Summary
The INternational VErapamil SR-Trandolapril STudy (INVEST), a randomized trial of 22,576 predominantly elderly patients with an average 2.7-year follow-up, compared a calcium antagonist led strategy (verapamil SR plus trandolapril) with a β blocker led strategy (atenolol plus hydrochlorothiazide) for hypertension treatment and prevention of cardiovascular outcomes in coronary artery disease. patients.
Patients received individualized dose and drug titration following a flexible, multi-drug, guideline-based treatment algorithm, with the objective of achieving optimal blood pressure (BP) control individualized for comorbidities (e.g., diabetes). The primary outcome (PO) was first occurrence of death (all-cause), nonfatal myocardial infarction or nonfatal stroke.
The strategies resulted in significant and very similar BP reduction with approximately 70% of patients in both strategies achieving BP control (< 140/90 mm Hg). Increasing number of office visits with BP in control was associated with reduced risk of the PO. Overall, there was no difference in the PO comparing the strategies, however new onset diabetes occurred more frequently in those assigned the atenolol strategy. This report summarizes findings from INVEST and puts them in perspective with our current state of knowledge derived from other large hypertension treatment trials. INVEST findings support that 1) BP reduction is important for prevention of adverse cardiovascular morbidity and mortality; and 2) selection of antihypertensive agents should be based on patient comorbidities and other risk factors (e.g. risk for diabetes) and not necessarily that any one drug be given to all.
Keywords: Coronary artery disease, hypertension, atenolol, verapamil SR, trandolapril, hydrochlorothiazide, INVEST, new onset diabetes
Introduction
Hypertension is the most prevalent risk factor for atherosclerotic coronary heart disease (CHD), and is present in ~70% of first myocardial infarction (MI) patients, first stroke patients and heart failure patients.[1] The risk of death, MI, and stroke for each age decade above 40 years and each 10 mm Hg above 115 systolic is incrementally increased and substantial [2,3]. The relationship between blood pressure (BP) lowering and morbidity and mortality reduction has been well documented, however because many early studies excluded patients with documented heart disease and many of the more contemporary studies only included small subsets of patients with documented heart disease, the relationship in contemporary patients with concomitant hypertension and documented coronary artery disease (CAD) is less well understood.
Additionally, which antihypertensives are best used and/or avoided has garnered increasing interest in recent years. Some believe β blockers no longer have a place in uncomplicated hypertension treatment based on data suggesting they inadequately protect from stroke [4,5] may not lower central BP adequately [6], and are not well tolerated compared with newer agents. Others believe that thiazide diuretics should be used as initial agents in all hypertensives, despite their metabolic complications, the most significant of which is diabetes [7]. Most recent, is the suggestion that BP lowering drugs should be used in everyone to protect against CHD and stroke, regardless of BP, without preference to any drug class [8].
Because we studied older and newer antihypertensives in a population of hypertensives with CAD, data from the INternational VErapamil SR-Trandolapril STudy (INVEST) can shed light on a number of issues and concerns related to antihypertensive drug use in this growing, high risk population. The purpose of this review is to summarize findings from INVEST published in the last several years, and compare and contrast results from other large hypertension treatment trials published during the same time frame.
Background and rationale
INVEST was conceived in the mid-nineties to address unanswered questions regarding hypertension management in patients with CAD. At that time, diuretics and β blockers were recommended as standard BP lowering therapy, however, reductions in morbidity and mortality were consistently less than predicted from epidemiologic studies and had plateaued in recent years [9]. Use of newer agents such as calcium antagonists and angiotensin converting enzyme (ACE) inhibitors was on the rise, however outcome data for these drugs were lacking, and there was concern regarding the safety of short-acting calcium antagonists, particularly in patients with ischemic heart disease.
To address the lack of outcome data related to newer antihypertensive agents, and the uncertainty surrounding the best treatment for hypertensive CAD patients, we undertook INVEST [10]. We focused on an older hypertensive population with evidence for CAD and opted to test verapamil SR, a long acting, non-dihydropyridine calcium antagonist with heart rate slowing properties and favorable results in patients with ischemic heart disease [11] and atenolol, the most widely prescribed β blocker, worldwide. Because we anticipated few patients would achieve BP control with monotherapy, we prespecified the ACE inhibitor, trandolapril as add-on therapy in the verapamil SR strategy (administered as the combination product Tarka®), and for all patients requiring protection from organ damage, and the thiazide diuretic hydrochlorothiazide (HCTZ) in the atenolol strategy [10]. Twice daily dosing was recommended after the initial step to assure sustained BP reduction with atenolol. HCTZ, atenolol, and verapamil SR are among the most frequent generic drugs prescribed worldwide. In the US in 2008, they totaled more than 96 million prescriptions [12], underscoreing the continued applicability and importance of findings from INVEST to guide treatment of hypertensive CAD patients.
Design
INVEST was an international, multicenter study with a prospective, randomized, open, blinded endpoint evaluation design [13], conducted in accordance with the principles of the Declaration of Helsinki. The hypothesis was that risk for adverse outcomes was equivalent comparing a verapamil SR-based with an atenolol-based strategy when the strategies were deployed to achieve the same BP control. Patients were eligible for inclusion if they were aged >50 years and had documented CAD with essential hypertension as defined by the Sixth Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC VI) [9] requiring drug therapy. Patients taking β blockers within two weeks of randomization, or taking β blockers for an MI that occurred in the previous 12 months were excluded to avoid withdrawal phenomena in patients randomized to the calcium antagonist strategy [10].
The protocol specified in-person clinic evaluations every 6 weeks for the first 6 months and then every six months thereafter until study end. Evaluations included physical exam, assessment of BP and pulse, angina symptoms in the prior 4 weeks, compliance with study medications and assessment of subjective well-being. The latter instrument allowed participants to rate their overall well-being in the prior 4 weeks as “excellent”, “good”, “fair”, or “poor”. BP was treated to a target of <140/90 mm Hg or BP <130/85 mm Hg when diabetes or renal impairment was present.
