • CG (N=17): SOC only. |
• Collected at 0, 12 wks; • substance use (self- report, UTox); • drug- and sex-related HIV risk behaviors; • impulsivity, spirituality, religiosity; • Tx experiences. |
• Retention: MM 67%, CG 65%; PP: • compared to CG, MM showed trend to decreased alcohol and drug use (p=0.08 [ES 0.25]), and improved impulsivity, spirituality and motivation for abstinence (p<0.05); • 3-S attendance correlated to decreased substance use (r= 0.49 [ES 0.2], impulsivity, and increased influence of spirituality on abstinence and HIV prevention motivation (p<0.05). |
MQS: 7/17; CBS: N/A per ITT (+2 PP) Subject meditation practice was not reported; adverse effects and side effects were not mentioned. |
|
Study 2a. Bowen et al. 2006 (44) |
Substance use among prisoners of the minimum- security jail. |
305 (63 F), mean age 37.5 (19–58 yrs); PSR 2; alcohol use 83%, tobacco use 83%, drug use 73% during 90 days prior to incarceration. |
Per subject choice: • MM (N=63): SOC + vipassana meditation (VM), 10 consecutive days: silent, gender- specific course, 8–10 hrs/day, led by a trained instructor; • CG (N=242): SOC only. |
• Collected at 0, 1 wk post-intervention, 3 & 6 mos post-release; • substance use (self- report), related adverse consequences; • psychological health. |
• Retention at 3 & 6 mos: MM 46% & 43%; CG 24% & 21%. PP: • at 3 mos, compared to CG, MM reduced (p<0.05) alcohol [ES 0.6], cocaine [ES 0.35] and marijuana [ES 0.5] use, and alcohol-related consequences [ES 0.35], and improved psychiatric symptoms, drinking-related locus of control and optimism; the changes were related to the VM participation; • at 6 mos, recidivism rates (the only results reported for 6 mos) were low, and comparable between the groups. |
MQS: 6/17; CBS: N/A per ITT (+2 PP) Subject post-intervention meditation practice was not recorded; adverse effects and side effects were not mentioned. |
|
Study 2b. Bowen et al. 2007 (45) (secondary analysis of Study 2a). |
Relationship between substance use & thought suppression; prisoners. |
See Bowen et al. 2006 (44) N=81 (for the main analysis of 0–3 month outcomes) |
See Bowen et al. 2006 (44) |
See Bowen et al. 2006 (44) |
PP: • at 3 mos, compared to CG, MM decreased thought avoidance (p<0.05); this change partially mediated the relationship between the VM participation, and alcohol use and related consequences. |
See Bowen et al. 2006 (44) |
|
Study 2c. Simpson et al. 2007 (48) (secondary analysis of Study 2a). |
Relationship between substance use & PTSD severity; prisoners |
See Bowen et al. 2006 (44) ~ 22% of the subjects scored positively for PTSD; N=88 : 29 MM and 59 CG (for the main analysis of 0–3 month outcomes) |
See Bowen et al. 2006 (44) |
See Bowen et al. 2006 (44) |
PP: • no differences in the PTSD symptom severity between the MM and CG groups; VM participation and baseline substance use, but not PTSD severity, predicted alcohol and drug use at 3 mos; baseline PTSD severity predicted adverse drinking consequences and psychological distress at 3 mos; PTSD subjects tolerated the VM course well; |
See Bowen et al. 2006 (44) |
|
Study 3. Altner 2002 (43) |
Smoking cessation among tobacco dependent hospital employees |
114 (71 F), mean age ~ 38.5 (20–65 yrs); PSR 2 |
Per subject choice: • MM (N=49): NRT + MBSR, 8 wks: therapist-led (2.5 hr/wk) group sessions; • CG (N=65): NRT only |
• Collected at 0, 1.5, 3, 6, 15 mos (quit date likely at ‘0’); • % subjects who stopped, reduced or did not reduce smoking (self-report, exhaled CO); • Tx experiences among meditators (N=23, qualitative) at 3 mos. |
• Retention: MM 100%; CG 97%. Descriptive statistics: • MM vs. CG subjects reported quit rate of 32.6% vs. 24.6% [ARR 8%, NNT 12.5], reduced smoking by 42.3% vs. 26.2%, continued smoking by 22.4% vs. 46.2%; • qualitative data: meditators reported positive opinions on the MBSR therapy and its usefulness as a coping strategy. |
MQS: 6/17; CBS: N/A* *No statistical assessment of the significance of in-between group differences or pre-post changes was provided; only descriptive statistics were used. Exhaled CO-related results were not reported. |
|
Study 4. Marcus et al. 2001 (46) |
Psychological health in alcohol or drug dependent patients of therapeutic community |
36 (2 F), mean age ~ 34 years; PSR 4 |
Per subject choice: • MM (N=18): SOC + MBSR, 8 wks, therapist-led (2.5 hrs/wk) group sessions; • CG (N=18): SOC only |
• Collected at 0, 8 wks; • psychopathology (SCL-90R), coping styles |
• Retention: MM & CG 100%; ITT=PP: • compared to CG, MM group tended to report a more self- controlling coping style (p=0.05, eta squared effect size: 0.11); no other differences between the groups were found (p>0.05); • effect sizes (eta squared 0.05–0.06) tended to favor the MM group in seeking social support, on hostility and paranoid ideation scores (per authors, eta squared effect size: 0.01– small, 0.06– medium, 0.14– large). |
MQS: 8/17; CBS: −1 per ITT (−1 PP) CES: −8 per ITT (−8 PP) Group were derived from separate residential facilities. |
| Case series | ||||||
|
Study 5. Zgierska et al. 2008 (53) |
Relapse prevention in alcohol dependent adults, graduates of the Intensive Outpatient Program |
19 (10 F), mean age 38.4 (21–50 yrs); PSR 4; alcohol-related Tx in the past: 63% |
Mindfulness Based Relapse Prevention, 8 wks, therapist-led (120 min/wk) group sessions; MM component was based on the MBSR & MBCT programs, and Relapse Prevention component was based on Cognitive Therapy. |
• Collected at 0, 4, 8, 12, 16 wks; • alcohol use (self- report); • severity of alcohol relapse triggers: stress, anxiety, depression, craving; • salivary cortisol, serum IL-6, liver enzymes at 0, 16 wks; • meditation-related outcomes; Tx services utilization; Tx experiences. |
• Retention: 78.9% PP: • During the study, HDD decreased (p=0.056, ES 0.3), total number of drinks (ES 0.3) and PDA (ES 0.03) did not significantly change; • stress, depression, anxiety severity improved (p<0.05, ES 0.7- 1.4); IL-6 level decreased (p=0.05, ES 0.6); craving severity (ES 0.5), cortisol and liver enzymes levels did not significantly change; • degree of mindfulness increased (p<0.05, ES 0.7); all completers meditated at 16 wks, on average 4.6 days/wk during the study; • high Tx satisfaction; no adverse events. |
MQS: 7/17; CBS: N/A per ITT (+1 PP) The only study that directly reports (lack of) side effects and adverse events, and describes evaluating distributional characteristics of the data, with the use of parametric or non-parametric tests, when appropriate. |
|
Study 6. Davis et al. 2007 (50) |
Smoking cessation, community setting |
18 (10 F), mean age 45.2 (22–67 yrs); PSR 2; on average, subjects smoked 19.9 cigarettes/day for 26.4 yrs. |
MBSR-based, with minor modifications, 8 wks, therapist-led group sessions (six 150 min/wk sessions + one 7 hr retreat); quit date at wk 7 (after the retreat). |
• Collected at 0–8 wks weekly, then 6 wks post-quit (12 wks post- entry); • smoking (self-report, exhaled CO); • stress, psychopathology symptom severity. |
• Retention: 72% (however, 100% data collection rate for self-reported smoking). ITT=PP: • 10/18 (56%) quit smoking; PP: • compared to non-quitters, those who quit meditated more (p<0.05), with a possible dose-effect: 100% highly compliant, 40% moderately compliant, and 0% non-compliant meditators quit; • compared to moderately compliant, highly compliant meditators decreased severity of stress one day post-quit (p<0.05); baseline interest in meditation and affective distress were related to abstinence (p<0.05). |
MQS: 8/17 CBS: +2 per ITT (+2 PP) Due to 100% data collection rate for the primary outcome, the primary analysis includes all subjects. |
|
Study 7a. Bootzin & Stevens 2005 (49) |
Sleep and sleepiness problems as relapse triggers among adolescents with SUDs |
55 (21 F), age 13–19 yrs; PSR 3; adolescents with sleep or daytime sleepiness problems, graduates or graduating from out- patient addiction Tx programs. |
MBSR-based, therapist-delivered in a small group format over 5 sessions, during 7 wks: 1st session – other interventions, not MBSR; 2nd-6th sessions: 45 min MBSR + 45 min other Tx (stimulus control, bright light therapy, sleep hygiene, CT). |
• Collected at 0–9 wks (weekly), 13, 52 wks; • self-reported and objective sleep and sleepiness related measures; • self-reported substance use and psychological distress. |
• Retention: 93%; PP: • drug use, low at baseline, increased during the Tx - no details provided; • substance problem index plateaued for Tx completers (42% of the subjects), while it kept rising for those who did not complete Tx (p<0.2, no details provided); • sleep improved (p<0.05) among Tx completers only; sleepiness, worry and mental health distress decreased during the study (p<0.05). |
MQS: 4/17; CBS: N/A per ITT (+2 PP) Study focused on methods description; only preliminary results were reported, without details on substance use outcomes. The study intervention included MM (MBSR-based; slightly less than 50%), but MM was not its primary focus. |
|
Study 7b. Haynes et al. 2006 (57) (secondary analysis of Study 3a) |
Is sleep improvement related to improved aggressive behavior among adolescents with SUDs |
23 (13 F), mean age 16.4 (13–19 years); see Bootzin & Stevens 200549 for other details |
See Bootzin & Stevens 2005 (49) |
• See Bootzin & Stevens 2005 (49); • two questions on presence or absence of aggressive thoughts or actions. |
• Retention: 91%; PP: • Those reporting aggression at baseline, compared to others, had lower self-efficacy in resisting substance use urges (p<0.05); • post- Tx, those reporting aggression, compared to others, reported more frequent substance use, especially alcohol use (p<0.05); • all subjects improved some aspects of their sleep; poor sleep was related to aggression, after controlling for substance use. |
See Bootzin & Stevens 2005 (49); Substance use was used as a covariate in the analysis, but was not the focus. No details on substance use are reported. |
|
Study 7c. Stevens et al. 2007 (52) (secondary analysis of Study 3a) |
