Abstract
Maternal mental health and prenatal stress are linked with neurobehavioral differences in the offspring. The majority of studies documenting this effect have been conducted using either predominantly European American infants or minority infants exposed to teratogens in utero. In this study, we focus on healthy African American women from low-income environments to investigate the earliest individual differences in biobehavioral regulation, including resting heart rate and variability (HRV). In 87 neonates, HRV was significantly lower in those born to mothers reporting past major depressive disorder (p = .01). The number of maternal life stressors also was associated with lower neonatal HRV (p = .03). Obstetrical complications were not associated with significant differences, but breast- versus bottle-feeding in the first few days of life was related to higher HRV (p = .04). Early variation in physiological regulation may be linked to subsequent individual differences in response to stress. Thus, identifying the earliest point in development when such differences can be reliably measured may result in opportunities for prevention of later deficits in regulating response to stress.
Introduction
There is growing evidence that the prenatal environment influences early brain development, with much of this research focused on the context of maternal exposure to stress during pregnancy (Huiznick, Mulder, & Buitelaar, 2004; Maccari et al., 2003; Wadhwa, 2004). Moderate to severe life events during pregnancy are associated with obstetric complications including preterm delivery and low birth weight (Lou et al., 1994; Wadhwa, Sandman, Porto, Dunkel-Schetter, & Garite, 1993). Over a dozen independent studies have linked prenatal maternal stress to differences in later child development, even when controlling for confounders such as postnatal mood (O'Connor et al., 2005; Van den Bergh, Mulder, Mennes, & Glover, 2005).
A smaller literature has demonstrated an association between maternal depression and lower cardiac variability in the neonate (Field et al., 2004). Other data have suggested that maternal depression during pregnancy rather than during the postpartum period is associated with negative affect and temperament differences in infants 6 months or older (Hout, Brennan, Stowe, Plotsky, & Walker, 2004). It is estimated that 10 to 20% of all pregnant women meet criteria for major depression at some point during the pregnancy (Gotlib, Whiffen, Mount, Milne, & Cordy, 1989; Thoppil, Riutcel, & Nalesnik, 2005), and that these rates are increased twofold among women who are impoverished or live in the inner city (Hobfoll, Ritter, Lavin, Hulsizer, & Cameron, 1995). Women with a past history for depression may be at greater risk for ongoing or recurring symptoms at various stages of the reproductive cycle.
The studies thus far, however, have mostly included women from a middle socioeconomic status who were predominantly European American or Hispanic. There are potential racial and ethnic differences in reproductive physiology, fetal reactivity, and maternal psychological functioning. For example, African American women have higher adrenocorticotropic hormone and beta-endorphin levels, but lower cortisol and placental CRH levels, than do European American and Hispanic women (Wadhwa, Glynn, Sandman, Chicz-DeMet, & Hobel, 2002; Weaver, Diorio, Seckl, Szyf, & Meaney, 2004). African American fetuses spend more time in quiet sleep than do European American fetuses (Paine, Strobino, Witter, & Johnson, 1991). In samples with greater representation of African American and Hispanic women, higher rates of depression during or after pregnancy have been reported (Yonkers et al., 2001; Zayas, Cunningham, McKee, & Jankowski, 2002). Given these significant racial and ethnic differences, studies of individual differences need to be conducted either within ethnic or racial groups or with large-enough samples sizes to test for individual differences across ethnic and racial groups.
This study was designed to investigate neonatal differences of biobehavioral regulation to explore factors that may influence very early differences in neuropsychological development. For our physiological measure of self-regulation, we chose heart rate variability (HRV) because it is a noninvasive measure associated with the autonomic nervous system and later development of behavior and emotion problems. Individual differences of HRV are parameters of parasympathetic nervous system functioning that have been related to a child's developing ability to control emotion, attention, and behavior (Calkins & Dedmon, 2000; DeGangi, DiPietro, Greenspan, & Porges, 1991; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996) and have been shown to be stable from the fetal period through the first year of life (DiPietro, Costigan, Pressman, & Doussard-Roosevelt, 2000). HRV is measured fluctuation of adjacent R-R cardiac wave-length intervals. HRV can be measured with short-term recordings lasting between 5 to 20 min to examine the high-frequency range that is generally reported (Longin, Schaible, Lenz, & Konig, 2005; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996).
