Abstract
The NEMO syndrome is a primary immunodeficiency with immune and non-immune manifestations. The immune deficiency is heterogeneous showing defects in humoral, innate, and cell-mediated immunity. While the clinical aspects of the immunodeficiency are increasingly well understood, little is known about autoimmune manifestations in NEMO patients. We therefore sought to examine serologic markers of systemic inflammation and intestinal pathology in a kindred of patients with the NEMO syndrome. We observed persistent elevation of erythrocyte sedimentation rates in five patients, and two were symptomatic, with a chronic but atypical enterocolitis. Though pathologic lesions in these two patients were consistent with acute inflammation, sustained clinical improvement was only achieved with systemic and/or topical glucocorticoid therapy. Our data suggest that some patients with the NEMO syndrome exhibit persistent elevation of inflammatory markers similar to systemic autoimmune diseases and may subsequently develop an atypical enterocolitis.
Keywords: Autoimmunity, Immunodeficiency, NEMO, Enteritis, Colitis, Inflammatory Bowel Disease, NF-κB, IKKγ
Introduction
The nuclear factor kappa B (NF-κB) family of transcription factors is central to the induction of both innate and adaptive immune responses to a variety of pathogens [1; 2]. The activation of NF-κB proteins is regulated by a multimeric complex consisting of two catalytic subunits, the inhibitor of kappa kinase alpha and beta (IKKα and IKKβ), and a regulatory unit, IKK gamma (IKKγ), also known as the NF-κB essential modulator (NEMO).
The critical role of NF-κB activity in host defense has in part been demonstrated by a group of human disorders resulting from hypomorphic alleles of NEMO that are inherited in an X-linked pattern. These mutations can result in defects in both innate and adaptive immunity. Innate immune defects include impairment of IL-12 production and of Toll-like receptor (TLR) signaling [3; 4], while adaptive immune defects include deficits in B cell responses to specific immunization, and profound T cell lymphopenia [4; 5].
While the clinical aspects of the immune deficiency are increasingly well characterized, the clinical effects of altered NF-κB activity on the inflammatory response are less clear. Given the central role of NF-κB in upregulating pro-inflammatory cytokines, loss of NF-κB activity might predict a diminished inflammatory response despite pervasive infection, as observed in some patients [6]. However, NF-κB activity also plays a role in the generation of natural killer T cells, CD4+CD25+ regulatory T cells, and memory T cells [7; 8; 9]. Therefore, immune dysregulation and organ-specific inflammatory disease might also be anticipated. To date, few studies to elaborate on this issue have been reported [2].
To better understand the clinical manifestations of the immune dysregulation in NEMO deficiency, we studied seven members of a kindred affected by this syndrome. Despite the absence of overt clinical infection or inflammation, most of the patients exhibited sustained periods of elevation of inflammatory markers, reminiscent of those with systemic inflammatory disorders such as systemic lupus erythematosus and inflammatory bowel disease (IBD) [10; 11]. More strikingly, two of our eldest patients have developed an atypical enterocolitis (beginning at 8-10 years of age) that was only responsive to anti-inflammatory therapy. These findings suggest that some NEMO syndrome patients exhibit immune dysregulation inclusive of organ-specific inflammation.
Materials and Methods
Patient care and data collection
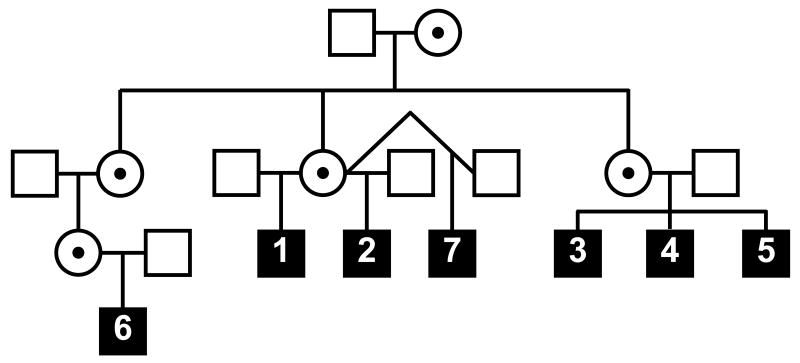
All seven patients in the cohort were seen monthly for intravenous immunoglobulin (IVIG) infusion (400 mg/kg every 4 weeks) at the University of California, San Francisco (UCSF) Pediatric Clinical Research Center (PCRC) of the UCSF Clinical and Translational Research Institute. Interval histories and physical examinations were obtained. The patients' antimicrobial prophylaxis regimen included penicillin (125 mg orally twice daily if <5 years of age and 250 mg orally twice daily if >5 years of age), trimethoprim/sulfamethoxazole (5 mg/kg/day of the trimethoprim component), and azithromycin (20 mg/kg/week). The 7 patients are spread across two generations (Fig. 1), and all had the E391X mutation in the tenth exon of the NEMO gene [12], which encodes for a zinc finger motif critical to NEMO function [4; 13].