Is sleep improvement related to improved trauma symptoms severity among adolescents with SUDs |
20 (10 F), mean age 16.3 (13–19 years); see Bootzin & Stevens 2005 (49) for other details. |
See Bootzin & Stevens 2005 (49) |
• See Bootzin & Stevens 2005 (49); • Trauma Severity Index. |
• Retention: unclear; PP: • Those with elevated trauma score at baseline, compared to others, had higher Substance Problem Index (p<0.05). Substance use did not play a significant role in the analyses. • Those with better sleep characteristics had greater improvements in trauma scores than others. |
See Bootzin & Stevens 2005 (49); Substance use was used as a covariate in the analysis, but was not the focus. No details on substance use are reported. |
|
Study 8. Marcus et al. 2003 (51) |
Stress severity among substance dependent patients in residential Tx settings |
21 (3 F), mean age 33.4 (21–51 yrs); PSR 4; therapeutic community patients with SUDs. |
SOC + MBSR, 8 wks, therapist-led (150 min/wk) group sessions. |
• Collected at 0, 8 wks; • salivary cortisol upon awakening; • Perceived Stress Scale. |
• Retention: 85.7% (data collection rate: 57% cortisol, 76% surveys); PP: • cortisol level decreased (p<0.05, ES 0.65); • perceived stress severity did not change (p>0.05, ES 0.44). |
MQS: 4/17; CBS: N/A per ITT (+1 PP) The study did not report substance use data. |
| Other studies | ||||||
|
Study 8 – case report. Twohig et al. 2007 (54) |
Marijuana dependence, community settings |
3 (1 F), ages 19, 20, 43; PSR 2; marijuana dependent (5 were enrolled, 2 dropped out) |
ACT, 8 wks, therapist-led (90 min/wk) individual therapy sessions. |
• Collected at 0–8 wks (daily), and 13 wks; • marijuana use (self- report, salivary swab); • withdrawal severity, psychological outcomes; |
• Retention: 3/5 (60%); • although the 3 subjects did not use marijuana at 8 wks, they resumed its use by 13 wks (one to the pre-Tx level, and two at less than pre-Tx levels); • withdrawal, anxiety and depression severity seemed to improve compared to baseline. |
Methodological quality not scored. Manualized intervention. |
|
Study 9- qualitative study. Carroll et al. 2008 (56) |
MBSR-related treatment experiences among substance dependent patients in residential Tx settings |
36 (6F), mean age 32.6 (19–54 yrs); PSR 4; residents of therapeutic community with SUDs. |
MBSR, adapted to therapeutic community settings, 6 wks, therapist-led (180 min/wk) group sessions. |
• 356 stories were reviewed (written as a part of guided expressive writing); • 38 stories of stress that referenced the MBSR therapy were identified and analyzed. |
Analysis of 38 stories identified 3 main MBSR qualities: • utility (usefulness for calming self, stress- reduction, coping), • portability (ability to apply learned skills in real- life), and • sustainability (application of skills to a variety of different situations, goals). |
This report was based on the ongoing unpublished trial.(72) Manualized intervention - the intervention description (MBSR for therapeutic community, MBSR-TC) was published elsewhere.(87) |
|
Study 10- qualitative study. Beitel et al. 2007 (55) (based on Margolin et al. 2006 (42) and 2007 (47) studies) |
3-S treatment experience in methadone maintenance patients, a part of the studies by Margolin et al. 2006 (42) and 2007.(47) |
39 (34 F), mean age 43 (28–54 yrs); PSR 4; opiate dependent patients of methadone maintenance program, cocaine use disorder 77%, HIV- positive 38%. |
3-S therapy: individual (46%) or individual + group (54%) – see Margolin et al. 2006 (42) and 2007 (47) for details. |
• Collected at post-Tx (8–12 wks post-entry); • Tx experiences questionnaire and interview. |
• Preferred Tx format: 43% group, 14% individual, and 43% equally liked individual and group sessions; • all subjects meditated, on average 26 min/day; • 3-S was viewed as helpful for recovery, and different from the received SOC; • meditation was the most liked and helpful aspect of the 3-S therapy; 49% reported a positive change resulting from 3-S therapy; subjects were satisfied with 3-S, and 97% would like to continue it; • no significant adverse events were reported. |
See Margolin et al. 2006 (42) and 2007 (47) for details. Subjects did not describe any significant negative effects (side effects or adverse events) or problems associated with 3-S therapy. |
Values (presented in [square brackets]) calculated for the systematic review: ARR: Absolute Risk Reduction; CBS: Clinical Benefit Score; CES: Cumulative Evidence Score; ES: Effect Size (Cohen's d); MQS: Methodological Quality Score; NNT: Number Needed to Treat; PSS: Population Severity Score.
CG: comparison group; CO: carbon monoxide; MM: mindfulness meditation; HIV: Human Immunodeficiency Virus; ITT: intention to treat analysis; MBCT: Mindfulness-Based Cognitive Therapy; MBSR: Mindfulness-Based Stress Reduction; min: minutes; mos: months; NRT: Nicotine Replacement Therapy; PP: per protocol analysis; PTSD: post-traumatic stress disorder; 3-S: Spiritual Self Schema; SOC: "standard of care" therapy; SUDs: Substance Use Disorders; Tx: treatment; UTox: urine toxicology test; VM: vipassana meditation; wks: weeks; yrs: years.
a) Studied population
Three studies focused on adolescents,(49,52,57) while the remaining 19 studies evaluated adults. Of the 22 studies, 12 evaluated severely impaired subjects (Population Severity Rating, PSR 4/4), treated for alcohol and/or drug dependence in residential (36,46,51,56) or outpatient settings,(37,40,41,47,53,55,62) three described a clinical sample of adolescents with SUDs (PSR 3/4),(49,52,57) three included a non-clinical sample of substance-using prisoners (PSR 2/4),(44,45,48) and four were based on community-recruited adults (PSR 2/4) with tobacco (38,43,50) or marijuana (54) dependence.
b) MM intervention
The MM interventions used in the included studies were based on 5 main models:
Vipassana meditation
(http://www.dhamma.org) is a form of MM that is deeply rooted in the Buddhist tradition. Most contemporary forms of MM derive from traditional Vipassana meditation. Typical Vipassana courses are group-based, last ten consecutive days, are conducted in silence, and involve meditating for 10–11 hours per day. They are available at no charge and follow a similar curriculum world-wide.
Three articles,(44,45,48) based on one study,(44) described the effects of a traditional vipassana training led by a traditionally trained teacher, in a prison settings. The intervention was standardized (followed a traditional vipassana format), but its delivery was not monitored.
Mindfulness Based Stress Reduction (MBSR)
Originally developed for management of chronic pain and stress-related disorders,(10) MBSR has been shown to be effective or potentially effective for many mental health and medical conditions.(16) MBSR is the most frequently cited method of meditation training in the medical context,(16) and has a published curriculum.(10) The usual MBSR course consists of 8 weekly, therapist-led group sessions (2–2.5 hours per session), one full-day retreat (7–8 hours) and daily home assignments. The MBSR curriculum served as a model for the manualized Mindfulness Based Cognitive Therapy (MBCT) (17) that combines meditation (10) and traditional, cognitive therapy strategies (63) to prevent relapse in recurrent depression. MBCT has been shown to reduce the rate of depressive relapse among persons with recurrent depression,(64,65) and may be efficacious for symptom reduction in "active" depression (66) and anxiety disorders as well.(67) Using the MBCT model in turn, a manualized Mindfulness Based Relapse Prevention (MBRP) program has been developed for outpatient clients with SUDs.(9,18) The elements of cognitive therapy in MBRP are based on relapse prevention cognitive therapy strategies (7) that have demonstrated efficacy for SUDs.(68) Evaluation of the MBRP program for SUDs is currently ongoing.(69)
Ten articles,(36,43,46,49–53,56,57) based on 8 separate studies,(36,43,46,49–51,53,56) reported use of the MBSR-based intervention. Only one study did not report modifications to the MBSR curriculum;(43) the other studies implemented modified MBSR programs, tailored to the targeted population. The modifications were reported as “minor” in 3 studies (46,50,51) – in these studies, the intervention, delivered by trained MBSR teachers, was labeled as "MBSR" and scored as “manualized.” Two studies (four reports: 36,49,52,57) used modified MBSR, with modifications being quite substantial and not manualized – therefore, these interventions were scored as “not manualized.” Two studies developed and used an MBSR-based, manualized intervention: one patterned after MBSR and adjusted to the needs of therapeutic community residents,(56) and one patterned after MBRP and adjusted to the needs of recovering, alcohol dependent adults.(53) The meditation course intensity in the included studies ranged from five 90-minute sessions over seven weeks (with less than 50% of the session content devoted to MM) (49,52,57) to eight 2–2.5-hour sessions over eight weeks, with the majority of each session devoted to MM.(36,43,46,50,53) In addition, two studies implemented a full-day retreat.(36,50) Only one study reported monitoring the integrity of intervention delivery.(56)
Spiritual Self Schema (3-S)
therapy has been developed for the treatment of addiction and HIV risk behavior.(21) Its curriculum is manualized (www.3-S.us) and consists of an 8- or 12-week long course, designed for clients at risk for, but not infected with HIV or clients with HIV, respectively. The 3-S therapy teaches meditation and mindfulness skills in the context of comprehensive psychotherapy, integrating Buddhist principles with modern cognitive self-schema theory that is tailored to each patient’s spiritual/religious faith.(21)
Four reports,(37,42,47,55) based on 3 separate studies,(37,42,47) used manualized 3-S therapy delivered in an individual and/or group format, during 1–2 hour-long sessions per week, by a trained therapist over eight (37,42,55) or twelve (47) weeks. Integrity of the intervention delivery was monitored in all the studies.