In the context of conducting a prospective, longitudinal study of infant development, we recruited African American mothers living in stressful socioeconomic conditions. Minority populations are often understudied and vulnerable to developing behavioral and emotional problems (Adams, Hillman, & Gaydos, 1994; Dohrenwend et al., 1992). To our knowledge, in no other study has an association between infant physiology and maternal psychological factors and perinatal experiences been tested within a sample of healthy African American newborns whose mothers reside in inner city, low-income environments. Since increased HRV is related to resilience and healthy states, we hypothesized that neonates born to mothers who have experienced more life stressors or who have been diagnosed with major depressive disorder would have lower resting HRV. Similarly, neonates born to mothers with more obstetrical complications would have lower resting HRV. Because type of feeding can affect cardiac patterns (Butte, Smith, & Garza, 1991; DiPietro, Larson, & Porges, 1987), we also expected lower resting HRV in bottle-fed infants.
Method
Participants
Participants were full-term neonates and their mothers who were recruited for a longitudinal study from the General Care Nursery at the University of Chicago. The nursery predominantly serves African American families with incomes below the poverty level. Inclusion criteria included healthy women and their newborns who did not require admission to Intensive Care Units immediately following delivery and who had no obvious congenital or chromosomal abnormalities. A total of 155 mothers and newborns met inclusion criteria, and 113 (73%) agreed to participate in a study requiring visits to the laboratory over the next 2 years to assess early child development. Thirteen (8%) of those who agreed to participate were not able to complete the neonatal assessment due to difficulties with data acquisition over the short course of the labor and delivery hospital stay. Ten of the remaining participants did not have cardiac data due to technical issues or extensive missing cardiac data. Three neonates had heart rate and variability values that were identified as extreme outliers (>3 SD above the mean). Examining their individual data did not show consistent patterns, so they were excluded from all further analyses.
All of the African American mothers were receiving some form of public assistance, and demographic details are provided in Table 1. One third of the births were first births. Newborns with obvious medical disorders or who had been exposed to illegal substances in utero by report and urine toxicology were excluded from the study. The women identified themselves as healthy and were screened for a history of taking medications, for chronic illness or infection, and for use of alcohol, nicotine, and illicit substances.
TABLE 1. Descriptive Statistics for the Study Sample.
| Neonatal characteristics | Mean (SD) | N | % |
| Gestational age (weeks) | 39 (1.3) | ||
| Apgar (5 minute) | 9 (0.2) | ||
| Birth weight (grams) | 3213 (4.2) | ||
| Females | 48 | 55 | |
| Circumcised males | 32 | 37 | |
| Uncircumcised males | 7 | 8 | |
| Perinatal and obstetrical factors | Mean (SD) | N | % |
| ROS Total Score | 3.17 (1.8) | ||
| ROS-Delivery Subscale | 2.38 (1.4) | ||
| Caesarean delivery | 19 | 22 | |
| Formula fed | 74 | 85 | |
| Maternal psychological factors | Mean (SD) | N | % |
| Maternal Life Stressors (DLC score) | 2.7 (1.7) | ||
| Past Major Depressive Disorder | 14 | 16 | |
| Current Major Depressive Disorder | 5 | 6 | |
| Maternal demographic summary | Mean (SD) | N | % |
| Maternal age | 23 (4.7) | ||
| Income per month (dollars) | 626 (645) | ||
| High-school completion | 62 | 71 | |
| Living with a partner | 65 | 74 | |
| Regular prenatal care | 80 | 92 |
Methods of delivery were comparable to the general population of this tertiary-care University hospital: 78% vaginal deliveries and 22% Caesarian sections. The newborns were full term, with a mean gestation of 39 weeks (by estimated date of conception), and of average weight (M = 3,213 g). The 5-min Apgar scores ranged from 8 to 10 (M = 9). Approximately half of the sample was male, and all but 7 of the males were circumcised at the time of data collection. Circumcisions were typically performed early in the morning without anesthesia. The circumcised males had their cardiac measures collected, on average, 10 hr (SD = 0.4) after the procedure. Institutional Review Board approval for this study was obtained through the University of Chicago Hospitals.