Figure 1.
Partial pedigree of the NEMO kindred. Symbols are as follows: open square (unaffected male), circle with black dot (carrier female), closed square (affected male). The patient numbers are depicted within the square of each affected male.
Upon diagnosis, the patients were enrolled in a study protocol that had been reviewed and approved by the Committee for Human Research (CHR) at UCSF. Control patients were similarly enrolled. For histologic studies related to enterocolitis, age-matched biopsy specimens from control patients with histologically and clinically defined IBD were examined [14; 15]. The use of these specimens was also approved by the UCSF CHR.
Laboratory studies
The serum erythrocyte sedimentation rate (ESR) was determined by the UCSF Clinical Laboratory using the Westergren method with a range from 1-100 and >100 mm per hour. Immunoglobulin G (IgG) and C-reactive protein (CRP) levels were determined by the UCSF Clinical Laboratory using rate nephelometry and rate turbidimetry, respectively.
Statistical analysis to examine differences in ESR values between patient results and published case series of ESR in acute infection was performed using an unpaired Student's t-test (Graphpad, San Diego, CA).
Longitudinal analysis of ESR was not carried out for Patient 1 as monthly ESR examination was initiated only after a symptomatic bout of colitis. Data on Patient 7 was limited by his young age.
Endoscopy
Endoscopy was performed with the consent of legal guardians and age-appropriate patient assent. Endoscopy was performed only when medically necessary. Two NEMO patients underwent upper and lower endoscopy a total of 5 times over a 2 year period. Colon biopsies were obtained at regions of visual abnormalities and/or at routine sites including rectum, sigmoid colon, descending colon, transverse colon, ascending colon, cecum, and distal ileum. All biopsies were fixed in formalin prior to transfer to the UCSF Department of Pathology for paraffin embedding and hematoxylin-and-eosin staining.
Results
Patient Demographics
Three of the patients (1-3) in the kindred have previously been described [12], and we have since diagnosed an additional four children (4-7) (Fig. 1). All patients, except patient 7 who was diagnosed perinatally, presented with meningitis in the first two years of life (Table 1). Amongst those old enough to determine, all had the characteristic dentition of NEMO syndrome with variable dermatologic manifestations, including hypohidrotic ectodermal dysplasia, sparse hair, and hypopigmentation. At the time of this study, those in the kindred ranged in age between 0.5-16 years.
Table 1.
Demographics, presenting infection, associated features, infectious complications, and organisms isolated from NEMO patients.