Acceptance and Commitment Therapy (ACT)
Theoretically based in contemporary behavior analysis, ACT applies both mindfulness/acceptance, as well as commitment and behavior change processes.(62) These core processes, conceptualized as positive psychological skills, aim to increase psychological flexibility that is defined as the ability to better connect to one’s experience, and to make overt behavioral choices in the service of chosen goals and values (“committed action”).(62) Originally developed for psychological disorders,(20) ACT has been applied to a variety of conditions, including SUDs.
Three studies used manualized ACT,(38,39,54) delivered by a trained therapist in either an individual (54) or both individual and group (38,39) therapy format. The ACT sessions took place weekly, ranging in duration from 1½ (54) to 3½ (39) hours per week, over seven (38) to sixteen (39) weeks. Integrity of intervention delivery was monitored in all the studies.
Dialectical Behavior Therapy (DBT)
DBT originated as a therapy for chronically suicidal clients with borderline personality disorder,(19,70) and was subsequently adapted for SUDs.(71) DBT comprises strategies from cognitive and behavioral therapies (with a problem-solving focus) and acceptance strategies (with mindfulness as its core) adapted from Zen teaching and practice. It provides a comprehensive long-term treatment that includes psychotherapy (therapist-led, in group and individual formats), case and medical management.
Two studies used a 1 year-long manualized DBT program for SUDs, with individual and group therapy lasting from three (41) to six (40) hours per week. Integrity of intervention delivery was monitored in these studies.
c) Methodological quality
Randomization
All 7 RCTs (Table 4) received 4 points for randomization on the MQS scores, including the study that reported unequal effects of randomization (36) – in this case, a full MQS score was assigned since the "unequality" favored the controls. Two of the 7 RCTs used randomization techniques that require a comment. One of these RCTs initially randomized subjects into 2 arms: MM and "standard of care" control, and then sub-randomized the MM group into two different protocols of MM delivery. Since no differences were found between the two MM subgroups, they were later combined into one group for the final analysis, and compared to controls.(42) Another RCT randomly assigned subjects to two different modes of MM delivery, but found no differences between these groups (no details described); thus, the groups were combined into one, and assessed as a prospective case series, with detailed results presented for pre-post analyses only.(37)
Table 4.
Published RCTs of mindfulness or mindfulness meditation based interventions (MM) used for the treatment of substance use, misuse or disorders. In the Outcomes section, values presented in [square brackets] were calculated by the authors for this systematic review.
| Feature | Study 1. Alterman et al. 2004 (36) |
Study 2. Avants et al. 2005 (37) |
Study 3. Margolin et al. 2006 (42) |
Study 4. Hayes et al. 2004 (39) |
Study 5. Gifford et al. 2004 (38) |
Study 6. Linehan et al. 1999 (41) |
Study 7. Linehan et al. 2002 (40) |
|---|---|---|---|---|---|---|---|
| Subjects | 31 (17 F); mean age 36.5 yrs |
29 (17 F); mean age 41.7 (28–51 yrs) |
72 (47 F); mean age ~ 41 (21–56 yrs) |
124 (63 F); mean age 42.2 (23–64 yrs); Axis II - 52%, anxiety and/or mood disorders ~ 42% |
76 (45 F); mean age 43 (19–71 yrs) |
28 F; mean age 30.4 (18–45 yrs); 50% depression, 38% PTSD |
23 F; mean age 36.1 (18–45 yrs); anxiety and/or mood disorders 40–50%; past suicide or self- injury attempt 65% |
|
Addictive disorder |
PSR 4; SUDs, recovery house residents; alcohol + drugs 68%; prior Tx: 4.9 times for drug, 1.9 times for alcohol abuse. |
PSR 4; opiate & cocaine dependent methadone maintenance out- patients; heroin use 17.1, cocaine use 16.5 yrs; majority with prior methadone Tx, and drug use in a prior month. |
PSR 4; opiate dependent methadone maintenance out- patients; heroin use 17.7 yrs; cocaine use disorder 89%; prior methadone Tx 57%; majority used drugs in a prior month. |
PSR 4; poly-SUDs; opiate dependent methadone maintenance out-patients, with other SUDs: 35% alcohol, 46% cocaine, 10% sedative dependent, who have continued drug use; prior residential or out-pt Tx: 6.3 times. |
PSR 2; tobacco dependent community- recruited adults, with at least one past-year quit attempt; on average, smoked 21.4 cig/day, had 4 prior quit attempts (median success: 30 days) in the past 2 yrs. |
PSR 4; BPD + SUDs; recruited from the clinic patients; 74% poly-SUDs, 58% cocaine, 52% alcohol; pre-study % days abstinent (alcohol, drugs): ~ 25–35%. |
PSR 4; BPD + opiate dependence; recruited from variety of out-pt settings; 52% cocaine, 26% alcohol, 13% sedative dependent; prior methadone Tx: 83%. |
|
MM group & MM intervention |
N=18; MBSR- based, 8 wks: therapist-led group sessions (120 min/wk) & one retreat (7 hrs) + 30–45min meditation group meetings (4 times / wk). |
N=11; 3-S (individual), 8 wks: therapist-led individual sessions (60 min/wk). |
N=38; 3-S (two groups), 8 wks: • one educational session (60 min), AND • N=20; therapist- led individual sessions (60 min/wk), OR • N=18; therapist- led individual (60 min/wk) & group (60 min/wk) sessions. |
N=42; ACT, 16 wks: therapist-led 32 individual (60 min) & 16 group (90 min) sessions. |
N=33; ACT, 7 wks: therapist-led 7 individual (50 min/wk) & 7 group (90 min/wk) sessions. |
N=12; DBT for SUDs, 52 wks: therapist-led individual (60 min/wk) & group (120 min/wk) sessions + skills coaching calls + optional 39-wk methadone or methylphenidate Tx for opiate or stimulant dependence. |
N=11; DBT for SUDs, 52 wks: therapist-led individual (40–90 min/wk) & group (150 min/wk) sessions + optional individual coaching (30 min/wk) + diary cards. |
|
Comparison group |
N=13, SOC only. |
N=18; 3-S (individual & group); 8 wks: therapist-led individual (60 min/wk) & group (60 min/wk) sessions. |
N=34; ‘waitlist’ SOC only. |
• N=44; ITSF, 16 wks: 32 individual (60 min) therapist or AA sponsor- led), + 16 group (90 min) therapist-led sessions. • N=38; SOC only. |
N=43; NRT + medical Tx (physician-led): one 90 min group education session; then weekly clinic visits (physician visit as needed). |
N=16; SOC only (subjects were referred to other clinics for SOC). |
N=12; CVT + 12- Step, 52 wks: individual (40–90 min/wk) + NA group (120 min/wk) + optional 12-Step sponsor meetings. |
|
Ancillary treatment (all groups) |
SOC | One 60 min individual HIV educational session + SOC |
SOC | SOC | None | None | ORLAAM (52 wks), physician-led medical Tx, weekly UTox for opiates during Tx; case management; PTSD Tx, crisis intervention, 12- Step. |
| Follow-up | 0, 8, 22 wks | 0, 8 wks | 0, 8 wks | 0, 8, 16, 42 wks | 0, 7, 26, 52 wks | 0, 16, 32, 52, 68 wks |
0, 16, 32, 52, 68 wks |
|
Retention (at the end of follow-up): |
• MM (MBSR- based) 83%; • SOC 77% |
• MM (3-S Individual) 64%; • MM (3-S Individual+Group) 89% |
• MM (3-S) 82%; • SOC 88% |
• MM (ACT) 43%; • ITSF 57%; • SOC 68% (differential retention: p<0.07) |
• MM (ACT) 61%; • NRT 81% (differential retention: p=0.07) |
• MM (DBT) 58%; • SOC 50% |
• MM (DBT) 82%; • CVT+12-Step 83% |
|
Outcome measures |
Drug use (self- report, UTox); psychological health, problem level (ASI composite scores); meditation practice |
Drug use (self- report, UTox); HIV risk behavior, spirituality, religious practices (surveys, reaction time task); Tx experiences. |
Drug use (HIV risk behavior survey, with "yes/no" question on injection drug use or unprotected sex; UTox), HIV prevention motivation; spirituality, religious practices (surveys, reaction time task); Tx experiences. |
Drug use (self-report, UTox); psychopathology; Tx satisfaction. |
Tobacco use (self-report, exhaled CO); dependence, withdrawal symptoms, coping styles, psychological health; Working Alliance Inventory; Tx satisfaction. |
Drug use (self- report, UTox); prior Tx, parasuicide history, the global adjustment and the global social adjustment, anger expression. |
Drug use (self- report, UTox); parasuicide history, the global adjustment and the global social adjustment, Brief Symptom Inventory. |
|
Substance use related outcomes (per ITT & PP analyses, at the end of follow-up, unless stated otherwise) |
ITT: N/A; PP: • no differences between the groups (ES < 0.1); • compared to baseline, both groups decreased substance use. |
ITT: N/A; PP: • no differences between the groups; • compared to baseline, the groups (combined into one sample) decreased heroin and cocaine use (p<0.05 [ES 0.5]) and HIV risk behavior (p=0.08 [ES 0.4]); • all but one subject reported positive 3-S effects on drug use, craving, motivation for abstinence and HIV prevention. |