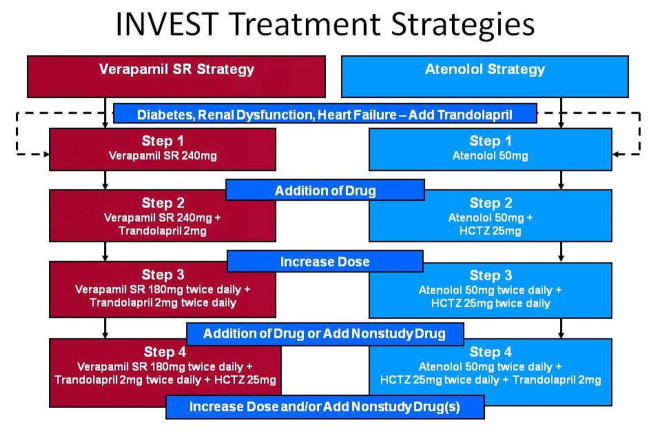
Patients were randomized to receive either verapamil SR or atenolol, and the addition of trandolapril and/or HCTZ was recommended when necessary to achieve BP goals, Figure 1. Importantly, drugs, doses and combinations were carefully selected for INVEST based on their relevant and complimentary actions for optimal BP treatment and control in the high risk CAD population.
Figure 1.
INVEST Treatment strategies. The drugs, order of addition, and recommended doses for each step of each strategy are summarized. Nonstudy antihypertensive drugs could be added to control blood pressure except for β blockers in those assigned to the verapamil SR strategy and calcium antagonists for those assigned to the atenolol strategy. Titration ranges: atenolol, 25–200mg/day; hydrochlorothiazide, 12.5–100mg/day; trandolapril, 1–8mg/day; and verapamil SR, 120–480mg/day. Reprinted with permission from [25]
A novel, study specific internet based data entry system was developed and utilized to collect data from sites in all 14 countries in real time [10]. The system had important validation logic built-in to identify nonphysiologic and/or highly variable BP responses. The system also included an electronic dosing/prescribing module which was developed with logic to automatically recommend uptitration of medication (i.e., dose increase or drug addition) when BPs were reported that did not meet goal, and with the exception of the initial dosage regimens, all regimens were dosed twice daily to ensure consistent 24 hour BP coverage. However, the electronic prescribing system was flexible to allow the practitioner to tailor the therapy (dose and drugs) for individual patients [14].
In addition to BP treatment, patients received concomitant guideline-based treatment for comorbidities like diabetes, renal impairment, lipid disorders, peripheral arterial disease and angina.
Patients were followed for BP control and outcomes for at least two years after randomization. The overall objective of INVEST was to compare the risk for the primary outcome (PO), defined as all-cause death, nonfatal MI or nonfatal stroke, following treatment with the two strategies. Secondary outcomes included not only all-cause death, nonfatal MI and nonfatal stroke individually, but also new onset diabetes and trends for cancer, Parkinson’s disease, Alzheimer’s disease, autoimmune disease and gastrointestinal bleeding, since these had all been anecdotally attributed to long term use of calcium antagonists.
Depression is common in CAD patients [15,16], and is an important risk factor for subsequent CHD events [16]. Because use of β blockers is associated with generalized fatigue and depression, we conducted the Study of Antihypertensive Drugs and Depressions Symptoms (SADD-Sx) substudy [17] to examine the tolerability of the two strategies and to assess for depression at baseline and after 1 year of treatment. For the substudy, 2317 consecutively randomized INVEST patients in the US were mailed questionnaires, including a sociodemographic survey at baseline and the Center for Epidemiologic Studies-Depression (CES-D) scale at baseline and after 1 year of study participation.
Another ongoing substudy includes ambulatory BP monitoring (ABPM), in which a portion of the INVEST population underwent ABPM at baseline and after 1 year of follow-up. Analysis from this substudy is underway and publications will be forthcoming. We also conducted the INVEST Genetics Substudy (INVEST GENES) in which almost 6000 INVEST participants provided a sample of DNA. While genetic analyses continue and many publications exist [18–24], summary of these data are beyond the scope of this review.
Data analysis
The study investigators had access to all of the data and performed or confirmed all of the analyses published to date. All of the main analyses were completed as specified in the protocol using the intent-to-treat population.
To estimate the impact of study drugs on outcomes, a drug dose model was developed using the prescribing information from the INVEST online system.[25] Drug variables in the model were the average daily dose for each of the four study drugs, ratios for the proportion of time that the first two drugs in each treatment strategy were prescribed at the same time (e.g., verapamil SR plus trandolapril, or atenolol plus HCTZ), and terms for interactions between both trandolapril and HCTZ and treatment strategy (since both drugs could have been prescribed in either strategy).
It was decided, a priori, that a 20% difference in the PO between the treatment strategies would be clinically relevant using the intention-to-treat population. Therefore the equivalence bound for the risk ratio was a confidence interval (CI) of 1.20 to 0.83. We assumed an annual PO rate of no less than 2% and α of 0.05 (two sided), and 90% power when estimating the number of patients required. On this basis, a tentative sample size of 27,000 patients was set with an anticipated dropout rate of 5–10%. The final target sample size was reduced to 22,000 patients because the longer-than-planned enrollment period resulted in an increased number of patient-years of follow-up [26].
Results
INVEST enrolled a total of 22,576 patients from 862 sites in 14 countries between September 1997 to December 2000 [26]. Investigators were practitioners in the ambulatory care setting across all practice types (academic, private practice, community health departments), making this a clinically relevant assessment of the implementation of a BP protocol with broad applicability.
Baseline Characteristics
Overall, the majority of patients were women (52%) with a mean age of 66 years. Approximately one third of patients were older than 70 years and more than 2,000 patients were >85 years old, making this one of the largest randomized subgroups to be reported among older patients.. The population was racially diverse, with 36% Hispanic [27] and 13% black. Baseline characteristics were very similar in both treatment strategies and generally reflected an elderly cohort with chronic CAD [26,28,29]. Prior MI was present in about a third, two thirds had angina, and over a quarter had diabetes. Mean BP among those using antihypertensive drugs was 149/86 mm Hg and among those untreated, 159/93 mm Hg. On average, patients were overweight [mean body mass index (BMI) 29 kg/m2], with additional risk factors for cardiovascular events. Baseline characteristics are summarized in Table 1 for the overall population and various subpopulations. Although the entire INVEST population would be considered high risk (age >50, hypertension, CAD), the study had many high-risk subgroups at baseline, including 6400 patients who also had diabetes [30], 7218 patients with a history of MI [31], and 2969 patients with class II or III obesity [32].