| Patient | Gender | Current Age (Years) | Age at Diagnosis (Years) | Presentation | Associated Features | Major Infectious Complications | Organisms Isolated and Site |
|---|---|---|---|---|---|---|---|
| 1 | M | 16 | 7 | Meningitis, pneumonia | Skin, Teeth | Pneumonia (PNA) × 5 | S. pneumoniae (CSF), S. aureus (skin, blood), Candida (GI tract), H. influenza (sputum) |
| 2 | M | 13 | 4 | Meningitis, pneumonia | Skin, Teeth | PNA × 4 | S. pneumoniae (CSF), Giardia lambia (stool) |
| 3 | M | 11 | 2 | Meningitis | Skin, Teeth | Cellulitis × 2, osteomyelitis | S. pneumoniae (CSF, blood), S. aureus (skin), Enterococcal species (blood), C. difficile (stool) |
| 4 | M | 7 | 0.5 | Meningitis | Skin, Teeth | skin abscess × 6, psoas abscess, PNA | S. pneumoniae (CSF), S. aureus (skin wound), |
| 5 | M | 3 | 0.5 | Meningitis | Skin, Teeth | Aspiration PNA, neck abscess | S. pneumoniae (CSF), S. aureus (skin wound), Candida albicans (VP shunt), Peptostreptococcus (wound), P. aeruginosa (urine, respiratory culture) |
| 6 | M | 9 | 1 | Meningitis | Skin, Teeth | PNA × 4, meningitis, sinusitis | S. pneumoniae (CSF, respiratory culture), H. influenzae (peri-shunt abscess, blood culture, respiratory culture), S. viridans (CSF), S. epidermidis (CSF), Candida albicans (otitis), C. difficile (stool), S. aureus (skin wound, ear drainage) |
| 7 | M | 0.5 | 0.2 | Perinatal Diagnosis | Skin | Urosepsis | E. Coli (urine) |
One of the primary immunologic manifestations of these patients' disease is dysgammaglobulinemia (characterized by normal immunoglobulin G levels but no humoral response to pneumococcal vaccination) [12]. Patients also demonstrated a consistent immunologic phenotype with modest decreases in absolute T cell numbers, elevated B cell numbers, and normal IgG levels in 6 out of 7 patients at the time of diagnosis (ref. [12] and Supplemental Table 1). T cell proliferative studies to antigen and mitogen showed variability. Patients 1-2 had diminished responses early in life but normalization over time(ref. [12] and Supplemental Table 1). Patients 3-5 showed slightly diminished proliferative responses, while patients 6 and 7 had normal responses to PHA stimulation (Supplemental Table 1).
Infections
Although we initially maintained these patients on monthly infusions of IVIG alone, it became apparent over time that IVIG was insufficient to suppress pneumococcal infections. In addition, with the description of susceptibility to other microorganisms including Gram negative bacteria and mycobacteria [3; 16], we modified the kindred's prophylactic regimen to include penicillin, azithromycin, and trimethoprim/sulfamethoxazole. All of the patients nevertheless developed recurrent infections, with a wide variety of causative organisms isolated (Table 1). During these infections, ESR levels were elevated compared to published case series for bacterial meningitis, upper respiratory tract infections, and pneumonia (Supplemental Table 2 and refs. [17; 18]).
Asymptomatic clinical periods with sustained elevation of ESR
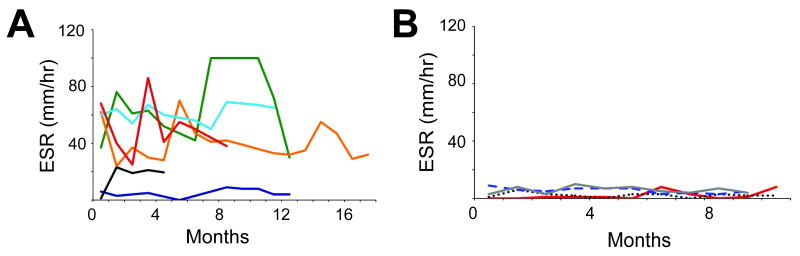
In addition to elevated inflammatory markers during episodes of documented infection, many of those in our NEMO kindred had elevated ESR levels in the absence of local or systemic symptoms of infection. Five of six patients examined showed persistently elevated ESR (mean ESR ranging between 21-67 mm/hr) (Fig. 2). In particular, the sibling triad of patients 3, 4, and 5 had mean ESR values of 67, 40, and 61, respectively, over the documented periods. For all patients with a persistently elevated ESR, the monthly values fluctuated (Fig. 2A), but were nevertheless elevated for age [19]. Only patient 2 had normal ESR values with a mean of 5.2. The ages of the patients at the start of the intervals examined were 1.7-11 years. None of the patients was hospitalized during the surveillance periods or received antibiotics beyond their prophylactic regimen. The patients reported no organ-specific disease, including weight loss or diarrhea, during the clinical evaluation performed prior to IVIG infusion. In addition, patients showed progressive weight and height gains during this time period (data not shown). A control group of 2 patients with combined immunodeficiency and 2 patients with CD40 ligand deficiency showed no elevation of ESR during similar 10-12 month surveillance periods (Fig. 2B). CRP levels during these same periods were available for only 5 of 6 patients. Interestingly, the patients showed normal levels (<6 mg/dl) 90% of the time (data not shown).