ITT: N/A; PP: • compared to SOC controls, fewer 3-S subjects injected drugs and/or had unsafe sex (53% vs. 23%, p<0.05 [ARR 30%, NNT 3.3]); • receipt of 3-S therapy was an independent protective factor against engaging in these behaviors (OR 8.9, p<0.05); • 3–S attendance was correlated to HIV risk behaviors (r= − 0.33, p<0.05; [ES 0.7]). |
ITT: • no significant differences between the ACT, ITSF and SOC groups (no details provided); PP: • the ACT and ITSF groups, compared to SOC, had more ‘clean’ UTox for opiates (61%, 50%, 28%, p<0.05 [ACT vs. SOC: ARR 33%, NNT 3.0; ACT vs. ITSF: ARR 11%, NNT 9.1]) and all drugs (50%, 38%, 12%, p<0.05 [ACT vs. SOC: ARR 38%, NNT 2.6; ACT vs. ITSF: ARR 12%, NNT 8.3]); • the ACT group tended to more accurately report drug use than the ITSF group (p<0.1). |
ITT: • no significant difference in quit rate between the ACT and NRT groups (21% vs. 9%, p>0.05 [ARR 12%, NNT 8.3]); PP: • ACT had a better quit rate than the NRT group (35% vs. 15%, p<0.05 [ARR 20%, NNT 5]); • quit status was predicted by change in acceptance skills in the ACT group, and change in acceptance skills mediated effects of ACT on smoking status (p<0.05). |
ITT: • DBT group had a higher proportion of drug/alcohol abstinent days compared to SOC (0.94 vs. 0.58, p<0.05, ES 0.59); PP: • The above difference was significant (ES 1.0). |
ITT: • at 52 wks, DBT group reduced opiate use compared to CVT group (p<0.05), however, this difference disappeared at 68 wks (positive UTox for opiates: 27% vs. 33%, p>0.05 [ARR 5%; NNT 22]); • during the 52-wk long Tx, the DBT group was more accurate in substance use reporting than CVT group (p<0.05). PP: N/A. |
|
Other outcomes (per ITT & PP analyses, at the end of follow-up, unless stated otherwise) |
ITT: N/A; PP: • the Addiction Severity Index medical composite score improved in the MM, but not SOC group (p<0.05, ES 0.195); • 47% MM subjects continued meditating (4 hrs during the prior month). |
ITT: N/A; PP: • compared to baseline, 3-S subjects increased spiritual practices, and showed a cognitive shift from ‘addict’ to ‘spiritual’ self (p<0.05); • spirituality correlated to drug abstinence (r= 0.6, p<0.05 [ES 0.4]) and to decrease in HIV risk behavior (r= 0.67, p<0.05 [ES 0.45]). |
ITT: N/A; PP: • compared to SOC, 3-S group increased spiritual practices and motivation for HIV prevention, and showed a cognitive shift from ‘addict’ to ‘spiritual qualities’ (p<0.05); • all 3-S subjects reported meditating (mean 25.7 min/day) and planned continuing it. |
ITT: • no differences between the groups in psychological health scores or Tx satisfaction ratings; • compared to baseline, the groups improved on majority of psychological outcomes (p<0.05). PP: Results as above. |
ITT: N/A; PP: • the ACT subjects endorsed better relationship with their providers than the NRT subjects (p<0.05); • Tx satisfaction ratings were comparable between the groups. |
(unclear whether ITT or PP was used): • compared to SOC, the DBT group received more psychotherapy (p<0.05), and improved the global adjustment and global social adjustment scores (p<0.05). |
ITT: • no differences between the groups in psychopathology or jail time; • compared to baseline, both groups improved the Brief Symptom Inventory scores and global adjustment ratings. |
|
Adverse effects |
Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| MQS / 17 | 8 | 8 | 11 | 12 | 13 | 13 | 14 |
|
CBS (ITT & PP analyses) |
ITT: N/A PP: +2 |
ITT: N/A PP: −1 |
ITT: N/A PP: +2 |
ITT: −1 PP: +2 |
ITT: −1 PP: +2 |
ITT: +1 PP: +1 |
ITT: +1 PP: N/A |
| Comments | All subjects lived in the same recovery house. |
The sample was randomized to individual or individual + group therapy, however, detailed results are reported as for pre-post one group. |
After the initial randomization, the MM group was sub-randomized to individual (N=20) or individual + group therapy (N=18); in the final analysis, these subgroups were combined into one MM group. |
3-arm RCT, with two "active" interventions (MM and ITSF) that were "matched" by subject involvement and therapy format (but not therapist contact time). |
None | Low Tx completion rate, especially in the SOC group (DBT 55%, SOC 19% per ITT). |
Two interventions were "matched" by subject involvement and therapy format (but not therapist contact time). One subject was randomized incorrectly, removed before the study onset and not analyzed (resulting in N=23). All drop- outs took place in the DBT group led by the only male therapist. |
Values (presented in [square brackets]) calculated for the systematic review: ARR: Absolute Risk Reduction; CBS: Clinical Benefit Score; CES: Cumulative Evidence Score; ES: Effect Size (Cohen's d); MQS: Methodological Quality Score; NNT: Number Needed to Treat; PSS: Population Severity Score.
ACT: Acceptance Commitment Therapy; BPD: borderline personality disorder; CO: carbon monoxide; CVT: Comprehensive Validation Therapy; DBT: Dialectical Behavior Therapy; MM: mindfulness meditation; HIV: Human Immunodeficiency Virus; ITSF: Intensive Twelve Step Facilitation; ITT: intention to treat analysis; MBSR: Mindfulness-Based Stress Reduction; min: minutes; mos: months; NRT: Nicotine Replacement Therapy; ORLAAM: oral solution levomethadyl acetate, opiate agonist; PP: per protocol analysis; PTSD: post-traumatic stress disorder; 3-S: Spiritual Self Schema; SOC: "standard of care" therapy; SUDs: Substance Use Disorders; TSF: Twelve Step Facilitation; Tx: treatment; UTox: urine toxicology test; wks: weeks; yrs: years.
Blinding
"Double-blinding," interventionist or subject blinding are not feasible and/or desirable in studies using MM interventions for practical and ethical reasons, and none of the treatment studies used such methods. Three RCTs reported assessor blinding.(39–41)
Sample size
The included studies were "pilots" with small sample sizes. One published RCT (39) and one unpublished RCT (59) provided sample size assumptions, however, a high attrition rate resulted in their being underpowered.
Analysis
Only one study described evaluation of the distributional characteristics of the variables, and the use of parametric or non-parametric tests, when appropriate.(53) One published study (43) and one unpublished study (58) did not use statistical analyses for the assessment of intervention effects. Other studies used parametric analyses that assume normal data distribution. However, in the context of small sample sizes and high variability of some data (as indicated by SDs), it is possible that some variables did not have normal distribution. Six studies either reported 100% follow-up data collection,(50) 100% retention (46) or analyzed data per intention-to-treat (ITT) protocol, imputing missing data.(38–41)
Quality of MM intervention delivery
MM was manualized in 15 separate studies, described in 18 articles.(37–48,50,51,53–56) Integrity of the intervention delivery (therapist protocol adherence and/or competence) was monitored in 10 separate studies.(37–42,47,50,54,56)
Retention rate
among the MM subjects ranged from about 45% (39,44) to 100%,(46) with an average rate of 75%. One study with a 72% retention rate collected primary outcome data for all enrolled subjects.(50)
Methodological Quality Score (MQS) and Cumulative Evidence Score (CES; Table 3)
Among the included 22 reports, 15 were based on separate studies. As expected, among these 15 studies, the highest MQSs were for RCTs, followed by non-randomized controlled trials and case series. Only one RCT (40) received a score of "excellent", defined as MQS ≥ 14.(23) Evaluating by the type of MM intervention used, the highest MQSs were achieved by studies of DBT and ACT, followed by 3-S and MBSR-based therapies. The MBSR-based intervention was the most commonly studied MM therapy (7/15 studies). Grouping by subject population, the highest mean MQSs were achieved by studies evaluating adults with SUDs in the outpatient setting, which were also the most common population / settings (N=7: opiate dependent, medically managed subjects – five studies; poly-SUDs – one study; and alcohol dependence – one study); these studies were followed by studies of community-recruited adults with tobacco dependence (N=3), and studies of adults treated for SUDs in residential settings (N=3). The Overall CES, calculated for subgroups of studies, indicated that, in general, the studies yielded positive evidence (Overall CES ranging from +10 to +143) with 50% to 85% of the studies reporting positive outcomes by ITT (N=6) or per protocol (N=13) analyses, respectively (Table 3).
d) Side effects or adverse events
Only two reports directly stated that no MM-related significant side effects, adverse events or problems occurred.(53,55) Other reports did not address this topic, implicitly suggesting lack of negative effects.