Procedures
Neonate Characteristics
Gestational age, sex, and birth weight were gathered from the medical record.
Maternal Characteristics
Sociodemographic questions were asked of mothers in a background interview during the hospitalization. The Difficult Life Circumstances Scale (DLC; Barnard, Johnson, Booth, & Bee, 1989) was used to assess maternal chronic life stressors during pregnancy. It was developed for use with mothers living in inner city poverty and has a 1-year test-retest correlation of 0.70 (Barnard et al., 1989). Twenty-eight questions assess stressful circumstances such as finding an affordable place to live, having problems with a former partner, or having a bad credit rating. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, Spitzer, Gibbon, & Williams, 1995) was administered to assess current and lifetime depression by either a bachelor-level research assistant or one of the coauthors (K.K.). Interrater reliability was calculated on 20% of the sample and was high (κ = 1.0 for diagnoses and ICC = .76 for symptoms).
Perinatal Experiences
The Rochester Research Obstetrical Scale (ROS; Sameroff, Seifer, & Zax, 1982) was used to measure delivery and postpartum complications (Molfese & Thomson, 1985). The ROS contains 37 items of potential prenatal, delivery, and postnatal complications, and the medical record was used to obtain this information. Breast- versus formula-feeding from a bottle was recorded since type of feeding can affect cardiac patterns (Butte et al., 1991; DiPietro et al., 1987).
Cardiac Data Acquisition and Quantification
Neonates were over 1 day old and in the hospital when the cardiac data were collected. Because state may influence heart rate, data were collected while newborns were sleeping and/or resting quietly with eyes closed. More detailed analyses of effects of sleep state cannot be tested since video, eye movements, and/or EEG monitoring were not simultaneously obtained. The protocol goal was to obtain at least 5 min of resting cardiac data similar to other postnatal collection methods (Longin et al., 2005). The average length of electrocardiography (ECG) recorded per participant was 8.01 min (SD = 1.0). Both heart rate and variability data were collected.
Two cardiac electrodes were placed axially on the left (+) and right (−) rib cage, approximately 10 to 15 cm below the armpits. The third electrode was placed on the sternum (ground). The bioamplifier was set for band-pass filtering with half-power cutoff frequencies of 0.1 and 1000 Hz. The signal was amplified with a gain of 250. Data were digitized at a sampling rate of 1000 Hz with a 12-bit A–D board connected to a laptop computer.
The physiology data reduction software identified R-waves in the ECG using an automated, multiple-pass, self-scaling algorithm (EKG data analysis program, Caroga Lake, NY). The first second of all data collected was excluded due to potential variability in starting at different phases of the heart period or measured wave form. A graphical program enabled visual inspection and editing of missed or misidentified R-waves. The R-wave times were converted to interbeat intervals (IBIs; or interval from one R wave to the next) and prorated into equal time intervals of 125 ms. IBIs that were suspiciously long or short were identified and examined along with any periods of artifact. Artifact was edited only after consensus by at least two of the investigators. In addition, all data were manually inspected, and the majority of the sample had no atypical IBIs over the full length of their data.
Prorated IBIs were detrended using a high-pass filter with a period of 10 s (∼0.1 Hz) to minimize variability from aperiodic shifts in the tonic heart period. Fourier analyses were then applied to the residual IBI data for each baseline epoch. The focus was on the frequency band of 0.24 to 1.04 Hz, similar to the frequency band examined in a recent study of healthy neonates (Longin et al., 2005). Heart period variability due to respiratory sinus arrhythmia was computed with the spectral method. The resulting spectral power was in the units of mean square seconds, and data were transformed to natural logarithms to normalize the distribution for statistical analyses. HRV data are reported as peak-to-peak amplitude measurements in the following results section. Calculations are based on the height of a sine wave (2√2) times the root mean square amplitude of the sine wave.