Figure 2.
Persistent elevation of ESR despite an asymptomatic clinical course for patients 2-7. A) ESR levels depicted for each patient over the examined intervals. Data from each patient correspond to the following colored lines: Patient 2 (Purple), 3 (Red), 4 (Orange), 5 (Light blue), 6 (Brown), and 7 (Black). B) Control ESR levels from 2 female patients with a combined immunodeficiency (Gray line and Blue dashed line) and 2 male patients with CD40 ligand deficiency (Red line and Black dots). These patients were between 8 and 28 years of age.
Atypical Enterocolitis
With increasing age, two patients (Patients 1 and 3) developed chronic abdominal pain secondary to enterocolitis. Patient 1 developed intermittent peri-umbilical pain at 8.5 years of age. Over the next 4 years, the patient had three partial colectomies secondary to severe abdominal pain with exploratory laparotomy demonstrating terminal ileum and ascending colon inflammation each time. The second episode was also complicated by an intra-abdominal abscess. Cultures obtained from this abscess as well as tissue cultures from the second and third colectomies yielded no viral, fungal, mycobacterial, or bacterial pathogens. (Culture results were not available from the first colectomy.) The first two resections led to resolution of the symptoms. However, after the third resection, the patient had intermittent attacks of abdominal pain, which did not abate with extended courses of empiric, broad-spectrum antibiotic therapy. Given this lack of benefit, an autoimmune or autoinflammatory etiology to the patient's disease was considered and methylprednisolone was delivered via intravenous infusion (30 mg a day for 14 days followed by a two month taper). The patient's abdominal pain resolved almost immediately. He has subsequently had four disease flares treated with pulse steroids each time. He is currently being treated with a combination of oral budesonide (9 mg oral daily), asacol (1 gram orally twice daily), and oral prednisone as maintenance therapy (10 mg orally daily).
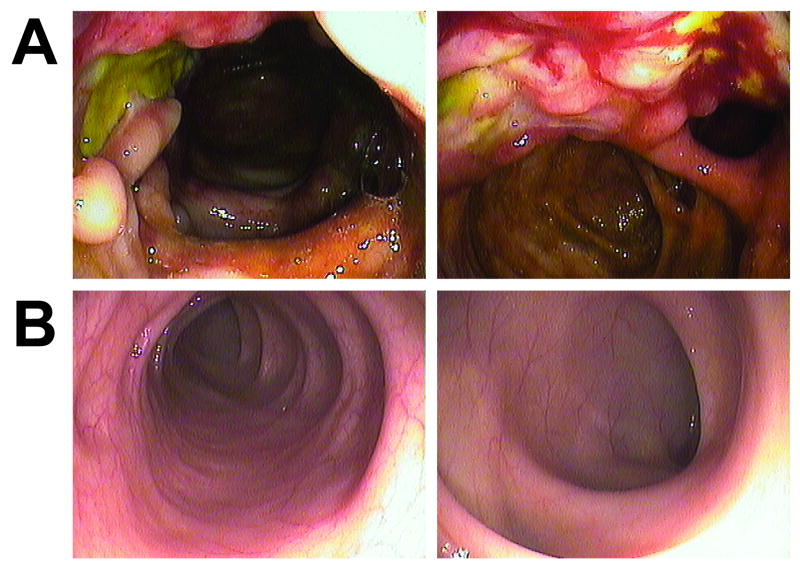
Patient 3 had a similar course though his bowel was never resected. He presented at 10 years of age with a two-week history of fever, anorexia, abdominal pain, and heme-positive stools. Colonoscopy revealed a focal area of exudate in the terminal ileum found to be secondary to C. difficile. After eradication of detectable C. difficile with a course of oral vancomycin and metronidazole, the abdominal pain persisted. Two months later, he exhibited worse symptoms and a 20% weight loss. Repeat colonoscopy revealed increasing areas of exudate, with new lesions involving the colon. Viral, mycobacterial, and fungal cultures were negative, but a tissue culture yielded C. perfringens. Despite a subsequent 21-day course of IV piperacillin/tazobactam and no further evidence of either C. perfringens or any other source of infection, the patient's condition worsened. As with patient 1, we initiated an empiric course of therapy to address an autoimmune cause. The patient was given budesonide (3 mg by mouth for 3 days, then increased to 6 mg once daily) and mesalamine (400 mg three times daily by mouth). Soon thereafter, the patient's pain decreased markedly and was able to tolerate a full oral diet. The patient's follow-up colonoscopy revealed normalization of the affected areas (Fig. 3B). The patient has since had a single flare requiring hospitalization, which was found to be secondary to medication non-compliance. Re-initiation of oral budesonide and mesalamine was sufficient to achieve remission. No organisms were found on tissue or stool cultures.