3. Results of the published treatment RCTs (Table 4)
Seven RCTs, including a total of 383 (63% female) adult subjects, followed for an average of 38 weeks post-entry (from eight (37,42) to sixty eight (40,41) weeks), offered the most detailed assessment of MM interventions. Methodological quality of the RCTs was moderate (MQS 8-14/17). Six RCTs evaluated severely impaired populations (PSR 4/4), and one (38) focused on tobacco dependent adults (PSR 2/4).
Six of 7 RCTs used two-arm, and one used a three-arm design.(39) Three studies compared MM + "standard of care" (SOC) to SOC alone,(36,42) with both MM and SOC provided at the same clinical site. One study compared MM to SOC, with SOC subjects referred out for therapy ("naturalistic control").(41) Finally, four studies (37–40) compared MM to an active intervention: three to a different therapy (behavioral (39,40) or pharmacotherapy (38)) and one compared MM delivered in "individual" versus "individual + group format".(37)
All RCTs reported some positive results. Compared to SOC, the MM intervention showed positive effects as indicated by the ITT (40,41) and per protocol (38–42) analyses. Differential improvement in both substance use-related and other outcomes was noted in 5 of 7 studies.(38–42) One study (36) noted a differential improvement in medical symptom severity only (p<0.05, effect size 0.2), and one study,(37) comparing 3-S "individual" to 3-S "individual + group" therapy did not find differences between the study arms, but noted pre-post improvements (p<0.05, effect size 0.5) in substance use outcomes.
Overall, studies using SOC (39,41,42) or active, but "non-matching" interventions (38) as a comparison group, tended to report greater statistically significant between-group differences, with results favoring MM, than studies using a "matching" behavioral intervention.(39,40) The 3-arm study by Hayes et al.,(39) with the largest sample size but low retention rate, of 124 poly-substance abusing methadone maintenance patients suggested, in a per protocol analysis, that MM + SOC may result in a substantial reduction of substance use compared to SOC alone (ARR 33%). This finding was consistent across 3 other RCTs comparing MM to SOC (ARR 30% (42) with medium to large effect size (41)) or to pharmacotherapy with medical management (ARR 12–20% per ITT and per protocol analyses).(38) Two methodologically strong RCTs compared MM to a different behavioral intervention that "matched" MM by subject involvement and therapy format.(39,40) These studies did not find significant in-between group differences in substance use (ARR 5–12%, p>0.05) or psychological outcomes at the study end-point at forty two (39) or sixty eight (40) weeks, however, they both noted a tendency to a more accurate drug use reporting among MM compared to comparison subjects.(39,40) Regarding non-substance use related measures, MM subjects, compared to SOC controls, increased their motivation for HIV prevention, their spiritual practices and showed a cognitive shift from "addict" to "spiritual qualities" (p<0.05);(42) they also improved their Global Adjustment and Global Social Adjustment Scale scores (p<0.05).(41)
4. Results of the published non-randomized controlled studies, case reports and case series (Table 5)
Table 5 summarizes four controlled non-randomized trials (described by 6 articles (43–48)), four case series (described by 6 articles (49–53,57)) and one case report.(54) These studies included 609 subjects, 13 to 67 years old, suffering from various SUDs, and recruited from the outpatient treatment,(46,47,49,51–53,57) community,(43,50) or jail (44,48) settings.
While the methodological quality of the scored studies was limited (MQS 4-8/17), collectively they reported overall positive outcomes. On average, substance use tended to decrease at follow-up compared to baseline (43,50,53) or compared to a control group,(44,47) with effect sizes ranging from small (47,53) to medium.(44) One study (49) reported an increase in substance use at follow-up compared to low-use at baseline, but no detailed results were presented, and substance use was not the primary focus of the study. Severity of potential relapse triggers (such as stress, mental health and sleep problems, certain coping styles) also tended to improve compared to both baseline (49,50,53) and control conditions,(44,46,47) and the average effect size for these changes ranged from medium to large. Bowen et al. (44) also noted a reduction of alcohol-related negative consequences (small effect size) among subjects who underwent MM training compared to controls. Only one uncontrolled trial (among all included studies) of alcoholics treated with MM therapy, adjunctive to SOC, assessed craving severity and the degree of mindfulness.(53) Although craving severity decreased (medium effect size), this change was not statistically significant; degree of mindfulness, or the ability to be attentive to a present-moment experience in daily life, improved (medium effect size), and this change correlated to improved stress severity (large effect size) at 16 week follow-up.(53)
Participation in the MM therapy correlated to or mediated the improvements in substance use and relapse-related outcomes, with small-to-large effect size.(44,45,47,48,50,53) One uncontrolled study compared "relapsers" (N=7; subjects reporting at least one heavy drinking day during the study) to non-relapsers (N=8), and found that relapsers had more severe symptoms of anxiety, depression and craving, reported lower degree of mindfulness and meditated fewer minutes per day (p<0.05).(53) The same study also reported that drinking level as well as the change in drinking correlated to the severity of relapse risk factors such as anxiety, depression, stress, and craving (large effect sizes) and the change in their severity, respectively. In turn, severity of relapse risk factors negatively correlated with the intensity of a daily meditation practice (large effect size).(53)
Two uncontrolled trials assessed pre-post levels of biological outcomes: stress-responsive and illness-sensitive biomarkers, in addition to self-reported psychological measures. A study of recovering alcoholics evaluated serum interleukin-6 (IL-6), liver enzymes and diurnal profile of salivary cortisol at baseline and 16-week follow-up.(53) While cortisol (N=10) and liver enzymes (N=12) did not significantly change over time (small effect size), IL-6 level decreased (N=12; medium effect size, p=0.05), suggesting a reduction in chronic stress level and improved health. After MM intervention, SUD-affected residents of a therapeutic community showed a decrease in an awakening salivary cortisol level compared to baseline (medium effect size).(51)
5. Subject treatment experiences related to MM intervention (Table 4 and Table 5)
Two qualitative studies,(55,56) derived from mixed-methods primary projects,(42,47,72) focused on subject treatment experiences related to MM therapy (Table 5). Several other studies also evaluated subject experiences, using quantitative (e.g. Likert scales) or qualitative techniques.(38,39,43,53) Taken together, MM therapy was well-received by the subjects with different degrees of problem severity (PSR 2-4/4) and in various settings: residential,(56) outpatient (39,53,55) and community.(38,43) Subjects reported high degree of satisfaction with MM therapy and its usefulness as a recovery-enhancing tool. They also viewed MM-related skills as unique, "brand new" and different from those taught in a traditional, professional addiction treatment.(53,55)
Four studies evaluated individual ("at-home") MM practices among the subjects who underwent an 8-week MM intervention.(36,42,43,53) Two of these studies, using the MBSR-based intervention, reported that about 47% of the subjects continued meditation practice at twleve (43) and twenty two (36) weeks post-entry. A study evaluating MBSR + CBT-based intervention found that 100% of the study completers (79% of the sample, with all drop-outs occurring after the 1st or 2nd MM session) meditated at 16 weeks, on average 3.9 days/week, 27.4 minutes/meditating day.(53) Likewise, an RCT evaluating 3-S therapy found that at 8 weeks all study completers (82%) continued meditating, on average 26 minutes/day.(42)
6. Unpublished studies (Table 6)
Table 6.
Unpublished treatment trials and a laboratory-based study of mindfulness or mindfulness meditation based interventions (MM) used for the treatment of substance use, misuse or disorders: methods and results. Results from the final follow-up are reported, unless stated otherwise.