Table 1.
INVEST baseline characteristics, overall and in selected subgroups. Values reported are % unless otherwise indicated.
| All Patients [26] (n = 22,576) | Hispanics [27] (n = 8045) | Diabetics [30] (n = 6400) | Post-MI [31] (n = 7218) | |
|---|---|---|---|---|
| mean Age, yrs (SD) | 66 (9.8) | 66 (9.8) | 66 (9.1) | 67 (9.7) |
| Female | 52 | 61 | 54.0 | 38.3 |
| mean BMI kg/m2 (SD) | 29 | 29 (6.2) | 30 (5.8) | 29 (7.2) |
| Caucasian | 48 | 0 | 44 | 64 |
| Hispanic | 36 | 100 | 38 | 21 |
| Black | 13 | 0 | 16 | 13 |
| Prior MI | 32 | 19 | 34 | 100 |
| Angina pectoris | 67 | 87 | 67 | 43 |
| Heart Failure (class I-III) | 5.6 | 3.0 | 8.1 | 8.3 |
| Prior Stroke | 5.1 | 5 | 6.8 | 9.8* |
| Hypercholesterolemia† | 56 | 48 | 62 | 65 |
| Diabetes† | 28 | 30 | 100 | 30 |
| Renal impairment†† | 1.9 | 1.0 | 3.7 | 2.7 |
| Past smoker | 46 | 38 | 46 | 58 |
| Current smoker | 12 | 7 | 10 | 15 |
Includes transient ischemic attack,
includes coronary artery bypass graft and/or percutaneous coronary intervention,
Based on patient history or use of antidiabetic or lipid-lowering medication,
History of or currently have elevated serum creatinine level but less than 4 mg/dl (<354 μmol/L)
MI = myocardial infarction; SD = standard deviation; BMI = body mass index.
Outcomes
Primary Outcome
After 61,835 patient-years of follow-up, the PO rate was not statistically significantly different between the two treatment strategies [verapamil SR strategy, 9.4%, atenolol strategy, 10.2%; relative risk (RR) 0.98, 95% CI 0.90–1.06] in the overall population or any of the subpopulations. The PO rate was highest in subgroups of patients with heart failure, age >70 years old, diabetes, coronary revascularization, and prior MI. Also within these subgroups there was no difference in PO by treatment strategy [26].
In the overall population, baseline predictors of the PO in descending order of risk increase were heart failure, diabetes, increasing age, US residency, renal impairment, stroke/transient ischemic attack (TIA), smoking, prior MI, peripheral arterial disease (PAD), and coronary revascularization [33].
Overall, higher baseline BMI was not a risk factor for the PO. In a stepwise model with normal BMI (20 to <25 kg/m2) as the reference point, the relationship between BMI and the PO was found to be quadratic rather than linear. The risk of the PO was highest for thin patients (BMI <20 kg/m2: adjusted hazard ratio [HR] = 1.52; 95% CI 1.24–1.86) and lowest for class I obesity patients (BMI 30 to <35: adjusted HR = 0.68; 95% CI 0.59–0.77) [32].
In a model adjusted for baseline covariates, multi-drug therapy was associated with reduced risk for the PO in both strategies compared with monotherapy, and with similar results when the model was adjusted for average follow-up systolic BP (SBP) and diastolic BP (DBP) [25].
Outcomes and predictors of outcomes are summarized in Table 2 for the randomized population and clinically important subgroups.
Table 2.
Predictors of increased risk of adverse outcome from adjusted stepwise Cox proportional hazard models, overall and in selected subgroups. Data presented as hazard ratio (95% confidence interval).
| Primary Outcome | New Onset Diabetes | MI | Stroke | |||
|---|---|---|---|---|---|---|
| All Patients [33] (n = 22,576) | Diabetes (n =6400) | Post-MI [31] (n = 7218) | No Diabetes at Entry [34] (n = 16,176)† | Post-MI [31] (n = 7218) | All Patients [37] (n = 22,576)† | |
| Treatment strategy (Verapamil SR vs. Atenolol)* | 0.97 (0.89–1.05)# | 1.05 (0.93–1.18)# | 0.94 (0.83–1.06)# | 0.85 (0.76–0.95) | 0.99 (0.81–1.20)# | 0.87 (0.71–1.06)# |
| Baseline condition | ||||||
| Heart failure (class I – III)* | 1.96 (1.73–2.22) | 2.26 (1.89–2.7) | 1.89 (1.59–2.25) | 2.27 (1.75–2.94) | ||
| Diabetes | 1.77 (1.62–1.93) | 1.77 (1.55–2.02) | NA | 1.90 (1.55–2.33) | 1.43 (1.14–1.78) | |
| Age (10 year increments)* | 1.63 (1.56–1.71) | 1.04 (1.04–1.05) | 1.47 (1.37–1.58) | 0.90 (0.85–0.96) | 1.19 (1.07–1.32) | 1.55 (1.38–1.75) |
| age per year | ||||||
| US Residency | 1.61 (1.41–1.84) | 1.55 (1.24–1.93) | 1.57 (1.31–1.88) | 1.62 (1.37–1.91) | 1.75 (1.24–2.47) | |
| Renal impairment | 1.50 (1.24–1.82) | 1.77 (1.39–2.25) | 1.30 (0.98–1.72)# | |||
| Prior stroke/TIA | 1.43 (1.27–1.62) | 1.45 (1.21–1.74) | 1.30 (1.09–1.55) | 1.26 (1.03–1.56) | 1.40 (1.06–1.84) | 2.33 (1.78–3.04) |
| Smoking (ever) | 1.41 (1.29–1.54) | 1.23 (1.07–1.41) | 1.26 (1.10–1.45) | 1.33 (1.06–1.66) | ||
| Prior MI* | 1.34 (1.23–1.46) | 1.42 (1.24–1.63) | NA | NA | 1.29 (1.04–1.61) | |
| PVD | 1.27 (1.14–1.42) | 1.37 (1.18–1.60) | 1.28 (1.09–1.51) | 1.34 (1.04–1.73) | ||
| Coronary revascularization | 1.15 (1.05–1.26) | 1.07 (0.92–1.23) # | 1.18 (1.03–1.35) | |||
| Race: Black vs. Caucasian* | 1.12 (0.99–1.26)# | 1.06 (0.88–1.29) # | 1.12 (0.94–1.34)# | 1.22 (0.93–1.60)# | ||
| Male gender* | 1.08 (0.99–1.19)# | 1.00 (0.87–1.15) # | 0.97 (0.85–1.11)# | 1.05 (0.86–1.29)# | ||
| Race: Hispanic vs. Caucasian* | 0.88 (0.79–0.99) | 0.98 (0.83–1.16) # | 1.12 (0.94–1.34)# | 1.21 (1.05–1.39) | 1.04 (0.80–1.35)# | |