Figure 3.
Colonoscopy images from NEMO enterocolitis. A) Representative images from a study at Patient 3's initial presentation with abdominal symptoms, showing exudate (left frame) and mucosal inflammation (right frame). B) Two months after initiation of anti-inflammatory therapy, images were obtained from the same segment of bowel.
Endoscopic and histopathologic findings in NEMO enterocolitis
Patient 3 had separate colonoscopies at the onset of symptoms, after antibiotic therapy for C. difficile infection, and two months following initiation of glucocorticoid therapy. At presentation, endoscopy revealed severe colitis with exudate and marked mucosal bleeding (Fig. 3A). After eradication of C. difficile toxin, repeat colonoscopy revealed no improvement in the degree of mucosal damage and exudate (data not shown). However, following glucocorticoid therapy, repeat colonoscopy revealed normal colonic mucosa (Fig. 3B).
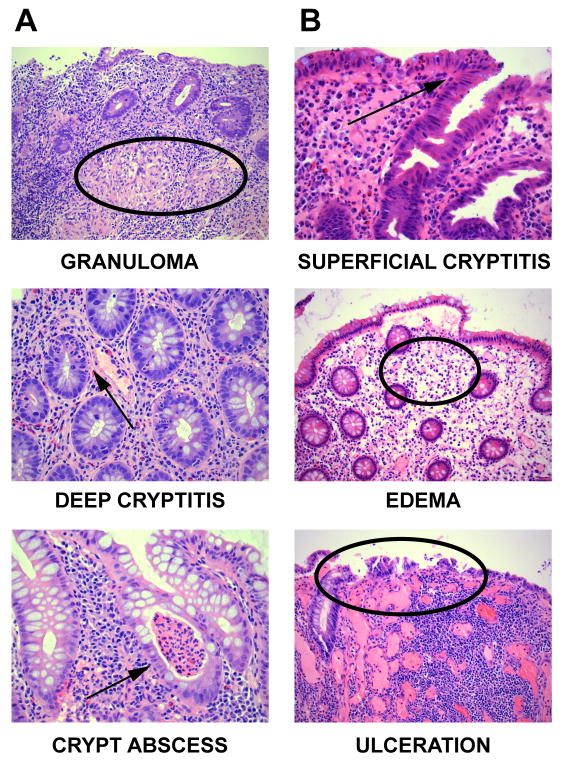
Since the clinical presentation and endoscopic appearance of NEMO enterocolitis were consistent with inflammatory bowel disease, biopsy specimens from patients 1 and 3 were examined by histopathology and compared with histopathologic slides of age- and gender-matched IBD patients. IBD patients had signs of chronic active enterocolitis, including granulomas, deep cryptitis, and abscesses with a predominance of lymphocytes present in the granulomas and crypts (Fig. 4A). By contrast, NEMO patient 3 did not manifest this pathology (Fig. 4B). Instead, patient 3 exhibited pathology more consistent with acute inflammation, including edema, superficial cryptitis, and mucosal ulceration. In addition, we observed an abundance of neutrophils within the lamina propria juxtaposed to the mucosal surface but not in the deeper anatomic region of the crypts. A relative paucity of lymphocytes also correlated with the absence of granulomas or deep cryptitis in the NEMO patients. Similar results were observed in biopsy specimens from patient 1 (data not shown).