| Study | Design & Indication |
Subjects | Intervention | Outcome measures | Results | MQS |
|---|---|---|---|---|---|---|
|
Study 1. Gifford et al., 2008, submitted (59) |
2-arm RCT: smoking cessation, community settings |
303 (10 F), mean age 46.0 (18–75 yrs); PSR 2; tobacco dependent, community- recruited adults; on average, 24 cigarettes/day, 2.2 prior quit attempts in the past 2 yrs, with a median abstinence of 21 days. |
• MM intervention, 10 wks: ACT + Functional Analytic Psychotherapy (therapist-led, weekly 120 min group and 50 min individual sessions) + bupropion. • Control group: bupropion only (medical management, one 60 min educational meeting, handouts) |
• Collected at 0, 10, 26, 52 wks; • smoking (7-day point prevalence; self-report, exhaled CO); • withdrawal severity, psychological health; • Working Alliance, Tx satisfaction. |
• Retention: 45.2%; PP: • quit rate was higher at the MM than control group (31.6% vs. 17.5%, p<0.05, ES 0.3 [ARR 14.1, NNT 7]). • during 52 wks, MM was more effective in reducing smoking than control Tx (OR 2.2, p<0.05). • acceptance- based responding and the therapeutic relationship mediated effects of MinM on Tx outcomes; • MM group reported higher Tx satisfaction than controls at all time points (p<0.05, ES 0.7 at 52 wks). |
MQS: 10/17 CBS: N/A per ITT (+2 PP) Manualized MM intervention. |
|
Study 2. Brown et al., 2008*, submitted (58) |
Case series: smoking cessation, community settings |
16 (12 F), mean age 41.9 (18–65 yrs); PSR 2; tobacco dependent, community- recruited adults; on average, 20.4 cigarettes/day, smoked for 26.3 yrs, unable to abstain for longer than 3 days in the past 10 yrs. |
• MM intervention, 10 wks, therapist-led: ACT + Cognitive Behavioral Therapy (wks 4–10) + NRT (wks 6–14). |
•Collected at 0, 10, 14, 19, 32 wks (quit date: week 6); • smoking (self-reported, exhaled CO); • withdrawal and depressive symptom severity. |
• Retention: 75%; • quit rates at 10, 14, 19, 32 wks: 31%, 25%, 19%, and 0%. • during the study, the longest continuous abstinence was median 24 days (mean 41.6), number of days abstinent was median 40.5 (mean 58.8, out of 180 study days), time to relapse (7 consecutive smoking days) was median 45.5 days (mean 49.9), and number of quit-smoking attempts was median 2.5 times (mean 4.1). • 82% reported that the learned skills were “very” or “extremely useful” in helping quit smoking. |
MQS: 5/17 CBS: N/A* *No statistical assessment of the significance of pre-post change was provided; only descriptive statistics were used. Follow-up psychological outcomes were not reported. Manualized intervention. |
|
Study 3. Bowen 2008, PhD dissertation (60) |
2-arm RCT, laboratory study: smoking, and related craving and negative affect; community settings |
123 (34 F), mean age 20.3 (18–46 yrs); PSR 2; undergraduate psychology students; on average, low dependence scores, smoked 5.3 cigarettes/day in the past week, had 8.4 quit attempts of minimum 24 hrs in the past year. |
Subjects did not smoke for 12 hrs prior, then underwent 4 brief cue (cigarette) exposures in a 90-min long laboratory session, during which they were asked to cope with arising thoughts, sensations and emotions using: • MM intervention (n=61): guided, audio- recorded, mindfulness- based coping strategies; • Controls (n=62): their "usual" coping strategies. |
• Collected post-exposure (4 in-person assessments), at 1 and 7 days; • smoking (self-reported); • smoking urges, affect; • brief written description of used coping strategies. |
• Retention: 90.2%; PP: • during the follow-up week, MM group smoked fewer cigarettes/day than controls (p<0.05, ES 0.6) – compared to baseline, MM decreased smoking by 26%, while controls increased it by 11%; • no significant differences between groups were found in latency to the first cigarette, negative affect and smoking urges; • during cue exposures, to cope with cravings and urges, MM group used MM strategies, while controls used primarily distraction-based techniques. |
MQS: 9/17 CBS: N/A per ITT (+2 PP) Manualized intervention. |
After completion of this manuscript, a report from this study has been published.(61)
Values (presented in [square brackets]) calculated for the systematic review: ARR: Absolute Risk Reduction; CBS: Clinical Benefit Score; CES: Cumulative Evidence Score; ES: Effect Size (Cohen's d); MQS: Methodological Quality Score; NNT: Number Needed to Treat; PSS: Population Severity Score.
ACT: Acceptance Commitment Therapy; CO: carbon monoxide; MM: mindfulness meditation; ITT: intention to treat analysis; min: minutes; mos: months; NRT: Nicotine Replacement Therapy; PP: per protocol analysis; Tx: treatment; UTox: urine toxicology test; wks: weeks; yrs: years.
Two treatment trials using ACT (58,59) and one laboratory-based study of a mindfulness-based coping technique (60) provide additional insights into the potential efficacy and mechanisms of MM interventions for smoking cessation. An RCT of 10-week MM intervention (ACT, combined with CBT and nicotine replacement therapy, NRT) found a significantly higher quit rate (small effect size) and higher treatment satisfaction (medium effect size) among the MM subjects compared to controls, receiving NRT alone, at 52 weeks; in addition, acceptance-based responding mediated effects of MM on smoking outcomes.(59) A set of prospective case series (N=16 subjects), designed for pilot-testing and refinement of the study methods, evaluated effects of combined ACT, CBT and NRT. Although smoking quantity and frequency have not been compared "pre-post," and all study completers resumed smoking at 26 weeks post-quit date, the longest continuous abstinence period was longer after the MM intervention than during subjects' previous attempts when abstinence had lasted less than 3 days. Further, 82% of the subjects reported that they learned skills that were “very” or “extremely useful” in helping them quit smoking.(58) Finally, one RCT (PhD dissertation) described an experiment evaluating efficacy of mindfulness-based coping compared to "usual" coping strategies aimed to prevent relapse after quitting smoking.(60) In this study, during a cue exposure paradigm, the MM group used MM strategies, as instructed, while controls used primarily distraction-based techniques to cope with smoking cravings and urges. During the 7 day follow-up, the MM group smoked fewer cigarettes/day than controls (medium effect size, p<0.05). Overall, the results of these unpublished trials support the existing preliminary evidence provided by published studies.
DISCUSSION
This is the first systematic review of mindfulness or mindfulness meditation based interventions (MM) for substance use, misuse or disorders. Although existing data is preliminary and does not allow a consensus recommendation for any particular type of MM intervention for any single substance use-related condition, several findings are of clinical, theoretical and research interest.
The majority of the reviewed studies showed some positive outcomes among SUD-affected subjects treated with MM intervention, compared to baseline or other therapy (most commonly SOC). The case studies illustrated how MM has been practiced from a historical and clinical perspective. The focus on real-life trial methods has been termed pragmatic.(73) Pragmatic studies have the advantage of assessing effectiveness under conditions that patients encounter in real-life settings, thereby avoiding confounders associated with highly standardized clinical trial settings. Though lacking methodological strengths of control and randomization, the case studies, documented here, consistently showed positive patient outcomes and the general treatment satisfaction of subjects with chronic, often refractory, SUDs who were treated with MM therapy. Pragmatic aspects of these studies included meeting patient's expectations of receiving a "promised treatment," the ability of the therapist to better select the patient, and study methods that more closely resemble real clinical settings. Data from the controlled trials suggest that subjects receiving MM, adjunctive to SOC or pharmacotherapy, do as well or better than those receiving SOC or pharmacotherapy alone. When compared to other behavioral interventions in RCT settings, MM appears to produce comparable results. All these conclusions require assessment with more formal methodology in adequately powered clinical trials.
The promise of MM as an efficacious treatment for SUDs is supported by the consistency of positive results, demonstrated in this review across different study designs, MM modalities, subject populations and addictive disorders treated. Additional support for the potential efficacy of MM in SUDs can be drawn from the results of studies of other clinical samples. MM-based therapies have been shown to be effective or potentially effective (with, on average, medium pooled effect size, Cohen’s d 0.5–0.7 (16,62)) for a variety of medical and mental health disorders, including stress, anxiety, depression, emotion dysregulation, avoidance coping,(16,62,74–77) all known risk factors for relapse in SUDs.(78,79) In this context, MM may be particularly helpful for patients with co-occurring substance use and mental health disorders ("dual diagnosis").
Although long-term MM practice patterns have not been assessed in the context of SUDs, its use in other clinical samples suggests that MM can have long-lasting effects. For example, after a meditation course, 60–90% of subjects still meditated up to 4 years later, and reported that the course "had lasting value" and was highly important.(16) Patient satisfaction is an important consideration when choosing between treatment alternatives.(80) MM also appears safe - rigorous studies have not reported any side effects or adverse events.(64,65) This review corroborates these findings.
It is appealing to assume that the preliminary positive results in MM studies are direct outcomes of MM interventions; however, such a hypothesis is premature. The theoretical framework behind MM as well as early indirect evidence supports the use of MM for SUDs (8,9,13,14) and suggests unique therapeutic properties of MM. On a conceptual level, MM stands out as distinctive and, in many ways, different from other existing behavioral modalities, specifically from cognitive behavioral therapy (CBT) that is commonly used to treat SUDs.(81,82) While CBT promotes adaptive, antecedent-focused coping strategies (e.g. targeting emotion cues), meditation targets maladaptive, response-focused strategies, such as emotional avoidance, suppression or impulse control; thus, MM-related skills can complement skills acquired through CBT.(8,9,13,14) The integration of meditation and traditional CBT strategies may improve overall treatment efficacy, e.g. by increasing awareness of sensations, such as craving, emotional states, and physiological arousal.(83) Recent pilot trials of clinical cohorts add support to the above hypotheses, indicating that MM and CBT can produce different effects on mental health outcomes.(62,84,85) Two reviewed qualitative studies (53,55) reported that subjects viewed MM-related skills as different from those acquired through "traditional" SOC therapy for addiction.
Limitations
of this review include limiting the inclusion criteria to studies published in English, potentially excluding relevant studies. Lack of reviewer blinding and lack of assessment of inter-rater agreement could have introduced bias. Although the included interventions were based on the MM principles, they were heterogeneous; evaluating them together as one "MM intervention" could have introduced bias.
Strengths
of this review include an exhaustive literature search and application of statistical methods allowing direct comparison of included studies.
Directions for Further Research
Existing studies of MM therapy for addictive disorders are far from definitive and the research methods are evolving. These clinical and research fields would benefit from the development of: 1) standardized scientific parameters to be used in future studies of MM as a therapy for addictive disorders, 2) a comprehensive conceptual model of possible mechanisms underlying MM efficacy in SUDs, and 3) recommendations on MM implementation in clinical addiction medicine settings. Below we elaborate on each of these recommendations:
- Research methods of MM studies in addiction settings require refinement and standardization.
- A written manual should be used to guide the meditation intervention. It has been suggested that best therapeutic effects are achieved when the meditation intervention is adjusted to accommodate the specific needs of the targeted population.(86) While several "manualized" MM therapies are currently used in the treatment of SUDs (MBSR, MBRP, DBT, ACT, 3-S), most have been modified and adjusted to the needs of individual studies; however, only a minority of these studies developed a separate intervention manual. It is not known whether even relatively minor adaptations could have affected outcomes. Standardization of treatment manuals would facilitate study replication and data pooling.
- A theory for, and measures to assess, the evaluation of treatment outcomes in the context of MM also require development.