| Race: Other vs. Caucasian* | 0.86 (0.59–1.24)# | 0.80 (0.45–1.43) # | 0.98 (0.58–1.66)# | 1.64 (1.15–2.34) | 1.05 (0.47–2.37)# | |
| Race: Asian vs. Caucasian* | 0.49 (0.24–0.98) | 0.54 (0.20–1.46) # | 0.48 (0.18–1.30)# | |||
| LVH | 0.90 (0.76–1.05) # | 1.27 (1.10–1.46) | ||||
| Arrythmia | 1.21 (1.01–1.45) | 1.55 (1.14–2.13) | ||||
| Hypercholesterolemia | 1.17 (1.04–1.31) | |||||
| BMI (5 kg/m2 increments) | 1.05 (1.04–1.06) | |||||
| CABG vs. no CABG | 1.47 (1.15–1.88) | |||||
| Race: Black vs. Non-Black | 1.64 (1.25–2.14) | |||||
| Follow-up | ||||||
| Time-dependent SBP ≥ 140 mm Hg | 1.59 (1.29–1.96) | |||||
Prespecified covariates (age, gender, race/ethnicity, prior MI, and heart failure) were forced into the model; other covariates were selected at P <0.10.
Prespecified covariates that did not increase risk were not reported, even though they were included in the model to adjust for baseline differences between subgroups.
P-value not significant.
MI = myocardial infarction; Ve = verapamil; AT = atenolol; ND = no difference between treatment strategies, specific values not reported; NA = not applicable, since 100% of patients in this subgroup had the presence (or absence) of this factor; ND = no difference between treatment strategies, specific values not reported. TIA = transient ischemic attack; PVD = peripheral vascular disease; LVH = left ventricular hypertrophy; BMI = body mass index; CABG = coronary artery bypass graft; SBP = systolic blood pressure.
New Onset Diabetes
Among the 16,176 nondiabetics at baseline, new onset diabetes, a prespecified outcome, occurred in 1234 patients during follow-up. The rate of new diabetes was lower in the verapamil SR strategy than in the atenolol strategy (7.03% vs. 8.23%: RR 0.85; 95% CI 0.76–0.95) [26,34].
Baseline characteristics associated with increased risk of new onset diabetes included US residency, prior stroke/TIA, coronary revascularization, Hispanic ethnicity, other race (including multiple races), left ventricular hypertrophy, hypercholesterolemia and increasing BMI. While the risk of new onset diabetes decreased with increasing age above 50 years old, elevated follow-up SBP was associated with an increased risk of new onset diabetes. From a stepwise time-dependent model, risk of new diabetes was 53% higher with an SBP of 150 mm Hg than with an SBP of 120 mm Hg [34].
Using drug dose modelling [25] with atenolol 50 mg/day as reference, atenolol 50mg/day plus HCTZ 50mg/day increased the risk of new diabetes (HR 1.38, 95% CI 1.06–1.80), which was not reduced when trandolapril was added. Verapamil SR 240mg/day plus trandolapril 4mg/day reduced the risk of new diabetes (HR 0.58, 95% CI 0.44–0.78), which was not increased when HCTZ was added [34].
Preliminary data shows that while the death rate during INVEST in those who developed diabetes was low, after extended follow-up (five years after the end of INVEST), rate of all-cause death in those who develop diabetes surpasses those without diabetes and is similar to those who had diabetes at baseline [35].
Angina Episodes
While the prevalence of angina at baseline in this CAD population was high, as BP was controlled during follow-up, the percent of patients reporting angina declined by more than 50% in both strategies, with fewer angina episodes per week reported in the verapamil SR strategy [24 months, mean (SD): verapamil SR 0.77 (1.31), atenolol 0.88 (1.62); P = 0.02] [26]. Mean resting heart rate (RHR), which was 75.5 ± 9.6 beats per minutes (bpm) at baseline, was reduced more at 24 months in the atenolol strategy (69.2 bpm) than in the verapamil SR strategy (72.8 bpm) as was expected. Despite this difference in RHR, both strategies had equivalent outcomes. An analysis using a stepwise Cox proportional hazards model determined that the relationship between follow-up RHR and PO was quadratic. The RHR nadir was 59 bpm for the overall INVEST population and was lower for the atenolol strategy (51 bpm) than for the verapamil SR strategy (62 bpm), consistent with the differences in mean RHR between the two strategies [36].
Blood Pressure
As expected, baseline BP was lower in treated patients; however, at entry fewer than 20% of patients had BP in control. During follow-up, BP reductions were equivalent comparing the strategies (24 months, verapamil SR 18.7/10.0mmHg, atenolol 19.0/10.2, mmHg, p values not significant), with most of the BP reduction occurring in the first 6 months [26]. BP control was similar in both treatment groups (24 months, <140/90 mm Hg: verapamil SR 72%, atenolol 71%).
Most INVEST patients required multiple drugs for BP control. At 24 months in both strategies, 30% of patients were taking two antihypertensive medications and approximately half of the patients were taking three or more antihypertensive medications. At 24 months, only 2% of patients in each strategy were not taking any antihypertensive medication [26], and patients with diabetes were taking an average of 2.9 antihypertensive medications, compared to 2.8 for patients with and 2.0 for patients without new diabetes [30,34].