Figure 4.
Comparison between NEMO colitis and typical colitis in IBD. A) Histopathology specimens from an IBD control. B) Histopathology from NEMO patient 3. Descriptors are noted below each image with corresponding arrows or circles.
Discussion
We show here that, in a kindred of 7 patients with NEMO syndrome, most manifest signs of persistent elevation of erythrocyte sedimentation rate in the absence of clinical symptoms. Two of the patients also developed organ-specific pathology (atypical enterocolitis), with durable resolution only after glucocorticoid treatment. These data suggest that specific NEMO mutations predispose to the development of serologic signs of systemic inflammation, with the potential to progress to autoimmune disease.
The erythrocyte sedimentation rate (ESR) can track disease severity in a number of conditions, including systemic lupus erythematosus and IBD [10; 11]. Interestingly, most members of this NEMO kindred had persistent elevation of the ESR, even in the absence of symptoms or signs of an inflammatory condition. Despite the absence of demonstrable infection during these processes, infectious agents may still play a critical role in precipitating organ-specific disease. For instance, the patients may have had repeated sub-clinical infections and/or sub-optimal responses to specific pathogens. Such processes may lead to organ-specific autoimmune disease, either from an inappropriate immune response or from low-level persistence of the pathogen [20]. Though CRP levels did not show a similar persistent elevation, similar discordance between CRP and ESR levels in systemic lupus erythematosus has been observed [21].
Our data contrast with previous reports of decreased signs and symptoms of inflammation in patients with NEMO mutations [6]. The most likely explanation is the varied phenotypes amongst the many NEMO mutations [22]. Interestingly, we care for an additional family with two boys possessing a novel F312L mutation. These patients have shown similar hyperinflammatory responses during acute infection, with one of two boys also showing periods of persistent elevation of ESR despite clinical well-being. Further genotype-phenotype correlations may help further identify predisposing mutations and perhaps lend biologic insights.
Two of the patients progressed to develop chronic, organ-specific (intestinal) symptomotology, and notably, an unrelated child with the NEMO syndrome has had a similar clinical picture [2]. The clinical course of our patients suggests that, while an infectious component may have contributed to or precipitated events, we did not achieve clinical resolution with antibiotic treatment even when we identified and eradicated the organism. Instead, sustained resolution of disease required glucocorticoids. Such a disease course is more consistent with IBD than with a chronic infectious process, despite the absence of classic histopathologic findings of IBD. The reason for this incongruity is unclear. In animal models of IBD, neutrophils are recruited in abundance during the acute inflammatory phase, leading ultimately to the recruitment of effector lymphocytes [23; 24]. It is also known that diminished NF-κB activity interferes with normal antigen presentation and T cell effector function [25]. Such alterations could impact effector T cell differentiation and function, which may in turn affect the ability of T cells to infiltrate the bowel wall and produce the classic histopathology seen in IBD.
The anti-inflammatory effects of glucocorticoids are in large part dependent on inhibition of NF-κB activity [27]. Thus, the effectiveness of glucocorticoid therapy in treating the bowel disease of NEMO patients is somewhat counterintuitive. Although the immune deficiency in NEMO syndrome patients is often significant, NEMO syndrome patients have residual NF-κB activity as complete NEMO mutations are embryonic lethal [1; 2]. Molecular characterization of hypomorphic NEMO alleles would further define this residual activity and the implications for immune dysregulation as well as the role of glucocorticoids in treatment of disease [26].
Thus far, we have not needed to consider medications beyond glucocorticoids as the mainstay of our therapeutic regimen. However, the atypical enterocolitis raises the question of whether NEMO patients might require medications not typically used in the treatment of chronic enterocolitis. In IBD, glucocorticoids are used primarily during acute presentation or flares, with other medications used to control chronic disease [28; 29]. These medications include 5-ASA derivatives, such as sulfasalazine, or nucleoside synthesis inhibitors, such as 6-mercaptopurine. More recently, biologic agents such as infliximab, which inhibits TNF-α activity, have been used with success. Given the pathologic findings in our NEMO patients and the preponderance of neutrophils associated with disease, such medications may be less effective. Targeting TNF-α activity represents an additional option, but the NEMO patients' underlying susceptibility to mycobacterial disease must be taken into account before such therapy is considered.