Analytic Approach
ANOVA was used to compare continuous data for mutually exclusive groups, such as differences in HRV between circumcision versus none, neonates who were breast- versus bottle-fed, and between offspring of mothers with and without a history of depression. Associations between HRV and nonnormally distributed measures (e.g., difficult life circumstances) were tested via Kendall's τ b correlation coefficient. Multivariate models of the effects of perinatal experiences on HRV were tested using regression analysis.
Results
Descriptive Statistics for HRV
Among the final sample of 87 neonates, the average heart rate was 128.4 bpm (SD = 10.2), and the average HRV was 17.7 ms (SD = 9.9) peak-to-peak amplitude or a mean 6.26 ms root mean square amplitude (see Table 2). Descriptive plots showed heart-rate differences between males who were circumcised (n = 32) and those who were not (n = 7) at the time of data collection. Because sex and circumcision overlap, sex differences were explored in the context of circumcision and by comparing cardiac variables for females (n = 48) to circumcised (n = 32) and uncircumcised (n = 7) males. Females had an average heart rate of 131.5 bps (SD = 10.2) and HRV of 15.6 (SD = 9.4) peak-to-peak amplitude, circumcised males had 124.6 bps (SD = 9.2) and HRV of 21.2 (SD = 10.6), and uncircumcised males had 125.1 bps (SD = 8.7) and HRV of 15.8 (SD = 6.9). Overall, heart rate, F(2, 85) = 5.15, p = .01, and HRV, F(2, 85) = 3.39, p = .04, were significantly different for these three groups. Scheffe's post hoc tests indicated a significant difference between females and circumcised males for heart rate (p < .01) and HRV (p < .04).
TABLE 2. Descriptive Statistics for Cardiac Variables.
| Heart rate (bpm) | ||
|---|---|---|
| M (SD) | n | |
| Total sample | 128.4 (10.2) | 87 |
| Females | 131.5 (10.2) | 48 |
| Circumcised males | 124.6 (09.2) | 32 |
| Uncircumcised males | 125.1 (08.7) | 7 |
| Heart rate variability (peak-to-peak amplitude in ms) | ||
| M (SD) | n | |
| Total sample | 17.7 (09.9) | 87 |
| Females | 15.6 (09.4) | 48 |
| Circumcised males | 21.2 (10.6) | 32 |
| Uncircumcised males | 15.8 (06.9) | 7 |
Associations Between HRV and Neonatal Characteristics
None of the examined newborn characteristics, with the exception of sex and circumcision as stated earlier, were significantly associated with differences in heart rate or HRV. We tested whether circumcision/sex was associated with other newborn or maternal characteristics listed in Table 1, such as maternal depression, stressors, or feeding method; it was not.
Associations Between HRV and Perinatal Factors
The Total Obstetrical Scale, which includes prenatal, developmental risk factors, and methods of delivery, was not associated with heart rate or HRV (p ≥ 0.2). The Delivery subscale, including delivery methods and complications, did not significantly correlate with heart rate or HRV (p ≥ 0.4). More specifically, the delivery method of vaginal versus Caesarian was not significantly associated with cardiac reactivity in this study of healthy infants.
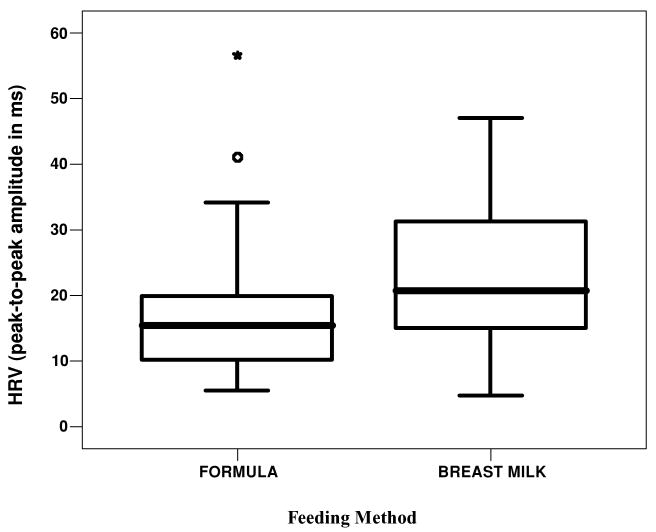
Feeding method was recorded based on maternal report in the hospital. Eighty-five percent of the neonates (n = 74) were bottle-fed in this sample. There were no differences in heart rate between breast- and bottle-fed neonates; however, breast-fed neonates (n = 13) had a significantly higher HRV than did those who were bottle-fed, F(2, 85) = 4.39, p = .04 (see Figure 1).