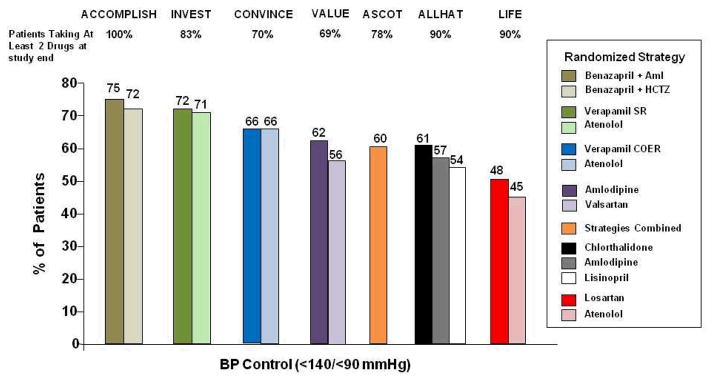
Table 3 summarizes baseline and follow-up BP in the overall population and multiple subgroups. Regardless of subgroup evaluated, like in the overall population there was no difference in BP reduction comparing the treatment strategies. At the completion of the trial, the level of BP control achieved in INVEST compares favourably to many other large BP treatment trials undertaken in recent years, Figure 2.
Table 3.
INVEST blood pressure and primary outcome rates, overall and in selected subgroups.
| All Patients [26] (n = 22,576) | Hispanics [27] (n = 8045) | Diabetics [30] (n =6400) | Post-MI [31] (n = 7218) | Stroke During Follow-up [37] (n = 377) | Without Stroke During Follow-up [37] (n = 22,199) | |
|---|---|---|---|---|---|---|
| Mean Baseline BP, mm Hg | 151/87 | 148/87 | 151/86 | 150/86 | 152/85 | 151/87 |
| Mean 24 month BP, mm Hg | 132/77 | 129/78 | 132/76 | 131/76 | SBP 142 | SBP 135 |
| Mean 24 month BP | 71 | 78 | 71 | 73 | 47 | 69 |
| <140/90 mm Hg, % |
MI = myocardial infarction; BP = blood pressure; SBP = systolic blood pressure.
Figure 2.
Percent of patients in contemporary blood pressure treatment trials achieving blood pressure control defined as <140/<90 mm Hg. Aml=amlodipine, SR=sustained release, COER=controlled onset extended release., ACCOMPLISH [47], INVEST [26], CONVINCE [52], VALUE [53], ASCOT [46], ALLHAT [7], LIFE [45].
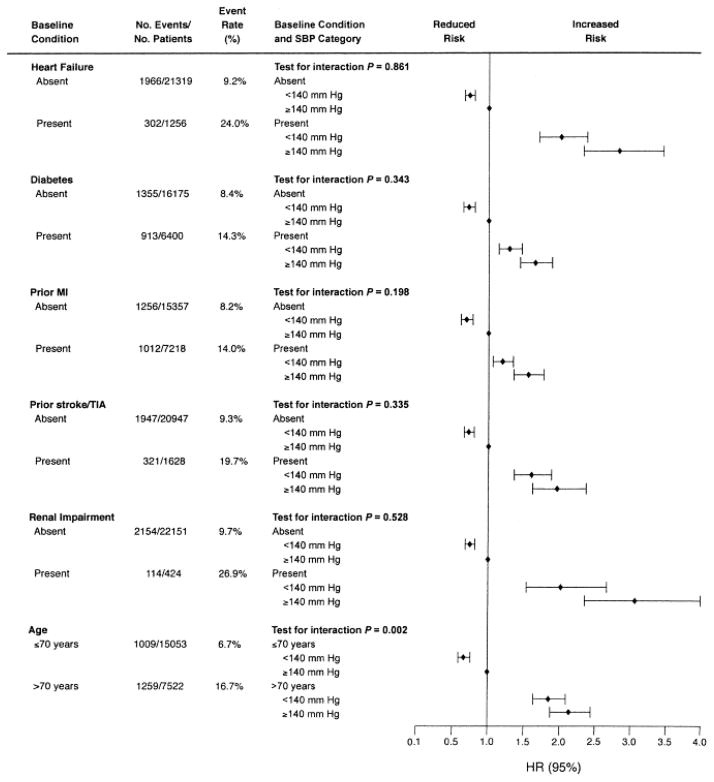
Baseline BP was not predictive, while follow-up BP was predictive of the PO. Patients with mean follow-up SBP <140 mm Hg or DBP <90 mm Hg had a lower risk of PO (adjusted HRs 0.82 and 0.70, respectively; P <0.001 for both comparisons) [33]. Lower on treatment SBP was associated with significant risk reduction for the PO, in the overall population as well as in multiple high risk subgroups, Figure 3 [33].
Figure 3.
Risk [adjusted hazard ratio (HR) and 95% confidence interval (CI)] for primary outcome associated with high-risk subgroups by time-dependent systolic blood pressure (SBP) category. In general, risk was lower when SBP <140 mm Hg. MI = myocardial infarction; TIA = transient ischemic attack. Reprinted with permission from [33]
Patients with mean follow-up SBP <140 mm Hg or DBP <90 mm Hg had a lower risk of stroke (adjusted HRs 0.63 and 0.50, respectively; P = 0.001 for both comparisons) [37]. Follow-up SBP <140 mm Hg was associated with a significant reduction in risk of stroke in subgroups with prior coronary artery bypass graft, prior stroke/TIA, age >70, US residency, diabetes, and a history of smoking [37].
The relationship between PO and follow-up BP followed a J-shaped curve, which when adjusted for baseline differences was flatter for SBP than for DBP and had a nadir of 129.5/73.8 mm Hg. Patients with follow-up DBP of 70 mm Hg or less accounted for 10.7% of patients (2415/22,576) and 19.6% of the PO events (445/2268). A similar relationship was found for DBP and all-cause death (the main component of the PO), MI (fatal and nonfatal), and, to a lesser extent, stroke (fatal and nonfatal) [38].
To investigate the impact of consistency of BP control on outcomes, INVEST patients were divided into four groups based on the proportion of visits with BP in control (<25%, ≥25% to <50%, ≥50% to <75%, ≥75%). All four groups experienced reductions in BP during the study, with the size of the reduction increasing for all but the group with ≥75% of visits in control (6.4/4.1, 13.9/7.4, 19.0/9.9, 17.3/9.3 mm Hg, respectively). Risk for PO, MI (fatal and nonfatal), and stroke (fatal and nonfatal) decreased as the proportion of office visits with BP in control increased and was lower in the group with ≥75% of visits in control compared to the group with <25% of visits in control by 40%, 42%, and 50%, respectively. When included together in a model, proportion of visits in control (continuous variable) and mean follow-up SBP were both predictive of PO risk. [39].