Numerous hypotheses might explain the ileitis and colitis observed in our two patients. First, the host response to bacteria likely plays a crucial role in the initiation of local inflammation, which can in turn lead to chronic ileocolitis despite clearance of the organism [24]. Patient 3 had C. difficile colitis at the onset of his symptoms. Antibiotic therapy was sufficient to eliminate fecal evidence of the organism, but the patient's symptoms continued to worsen. Thus, the pathogen may have initiated a host response which evolved into a chronic inflammatory state [20]. In particular, the newly appreciated role of NF-κB signaling in modulating NADPH oxidase activity might lead to delayed pathogen clearance and an altered host response to infection (even if the pathogen is ultimately eliminated) [30]. While pathogenic bacteria could certainly contribute to an aberrant intestinal inflammatory response, increasing evidence suggests that, in susceptible individuals, the host response may be driven primarily by commensal bacteria [31]. Specifically, diminished integrity of the epithelial gut barrier increases the inflammatory potential of commensal organisms, and animal models suggest a critical role for NEMO in maintaining the integrity of the epithelial barrier [32].
CD4+ T regulatory cells (TR) are also thought to play a role in maintaining immune homeostasis in the gastrointestinal tract. Selective loss of TR leads to severe inflammatory bowel disease in patients with the immune dysregulation, polyendocrinopahty, enteropathy, and X-linked syndrome (IPEX) [33]. While TR function in NEMO patients has not yet been examined, mice with T cells lacking NEMO exhibit impaired formation of TR cells [8]. Further investigation into TR numbers and function in NEMO patients may illuminate the specific role of TR cells in gastrointestinal disease.
Lastly, though NF-κB activity is traditionally associated with enhanced immune responses, recent studies suggest NF-κB may also contribute to downmodulation of the immune response. In particular, interleukin-1 beta processing and secretion appear to be negatively regulated by NF-κB signaling [34], and interference with IKK activity via pharmacologic inhibition or genetic deletion of IKKβ leads to hypersusceptibility to endotoxin mediated shock. It is plausible that diminished IKK activity secondary to defective NEMO function could have a similar effect.
Given the large numbers and distinct phenotypes of immune cells affected in the NEMO syndrome, these mechanisms are not mutually exclusive and multiple processes may be involved. Further investigation into the function of immune cells in NEMO syndrome patients and the phenotypic variations amongst the myriad NEMO mutations will likely provide insight to the cause of the aberrant immune responses seen in these individuals.
Supplementary Material
Acknowledgments
We would like to thank Jeff Mold for assistance in preparation of histopathologic specimens, and Michael Pickens for gathering endoscopic images. We thank Ashish Jain and colleagues for genotype analysis. We would also like to thank Emily von Scheven for helpful discussions. This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Laurence E. Cheng was supported by a NIH training grant (T32 HD044331) and an AAAAI career development award. Bittoo Kanwar was supported by a NIH training grant (T32 DK007762). Haig Tcheurekdjian was supported through a Glaser Pediatric network grant. Joseph M. McCune is the recipient of the NIH Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant number DPI OD00329. Melvin B. Heyman received support from the NIH (K24 DK060617). All of the authors declare no relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Puel A, Picard C, Ku CL, Smahi A, Casanova JL. Inherited disorders of NF-kappaB-mediated immunity in man. Curr Opin Immunol. 2004;16:34–41. doi: 10.1016/j.coi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Orange JS, Levy O, Geha RS. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol Rev. 2005;203:21–37. doi: 10.1111/j.0105-2896.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 3.Ku CL, Yang K, Bustamante J, Puel A, von Bernuth H, Santos OF, Lawrence T, Chang HH, Al-Mousa H, Picard C, Casanova JL. Inherited disorders of human Toll-like receptor signaling: immunological implications. Immunol Rev. 2005;203:10–20. doi: 10.1111/j.0105-2896.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 4.Yang F, Yamashita J, Tang E, Wang HL, Guan K, Wang CY. The zinc finger mutation C417R of I-kappa B kinase gamma impairs lipopolysaccharide- and TNF-mediated NF-kappa B activation through inhibiting phosphorylation of the I-kappa B kinase beta activation loop. J Immunol. 2004;172:2446–52. doi: 10.4049/jimmunol.172.4.2446. [DOI] [PubMed] [Google Scholar]