- Assessment of subjects' MM practice intensity and changes in the degree of mindfulness should be considered. These are essentially the "dose" and "quality" of the intervention and as such have the potential to influence findings similar to medication dose-response effects in pharmacotherapy trials. This data would also provide information on the ability of subjects to sustain MM practice over time. Many clinical MM programs "interview" potential clients before enrollment to ensure appropriate selection. In research settings, including the reviewed studies, the eligibility criteria are usually diagnosis-driven; results may therefore be skewed away from a positive effect, should one exist.
Research of MM has the potential to create a comprehensive bench-to-bedside explanatory model of its effects. Therefore, the mechanism of MM should be assessed in tandem with clinical assessment. Possible mechanisms have been hypothesized;(8,9,13,14) however, no comprehensive model, encompassing bio-psychological aspects of addiction, has been developed. None of the included studies was designed as a "dismantling" study or focused on "active ingredients" underlying MM effects. Appropriate control therapies and targeted assessments, including "objective" measures (e.g. biomarkers, imaging studies) are needed to assess treatment efficacy and to elucidate a mechanism of action for MM interventions in addictive disorders.
While MM techniques are already used in the clinical setting to treat addictive disorders,(14) it is premature to formally recommend for or against such practice. Researchers and clinicians involved in MM research should collectively address effectiveness of MM therapies in the form of consensus statements, and thereby provide guidelines on the best ways to implement MM therapies in clinical settings as a part of comprehensive addiction treatment programs.
CONCLUSIONS
Conclusive data for MM as a treatment for addictive disorders are lacking. However, the preliminary evidence indicate MM efficacy. MM therapies appear safe when performed in clinical research settings. Significant methodological limitations exist in most studies published to date, and it is unclear which persons with addictive disorders might benefit most from MM. Future clinical trials must be of sufficient sample size to answer a specific clinical question and should include carefully designed comparison groups that would allow assessment of both the effect size and mechanism of action of MM.
Acknowledgements
This study was supported by two grants from the National Institute on Alcohol Abuse and Alcoholism: 5T32 AA014845—A.Z. (P.I.: Michael Fleming, MD, MPH), and K23 AA017508—A.Z. (P.I.: A.Z.).
References
- 1.United Nations Office of Drugs and Crime (UNODC) Global Illicit Drug Trends 2007. New York: United Nations; 2007. [Google Scholar]
- 2. [accessed Jun 9, 2007];Substance Abuse and Mental Health Services Administration (SAMHSA), Office of Applied Studies: Results from the 2005 National Survey on Drug Use and Health: Detailed Tables; Prevalence Estimates, Standard Errors, P Values, and Sample Sizes. 2006 September; http:/www.oas.samhsa.gov.
- 3.Office of National Drug Control Policy (ONDCP) The Economic Costs of Drug Abuse in the United States 1992–2002. Washington, D.C.: 2004. [Google Scholar]
- 4.Connors GJ, Maisto SA, Donovan DM. Conceptualizations of relapse: a summary of psychological and psychobiological models. Addiction. 1996;91 Suppl:S5–S13. [PubMed] [Google Scholar]
- 5.Mackay PW, Marlatt GA. Maintaining sobriety: stopping is starting. Int J Addict. 1990;25(9A–10A):1257–1276. doi: 10.3109/10826089109081045. [DOI] [PubMed] [Google Scholar]
- 6.Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62(2):211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 7.Marlatt GA, Grodon JR. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. [Google Scholar]
- 8.Breslin FC, Zach M, McMain S. An information-processing analysis of mindfulness: implications for relapse prevention in the treatment of substance abuse. Clin Psychol Sci Prac. 2002;9:275–299. [Google Scholar]
- 9.Marlatt GA, Chawla N. Meditation and alcohol use. South Med J. 2007;100(4):451–453. doi: 10.1097/SMJ.0b013e3180381416. [DOI] [PubMed] [Google Scholar]
- 10.Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York: Delta; 1990. [Google Scholar]
- 11.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 12.Kutz I, Borysenko JZ, Benson H. Meditation and psychotherapy: a rationale for the integration of dynamic psychotherapy, the relaxation response, and mindfulness meditation. Am J Psychiatry. 1985;142(1):1–8. doi: 10.1176/ajp.142.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann SG, Asmundson GJ. Acceptance and mindfulness-based therapy: new wave or old hat? Clin Psychol Rev. 2008;28(1):1–16. doi: 10.1016/j.cpr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Hoppes K. The application of mindfulness-based cognitive interventions in the treatment of co-occurring addictive and mood disorders. CNS Spectr. 2006;11(11):829–851. doi: 10.1017/s1092852900014991. [DOI] [PubMed] [Google Scholar]
- 15.Salmon PG, Santorelli SF, Kabat-Zinn J. Handbook of Health Behavior Change. New York: Springer; 1998. Intervention elements promoting adherence to mindfulness-based stress reduction programs in the clinical behavioral medicine setting; pp. 239–268. [Google Scholar]
- 16.Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Prac. 2003;10:125–143. [Google Scholar]
- 17.Segal ZV, Williams JM, Teasdale JD. Mindfulness-based Cognitive Therapy for Depression: a new approach to preventing relapse. New York: Guilford Publications; 2002. [Google Scholar]
- 18.Witkiewitz K, Marlatt GA. Mindfulness-based relapse prevention for alcohol and substance use disorders: the meditative tortoise wins the race. J Cognit Psychother. 2005;19:221–230. [Google Scholar]
- 19.Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: Guilford; 1993. [Google Scholar]
- 20.Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment therapy: an experimental approach to behavior change. New York: Guilford Press; 1999. [Google Scholar]
- 21.Avants SK, Margolin A. Development of Spiritual Self-Schema Therapy for the treatment of addictive and HIV risk behavior: A convergence of cognitive and Buddhist psychology. Journal of Psychotherapy Integration. 2004;14(3):253–289. [Google Scholar]
- 22.Manheimer E, Anderson BJ, Stein MD. Use and assessment of complementary and alternative therapies by intravenous drug users. Am J Drug Alcohol Abuse. 2003;29(2):401–413. doi: 10.1081/ada-120020522. [DOI] [PubMed] [Google Scholar]
- 23.Miller RM, Wilbourne PL, Hettema JE. What works? A summary of alcohol treatment outcome research. In: Hester RK, Miller WR, editors. Handbook of Alcoholism Treatment Approaches. 3rd ed. Boston: Pearson Education; scoring manual available at http://casaa.unm.edu/download/mesa.pdf2003. [Google Scholar]
- 24.Cropley M, Ussher M, Charitou E. Acute effects of a guided relaxation routine (body scan) on tobacco withdrawal symptoms and cravings in abstinent smokers. Addiction. 2007;102(6):989–993. doi: 10.1111/j.1360-0443.2007.01832.x. [DOI] [PubMed] [Google Scholar]
- 25.Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994;62(4):809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert GS, Parker JC, Claiborn CD. Differential mood changes in alcoholics as a function of anxiety management strategies. J Clin Psychol. 1978;34(1):229–232. doi: 10.1002/1097-4679(197801)34:1<229::aid-jclp2270340147>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.McIver S, O'Halloran P, McGartland M. The impact of Hatha yoga on smoking behavior. Altern Ther Health Med. 2004;10(2):22–23. [PubMed] [Google Scholar]
- 28.Niederman R. The effects of Chi-Kung on spirituality and alcohol/other drug dependency recovery. Alcoholism Treatment Quarterly. 2003;21(1):22–23. [Google Scholar]
- 29.Parker JC, Gilbert GS, Thoreson RW. Reduction of autonomic arousal in alcoholics: a comparison of relaxation and meditation techniques. J Consult Clin Psychol. 1978;46(5):879–886. doi: 10.1037//0022-006x.46.5.879. [DOI] [PubMed] [Google Scholar]
- 30.Rohsenow DJ, Monti PM, Martin RA, Colby SM, Myers MG, Gulliver SB, Brown RA, Mueller TI, Gordon A, Abrams DB. Motivational enhancement and coping skills training for cocaine abusers: effects on substance use outcomes. Addiction. 2004;99(7):862–874. doi: 10.1111/j.1360-0443.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 31.Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96(8):1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- 32.Rohsenow DJ, Smith RE, Johnson S. Stress management training as a prevention program for heavy social drinkers: cognitions, affect, drinking, and individual differences. Addict Behav. 1985;10(1):45–54. doi: 10.1016/0306-4603(85)90052-8. [DOI] [PubMed] [Google Scholar]
- 33.Shaffer HJ, LaSalvia TA, Stein JP. Comparing Hatha yoga with dynamic group psychotherapy for enhancing methadone maintenance treatment: a randomized clinical trial. Altern Ther Health Med. 1997;3(4):57–66. [PubMed] [Google Scholar]
- 34.Ussher M, West R, Doshi R, Sampuran AK. Acute effect of isometric exercise on desire to smoke and tobacco withdrawal symptoms. Hum Psychopharmacol. 2006;21(1):39–46. doi: 10.1002/hup.744. [DOI] [PubMed] [Google Scholar]
- 35.Rohsenow DJ, Monti PM, Martin RA, Michalec E, Abrams DB. Brief coping skills treatment for cocaine abuse: 12-month substance use outcomes. J Consult Clin Psychol. 2000;68(3):515–520. doi: 10.1037//0022-006x.68.3.515. [DOI] [PubMed] [Google Scholar]