Depression and Quality of Life
Among INVEST participants who responded to surveys at baseline and year 1, CES-D scores improved significantly from baseline to 1 year in the verapamil SR strategy (n = 617, 14.00 vs. 12.54; P <0.001) while remaining unchanged in the atenolol strategy (n = 575, 14.27 vs. 14.00; P = 0.44). Baseline predictors of higher CES-D scores (more depression) at 1 year were higher CES-D scores at entry (P <0.001), history of depression diagnosis (P = 0.03), history of stroke (P <0.001), and randomization to the atenolol strategy (P <0.001) [17].
With regard to quality of life, follow-up SBP >150-≤160 mm Hg and > 160 mm Hg were associated with a significant increase in the odds of feeling fair/poor [adjusted OR(95% CI) 1.90 (1.81–2.00) and 2.53 (2.41–2.66), respectively]. Those who reported angina in the 4 weeks prior to a protocol visit had 2.2 times greater odds of reporting fair/poor subjective well being [adjusted OR 2.2, 95% CI (2.13–2.27)] [17,40].
Safety and Tolerability
Both treatment strategies were well tolerated. Adverse event rates were low and consistent with what has been previously reported for the study drugs. Patients in the verapamil SR strategy reported more constipation, while patients in the atenolol strategy reported more symptomatic bradycardia, and wheezing. Importantly, there were no differences in episodes of gastrointestinal bleeding, cancer, Parkinson’s disease, Alzheimer’s disease, autoimmune disease or other adverse events comparing the strategies.
Conclusion
Across the spectrum of hypertensive CAD patients studied in INVEST, we demonstrated that 1) BP can be controlled in a high-risk population using a verapamil SR - based or an atenolol-based multi-drug treatment strategy without concern for safety or tolerance, 2) lowering BP is associated with reduced risk of cardiovascular events, including all-cause death, MI and stroke, 3) risk of new onset diabetes is reduced when BP is lowered with the combination of verapamil SR plus trandolapril, and 4) both BP lowering strategies reduce the occurrence of angina episodes, and this along with BP reduction is associated with improved feeling of well being.
Expert Commentary and Five Year View
Findings from INVEST demonstrate that both readily available therapeutic strategies, which now contain only generic medications, when deployed to lower BP to goal result in equivalent outcomes. This supports the notion that selection of antihypertensive agents should be based on the patients’ comorbidities and other risk factors (e.g. risk for diabetes), and not necessarily any given drug should be used in all hypertensive patients. Conclusions from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) included the recommendation that thiazide diuretics should be used as first-line BP lowering drugs, regardless of patient characteristics, and despite the increased risk of diabetes in patients who received chlorthalidone [7]. While BP was lower in the chlorthalidone treated group, there was no significant difference in the occurrence of CV related adverse outcomes evaluated, with the exception of heart failure [7]. It is not clear why this lower BP did not result in reduced outcomes (particularly death, MI and stroke)
In a recently published summary from ALLHAT, it was reiterated that because thiazide diuretics are superior in preventing heart failure, and new onset diabetes, which occurred more frequently in the chlorthalidone treated patients, was not associated with increased CV outcomes, thiazides should continue to be the drug of first choice [41]. However, we and others have shown that risk from diabetes that develops during antihypertensive treatment is associated with significant morbidities and mortality which is similar to diabetes of other etiologies, although there may be a lag in onset [41–44].
β blockers have recently fallen out of favor, in part related poor BP control among elderly patients and metabolic and safety concerns. In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study, which enrolled 9,193 hypertensives with left ventricular hypertrophy, and compared once daily atenolol to losartan with add-on HCTZ in both groups, superior BP lowering and CV outcome prevention was observed in the losartan group [45]. Similarly, in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), which compared once daily atenolol plus HCTZ to amlodipine plus perindopril, there was similar brachial BP lowering in the two groups, but superior reduction in CV outcomes in the amlodipine treated patients [46]. In a substudy of ASCOT patients who underwent central BP assessment, it was determined that patients in the amlodipine group had lower central pressure, and it was hypothesized that it may be this differential effect on central pressure that was responsible for the decreased risk for CV outcomes [6]. However, in INVEST, where atenolol was dosed twice daily rather than once daily as in LIFE and ASCOT, BP lowering and CV outcomes were equivalent. Importantly, we observed no increased risk of stroke in atenolol treated patients as has been observed in some metaanalyses [4,5].
For the first time in high risk CAD patients, a calcium antagonist has been shown to be a safe and beneficial component of a BP lower regimen, including subpopulations with diabetes, post MI, prior coronary revascularization, and the elderly. In the period since INVEST was completed, ASCOT [46] and the Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) study [47] which compared benazepril plus amlodipine to benazepril plus HCTZ, have confirmed these findings. In the overall population, ACCOMPLISH demonstrated superior BP reduction and CV risk reduction in the benazepril plus amlodipine group. While some suggested the CV benefit was derived solely from the greater BP reduction, recently data from an ABPM substudy in 573 patients showed a mean 1.6 mmHg lower SBP in the benazepril plus HCTZ patients, but this was not statistically significant, and over the 24 hour mean daytime and nighttime periods, pressures and surges in BP showed the combinations were equivalentsimilar, which does not explain the CV benefit observed in the amlodipine treated group [48]. Opie [49] recently suggested that in patients with stable angina and no prior MI, a calcium antagonist may be as beneficial as a β blocker, but without the adverse effects of insulin resistance, weight gain, decreased exercise tolerance, and sexual dysfunction.
INVEST, through its recruitment of large numbers of elderlies, women, Hispanics, and blacks, provides an important and here-to-for unknown, understanding of the response to treatment in these subgroups and direct evidence for the generalizability of the findings to these growing populations worldwide. We demonstrated that in some patients, particularly the lean elderly, risk for adverse CV outcomes increased as BP was lowered, suggesting the need to question recommendations for significant BP lowering in all, especially in those with CAD.