- 5.Jain A, Ma CA, Lopez-Granados E, Means G, Brady W, Orange JS, Liu S, Holland S, Derry JM. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004;114:1593–602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bernuth H, Puel A, Ku CL, Yang K, Bustamante J, Chang HH, Picard C, Casanova JL. Septicemia without sepsis: inherited disorders of nuclear factor-kappa B-mediated inflammation. Clin Infect Dis. 2005;41 7:S436–9. doi: 10.1086/431994. [DOI] [PubMed] [Google Scholar]
- 7.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–21. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–89. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–71. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachar DB, Luppescu NE, Bodian C, Shlien RD, Fabry TL, Gumaste VV. Erythrocyte sedimentation as a measure of Crohn's disease activity: opposite trends in ileitis versus colitis. J Clin Gastroenterol. 1990;12:643–6. doi: 10.1097/00004836-199012000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Vila LM, Alarcon GS, McGwin G, Jr, Bastian HM, Fessler BJ, Reveille JD. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXIX. Elevation of erythrocyte sedimentation rate is associated with disease activity and damage accrual. J Rheumatol. 2005;32:2150–5. [PubMed] [Google Scholar]
- 12.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, Shapira SK, Farndon PA, Wara DW, Emmal SA, Ferguson BM. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–62. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makris C, Roberts JL, Karin M. The carboxyl-terminal region of IkappaB kinase gamma (IKKgamma) is required for full IKK activation. Mol Cell Biol. 2002;22:6573–81. doi: 10.1128/MCB.22.18.6573-6581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV, Jr, Pena AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 A:5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 15.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113:725–33. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 17.Tatara R, Imai H. Serum C-reactive protein in the differential diagnosis of childhood meningitis. Pediatr Int. 2000;42:541–6. doi: 10.1046/j.1442-200x.2000.01285.x. [DOI] [PubMed] [Google Scholar]
- 18.Korppi M, Heiskanen-Kosma T, Leinonen M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur Respir J. 1997;10:1125–9. doi: 10.1183/09031936.97.10051125. [DOI] [PubMed] [Google Scholar]
- 19.Robertson J, Shilkofski N, Johns Hopkins Hospital . The Harriet Lane handbook : a manual for pediatric house officers. Elsevier Mosby; Philadelphia, Pa: 2005. Children's Medical and Surgical Center. [Google Scholar]
- 20.Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–702. doi: 10.1182/blood.v99.8.2694. [DOI] [PubMed] [Google Scholar]
- 21.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 22.Uzel G. The range of defects associated with nuclear factor kappaB essential modulator. Curr Opin Allergy Clin Immunol. 2005;5:513–8. doi: 10.1097/01.all.0000191241.66373.74. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, Imaoka A, Okada Y, Umesaki Y. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 26.Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, Orange JS. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122:1169–1177 e16. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007;275:79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 29.Bayless TM. Maintenance therapy for Crohn's disease. Gastroenterology. 1996;110:299–302. doi: 10.1053/gast.1996.v110.agast960299. [DOI] [PubMed] [Google Scholar]
- 30.Luengo-Blanco M, Prando C, Bustamante J, Aragao-Filho WC, Pereira PV, Rehder J, Padden C, Casanova JL, Newburger PE, Condino-Neto A. Essential role of nuclear factor-kappaB for NADPH oxidase activity in normal and anhidrotic ectodermal dysplasia leukocytes. Blood. 2008;112:1453–60. doi: 10.1182/blood-2007-07-099267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 32.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–61. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 33.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 34.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O'Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-kappaB Is a Negative Regulator of IL-1beta Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKbeta. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.