- 36.Alterman AI, Koppenhaver JM, Mulholland E, Ladden LJ, Baime MJ. Pilot trial of effectiveness of mindfulness meditation for substance abuse patients. J Subst Use. 2004;9(6):259–268. [Google Scholar]
- 37.Avants SK, Beitel M, Margolin A. Making the shift from 'addict self' to 'spiritual self' Results from a Stage 1 study of Spritual Self-Schema (3-S) therapy for the treatment of addiction and HIV risk behavior. Mental Health, Religion, and Culture. 2005;8(3):167–177. [Google Scholar]
- 38.Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki M, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behavior Therapy. 2004;35:689–705. [Google Scholar]
- 39.Hayes SC, Wilson KG, Gifford EV, Bissett R, Piasecki M, Batten S, Byrd M, Gregg J. A preliminary trial of twelve-step facilitation and acceptance and commtiment therapy with polysubstance-abusing methadone-maintained opiate addicts. Behavior Therapy. 2004;35:667–688. [Google Scholar]
- 40.Linehan MM, Dimeff LA, Reynolds SK, Comtois KA, Welch SS, Heagerty P, Kivlahan DR. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13–26. doi: 10.1016/s0376-8716(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 41.Linehan MM, Schmidt H, 3rd, Dimeff LA, Craft JC, Kanter J, Comtois KA. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addict. 1999;8(4):279–292. doi: 10.1080/105504999305686. [DOI] [PubMed] [Google Scholar]
- 42.Margolin A, Beitel M, Schuman-Olivier Z, Avants SK. A controlled study of a spiritually-focused intervention for increasing motivation for HIV prevention among drug users. AIDS Education and Prevention. 2006;18(4):311–322. doi: 10.1521/aeap.2006.18.4.311. [DOI] [PubMed] [Google Scholar]
- 43.Altner N. Mindfulness practice and smoking cessation: The Essen Hospital Smoking Cessation Study (EASY) Journal for Meditation and Meditation Research. 2002;1:9–18. [Google Scholar]
- 44.Bowen S, Witkiewitz K, Dillworth TM, Chawla N, Simpson TL, Ostafin BD, Larimer ME, Blume AW, Parks GA, Marlatt GA. Mindfulness meditation and substance use in an incarcerated population. Psychol Addict Behav. 2006;20(3):343–347. doi: 10.1037/0893-164X.20.3.343. [DOI] [PubMed] [Google Scholar]
- 45.Bowen S, Witkiewitz K, Dillworth TM, Marlatt GA. The role of thought suppression in the relationship between mindfulness meditation and alcohol use. Addict Behav. 2007;32(10):2324–2328. doi: 10.1016/j.addbeh.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcus MT, Fine M, Kouzekanani K. Mindfulness-based meditation in a therapeutic community. J Subst Use. 2001;5:305–311. [Google Scholar]
- 47.Margolin A, Schuman-Olivier Z, Beitel M, Arnold A, Fulwiler CE, Avants SK. A preliminary study of Spiritual Self-Schema (3-S) therapy for reducing impulsivity in HIV-positive drug users. Journal of Clinical Psychology. 2007;63(10):979–999. doi: 10.1002/jclp.20407. [DOI] [PubMed] [Google Scholar]
- 48.Simpson TL, Kaysen D, Bowen S, MacPherson LM, Chawla N, Blume A, Marlatt GA, Larimer M. PTSD symptoms, substance use, and vipassana meditation among incarcerated individuals. J Trauma Stress. 2007;20(3):239–249. doi: 10.1002/jts.20209. [DOI] [PubMed] [Google Scholar]
- 49.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev. 2005;25(5):629–644. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Davis JM, Fleming MF, Bonus KA, Baker TB. A pilot study on mindfulness based stress reduction for smokers. BMC Complement Altern Med. 2007;7:2. doi: 10.1186/1472-6882-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcus MT, Fine M, Moeller FG, Khan MM, Pitts K, Swank PR, Liehr P. Change in Stress Levels Following Mindfulness-Based Stress Reduction in a Therapeutic Community. Addictive Disorders and Their Treatment. 2003;2:63–68. [Google Scholar]
- 52.Stevens S, Haynes PL, Ruiz B, Bootzin RR. Effects of a behavioral sleep medicine intervention on trauma symptoms in adolescents recently treated for substance abuse. Substance Abuse. 2007;28(2):21–31. doi: 10.1300/J465v28n02_04. [DOI] [PubMed] [Google Scholar]
- 53.Zgierska A, Rabago D, Zuelsdorff M, Coe C, Miller M, Fleming M. Mindfulness meditation for alcohol relapse prevention: A feasibility pilot study. Journal of Addiction Medicine. 2008;2(3):165–173. doi: 10.1097/ADM.0b013e31816f8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Twohig MP, Shoenberger D, Hayes SC. A preliminary investigation of acceptance and commitment therapy as a treatment for marijuana dependence in adults. J Appl Behav Anal. 2007;40(4):619–632. doi: 10.1901/jaba.2007.619-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beitel M, Genova M, Schuman-Olivier Z, Arnold R, Avants SK, Margolin A. Reflections by inner-city drug users on a Buddhist-based spirituality-focused therapy: a qualitative study. Am J Orthopsychiatry. 2007;77(1):1–9. doi: 10.1037/0002-9432.77.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Carroll D, Lange B, Liehr P, Raines S, Marcus MT. Evaluating Mindfulness-Based Stress Reduction: analyzing stories of stress to formulate focus group questions. Arch Psychiatr Nurs. 2008;22(2):107–109. doi: 10.1016/j.apnu.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haynes PL, Bootzin RR, Smith L, Cousins J, Cameron M, Stevens S. Sleep and aggression in substance-abusing adolescents: results from an integrative behavioral sleep-treatment pilot program. Sleep. 2006;29(4):512–520. [PubMed] [Google Scholar]
- 58.Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Hayes SC, Wilson KG, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. 2008 doi: 10.1177/0145445507309024. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gifford EV, et al. Applying acceptance and the therapeutic relationship to smoking cessation: A randomized controlled trial. 2008 In preparation. [Google Scholar]
- 60.Bowen S. Doctoral Dissertation. Seattle, WA: University of Washington; 2008. Effects of Mindfulness-Based Instructions on Negative Affect, Urges and Smoking. (unpublished) [Google Scholar]
- 61.Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Hayes SC, Wilson KG, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behav Modif. 2008;32(3):302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- 64.Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- 65.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 66.Kenny MA, Williams JM. Treatment-resistant depressed patients show a good response to Mindfulness-based Cognitive Therapy. Behav Res Ther. 2007;45(3):617–625. doi: 10.1016/j.brat.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2007 doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Exp Clin Psychopharm. 1996;4(1):46–54. [Google Scholar]
- 69.Marlatt GA. Efficacy of Mindfulness-Based Relapse Prevention. National Institute of Drug Abuse, Grant #1R21DA0119562, Years 2006–2008. Seattle: University of Washington; [Principal Investigator] [Google Scholar]
- 70.Linehan MM, Armstrong HE, Suarez A, Allmon D, Heard HL. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry. 1991;48(12):1060–1064. doi: 10.1001/archpsyc.1991.01810360024003. [DOI] [PubMed] [Google Scholar]
- 71.Linehan MM, Dimeff LA. Dialectical Behavior Therapy for Substance Abuse Treatment Manual. Seattle, WA: University of Seattle; 1997. [Google Scholar]
- 72.Marcus MT. Stress Reduction in Therapeutic Community Treatment. National Institute of Drug Abuse, Grant #5R01DA017719 Years 2004–2008. Houston: University of Texas; [Principal Investigator] [Google Scholar]
- 73.Ernst E, Pittler MH, Stevinson C, White A. Ramdomised clinical trials: pragmatic or fastidious? Focus Alt Compl Ther. 2001;63:179–180. [Google Scholar]
- 74.Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 75.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 76.Praissman S. Mindfulness-based stress reduction: a literature review and clinician's guide. J Am Acad Nurse Pract. 2008;20(4):212–216. doi: 10.1111/j.1745-7599.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- 77.Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciraulo DA, Piechniczek-Buczek J, Iscan EN. Outcome predictors in substance use disorders. Psychiatr Clin North Am. 2003;26(2):381–409. doi: 10.1016/s0193-953x(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 79.Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- 80.Kazdin AE. Methodology, design, and evaluation of in psychotherapy research. In: Bergin AE, Garfield SL, editors. Handbook of Psychotherapy and Behavior Change. New York: Wiley; 1994. pp. 19–71. [Google Scholar]
- 81.Project MATCH Research Group. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58(1):7–29. [PubMed] [Google Scholar]
- 82.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 83.Marlatt GA. Buddhist philosophy and the treatment of addictive behavior. Cogn Behav Pract. 2002;9:44–50. [Google Scholar]
- 84.Smith BW, Shelley BM, Dalen J, Wiggins K, Tooley E, Bernard J. A pilot study comparing the effects of mindfulness-based and cognitive-behavioral stress reduction. J Altern Complement Med. 2008;14(3):251–258. doi: 10.1089/acm.2007.0641. [DOI] [PubMed] [Google Scholar]
- 85.Zautra AJ, Davis MC, Reich JW, Nicassario P, Tennen H, Finan P, Kratz A, Parrish B, Irwin MR. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. 2008;76(3):408–421. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- 86.Teasdale JD, Segal ZV, Williams JM. Mindfulness training and problem formulation. Clin Psychol - Sci Pr. 2003;10(2):157–160. [Google Scholar]
- 87.Marcus MT, Liehr PR, Schmitz J, Moeller FG, Swank P, Fine M, Cron S, Granmayeh LK, Carroll DD. Behavioral Therapies Trials: A Case Example. Nurs Res. 2007;56(3):210–216. doi: 10.1097/01.NNR.0000270024.52242.39. [DOI] [PMC free article] [PubMed] [Google Scholar]