In the recent past, many of the professional societies have revised and updated their guidelines and recommendations for the treatment of hypertension. The European Society for Hypertension/European Society of Cardiology are no longer endorsing thiazide diuretics or β-blockers in hypertensive patients with diabetes[50] and the American Association of Clinical Endocrinologists recommends thiazide diuretic use only at low dosage and only with adequate potassium replacement and β-blocker use only as second- or third-line agents in patients with diabetes.[51] The National Institute for Health and Clinical Excellence, together with the British Hypertension Society, recently published guidelines that indicate β-blockers are no longer a suitable first-line treatment option in uncomplicated hypertensive patients largely due to increased incident diabetes and they recommend use of RAS inhibitors as first-line therapy in younger patients, with diuretics reserved for the elderly or black patients of any age.[46] In the US, the National Heart Lung and Blood Institute recently constituted and convened JNC 8, which will synthesize and deliberate data from INVEST as well as all of the other hypertension mega-trials published in the last decade to establish new guidelines and recommendations which will inform treatment of hypertension in the next five years and beyond.
Key issues
A verapamil SR plus trandolapril strategy was equivalent to an atenolol plus hydrochlorothiazide strategy with regard to reduction in CV outcomes, with similar BP reduction and control
The verapamil SR plus trandolapril strategy was associated with a reduced risk for new onset diabetes
Elderly patients with hypertension and CAD require multi-drug therapy for BP control
BP reduction and reduction in angina episodes were associated with improved feeling of well being
Atenolol, when dosed twice daily was not associated with increased risk of stroke or other adverse CV outcomes
Very low diastolic BP (< 70 mmHg) was associated with increased risk of CV outcomes, raising concerns about optimal BP targets in special populations
Acknowledgments
7. Financial disclosure/acknowledgments
INVEST was funded by Abbott and the University of Florida Opportunity Fund. RMC-D, EMH and CJP are co-inventors of the online data capture and prescribing system used in INVEST, which is licensed to the University of Florida and co-inventors for a patent pending for the triple combination of verapamil SR, trandolapril and HCTZ, and have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony. QZ, AC and UL are employees of Abbott. This work is supported in part by NIH grants K23HL086558 and GM074492 (RMC-D).
Annotated bibliography
- 1•.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. Most current statistics related to heart disease and stroke. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Neal B, MacMahon S. The World Health Organization--International Society of Hypertension Blood Pressure Lowering Treatment Trialists’ Collaboration: prospective collaborative overviews of major randomized trials of blood pressure-lowering treatments. Curr Hypertens Rep. 1999;1(4):346–356. doi: 10.1007/s11906-999-0045-2. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366(9496):1545–1553. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 5.Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706–2715. doi: 10.1161/CIRCULATIONAHA.107.695007. discussion 2715. [DOI] [PubMed] [Google Scholar]
- 6.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 7.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 8.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 10•.Pepine CJ, Handberg-Thurmond E, Marks RG, et al. Rationale and design of the International Verapamil SR/Trandolapril Study (INVEST): an Internet-based randomized trial in coronary artery disease patients with hypertension. J Am Coll Cardiol. 1998;32(5):1228–1237. doi: 10.1016/s0735-1097(98)00423-9. Full details describing the rationale and design of the INVEST clinical trial. [DOI] [PubMed] [Google Scholar]
- 11.Pepine CJ, Faich G, Makuch R. Verapamil use in patients with cardiovascular disease: an overview of randomized trials. Clin Cardiol. 1998;21(9):633–641. doi: 10.1002/clc.4960210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Accessed June 11, 2009];Drug Topics. http://www.modernmedicine.com/modernmedicine/data/articlestandard/drugtopics/192009/597084/article.pdf.
- 13.Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press. 1992;1(2):113–119. doi: 10.3109/08037059209077502. [DOI] [PubMed] [Google Scholar]
- 14.Cooper-DeHoff R, Handberg E, Heissenberg C, Johnson K. Electronic prescribing via the internet for a coronary artery disease and hypertension megatrial. Clin Cardiol. 2001;24(11 Suppl):V14–16. doi: 10.1002/clc.4960241706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thombs BD, Bass EB, Ford DE, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21(1):30–38. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 17.Ried LD, Tueth MJ, Handberg E, Kupfer S, Pepine CJ. A Study of Antihypertensive Drugs and Depressive Symptoms (SADD-Sx) in patients treated with a calcium antagonist versus an atenolol hypertension Treatment Strategy in the International Verapamil SR-Trandolapril Study (INVEST) Psychosom Med. 2005;67(3):398–406. doi: 10.1097/01.psy.0000160468.69451.7f. [DOI] [PubMed] [Google Scholar]
- 18.Beitelshees AL, Gong Y, Wang D, et al. KCNMB1 genotype influences response to verapamil SR and adverse outcomes in the INternational VErapamil SR/Trandolapril STudy (INVEST) Pharmacogenet Genomics. 2007;17(9):719–729. doi: 10.1097/FPC.0b013e32810f2e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunner M, Cooper-DeHoff RM, Gong Y, et al. Factors influencing blood pressure response to trandolapril add-on therapy in patients taking verapamil SR (from the International Verapamil SR/Trandolapril [INVEST] Study) Am J Cardiol. 2007;99(11):1549–1554. doi: 10.1016/j.amjcard.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhard T, Gong Y, Beitelshees AL, et al. Alpha-adducin polymorphism associated with increased risk of adverse cardiovascular outcomes: results from GENEtic Substudy of the INternational VErapamil SR-trandolapril STudy (INVEST-GENES) Am Heart J. 2008;156(2):397–404. doi: 10.1016/j.ahj.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AD, Gong Y, Wang D, et al. Promoter polymorphisms in ACE (angiotensin I-converting enzyme) associated with clinical outcomes in hypertension. Clin Pharmacol Ther. 2009;85(1):36–44. doi: 10.1038/clpt.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langaee TY, Gong Y, Yarandi HN, et al. Association of CYP3A5 polymorphisms with hypertension and antihypertensive response to verapamil. Clin Pharmacol Ther. 2007;81(3):386–391. doi: 10.1038/sj.clpt.6100090. [DOI] [PubMed] [Google Scholar]
- 23.Pacanowski MA, Gong Y, Cooper-Dehoff RM, et al. beta-adrenergic receptor gene polymorphisms and beta-blocker treatment outcomes in hypertension. Clin Pharmacol Ther. 2008;84(6):715–721. doi: 10.1038/clpt.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacanowski MA, Zineh I, Cooper-Dehoff RM, Pepine CJ, Johnson JA. Genetic and Pharmacogenetic Associations Between NOS3 Polymorphisms, Blood Pressure, and Cardiovascular Events in Hypertension. Am J Hypertens. 2009 doi: 10.1038/ajh.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott WJ, Hewkin AC, Kupfer S, Cooper-DeHoff R, Pepine CJ. A drug dose model for predicting clinical outcomes in hypertensive coronary disease patients. J Clin Hypertens (Greenwich) 2005;7(11):654–663. doi: 10.1111/j.1524-6175.2005.04800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290(21):2805–2816. doi: 10.1001/jama.290.21.2805. Primary findings of the INVEST clinical trial. Data includes baseline characteristics, BP and drug treatment data and primary outcomes. [DOI] [PubMed] [Google Scholar]
- 27••.Cooper-DeHoff RM, Aranda JM, Jr, Gaxiola E, et al. Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients--findings from the International Verapamil SR/Trandolapril Study (INVEST) Am Heart J. 2006;151(5):1072–1079. doi: 10.1016/j.ahj.2005.05.024. INVEST findings in the Hispanic cohort of patients enrolled in INVEST. INVEST has the largest cohort of Hispanic hypertensive CAD patients published to date. [DOI] [PubMed] [Google Scholar]
- 28.Cooper-DeHoff RM, Handberg EM, Cohen J, et al. Characteristics of contemporary patients with hypertension and coronary artery disease. Clin Cardiol. 2004;27(10):571–576. doi: 10.1002/clc.4960271010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Zineh I, Cooper-Dehoff RM, Wessel TR, et al. Global differences in blood pressure control and clinical outcomes in the INternational VErapamil SR-Trandolapril STudy (INVEST) Clin Cardiol. 2005;28(7):321–328. doi: 10.1002/clc.4960280704. INVEST baseline characteristic and primary outcome data according to geographic regions of enrollment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Bakris GL, Gaxiola E, Messerli FH, et al. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension. 2004;44(5):637–642. doi: 10.1161/01.HYP.0000143851.23721.26. INVEST findings in the cohort with diabetes at baseline. [DOI] [PubMed] [Google Scholar]
- 31.Bangalore S, Messerli FH, Cohen JD, et al. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: an INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J. 2008;156(2):241–247. doi: 10.1016/j.ahj.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120(10):863–870. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 33••.Pepine CJ, Kowey PR, Kupfer S, et al. Predictors of adverse outcome among patients with hypertension and coronary artery disease. J Am Coll Cardiol. 2006;47(3):547–551. doi: 10.1016/j.jacc.2005.09.031. Predictors of adverse outcomes in the INVEST clinical trial. [DOI] [PubMed] [Google Scholar]
- 34••.Cooper-Dehoff R, Cohen JD, Bakris GL, et al. Predictors of development of diabetes mellitus in patients with coronary artery disease taking antihypertensive medications (findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]) Am J Cardiol. 2006;98(7):890–894. doi: 10.1016/j.amjcard.2006.04.030. Description of the cohort that developed diabetes during participation in INVEST and predictors of diabetes develoment. [DOI] [PubMed] [Google Scholar]
- 35.Cooper-DeHoff RM, Gong Y, Marin J, et al. Long-term Mortality Associated with New Onset Diabetes in Hypertensive CAD Patients Following Exposure to Antihypertensive medications: Findings from the INternational VErapamil SR/Trandolapril Study. J Am Coll Cardiol. 2009 Abstr., 0910–0917:A0224. [Google Scholar]
- 36.Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil-SR/trandolapril STudy (INVEST) Eur Heart J. 2008;29(10):1327–1334. doi: 10.1093/eurheartj/ehn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coca A, Messerli FH, Benetos A, et al. Predicting stroke risk in hypertensive patients with coronary artery disease: a report from the INVEST. Stroke. 2008;39(2):343–348. doi: 10.1161/STROKEAHA.107.495465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144(12):884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. Data from INVEST documenting a J shaped curve associated with diastolic BP and adverse outcomes. [DOI] [PubMed] [Google Scholar]
- 39.Mancia G, Messerli F, Bakris G, et al. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. 2007;50(2):299–305. doi: 10.1161/HYPERTENSIONAHA.107.090290. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y, Handberg EM, Gerhard T, et al. Systolic Blood Pressure and Subjective Well-Being in Patients with Coronary Artery Disease. Clinical Cardiology. 2009 doi: 10.1002/clc.20501. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright JT, Jr, Probstfield JL, Cushman WC, et al. ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analyses. Arch Intern Med. 2009;169(9):832–842. doi: 10.1001/archinternmed.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14(7):933–946. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 43.Aksnes TA, Kjeldsen SE, Rostrup M, et al. Impact of new-onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial population. Hypertension. 2007;50(3):467–473. doi: 10.1161/HYPERTENSIONAHA.106.085654. [DOI] [PubMed] [Google Scholar]
- 44.Almgren T, Wilhelmsen L, Samuelsson O, et al. Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28-year follow-up. J Hypertens. 2007;25(6):1311–1317. doi: 10.1097/HJH.0b013e328122dd58. [DOI] [PubMed] [Google Scholar]
- 45.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 46.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 47.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 48.Jamerson K. ACCOMPLISH Ambulatory Blood Pressure Monitoring Substudy. data presented as Late Breaking Clinical Trial at the American Society of Hypertension, 2009 Scientific Sessions; San Francisco, California. 2009. [Google Scholar]
- 49.Opie LH. Controversies in cardiology. Lancet. 2006;367(9504):13–14. doi: 10.1016/S0140-6736(06)67903-8. [DOI] [PubMed] [Google Scholar]
- 50.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 51.American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of hypertension. Endocr Pract. 2006;12(2):193–222. [PubMed] [Google Scholar]
- 52.Black HR, Elliott WJ, Grandits G, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289(16):2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 53.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]