FIGURE 1.
Boxplots of heart rate variability (HRV) of neonates who were breast- or bottle-fed.
Associations Between HRV and Maternal Psychological Factors
Maternal stressors, as reported on the DLC, were relatively common in this sample: Ninety percent of the women reported at least one life stressor. The most commonly reported stressors included problems with credit rating (35%), someone in the house with a long-term illness (30%), a partner being away from home more than half the time (25%), and looking for a job and cannot find one (23%). The number of maternal stressors was associated with neonatal HRV (r = −.21, p = .03).
Based on the data generated from the diagnostic clinical interview, 22% of the women reported one or more current depressive symptom, and 31% reported one or more past depressive symptoms. The number of past depressive symptoms, rather than current depressive symptoms, was associated with neonatal cardiac variables (heart rate: Kendall's τ b = −.17, p = .02; HRV: −.17, p = .02). The specific symptoms reported by the subset of women who reported depressed mood or anhedonia in the past included sleep problems (73%), weight loss (54%), feelings of worthlessness or guilt (50%), fatigue (46%), psychomotor agitation/retardation (35%), indecision or diminished ability to think (31%), and recurrent thoughts of death (23%).
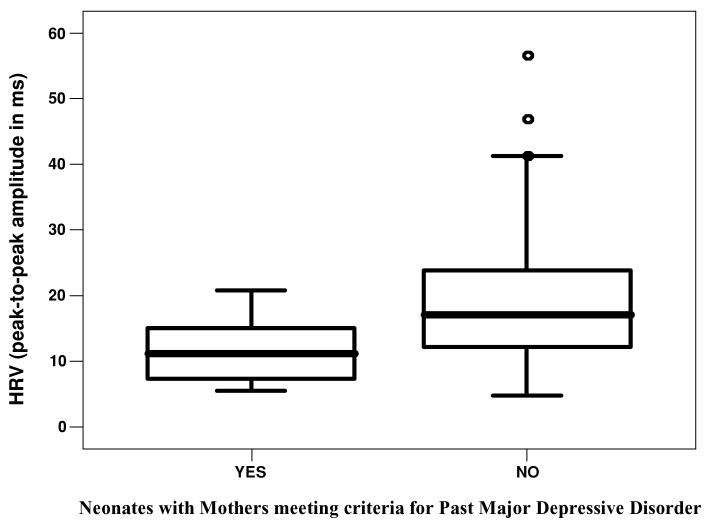
A total of 14 women met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) criteria for past major depressive disorder (see Figure 2). HRV was significantly lower among those neonates born to mothers with a past history of major depressive disorder than those born to mothers without past history, F(2, 83) = 6.39, p = .01. Only 2 women fulfilled criteria for current major depressive disorder, but not past major depressive disorder. A linear regression was computed to evaluate the amount of variance in individual differences of neonatal HRV accounted for by a DSM-IV diagnosis of past maternal depression, maternal life stressors, feeding method, and circumcision. The linear combination of these measures was significantly related to HRV (p = .001). Past maternal depression (t = −2.2, p = .03), feeding method (t = 2.5, p = .02), and circumcision (t = −2.1, p = .04) all demonstrated significant predictive associations with HRV. The sample multiple correlation coefficient was 0.5, indicating that approximately 18% of the variance of HRV in the sample was accounted for by the linear combination of these three variables.
FIGURE 2.
Boxplots of heart rate variability (HRV) of neonates born to mothers with and without a past history of major depressive disorder.
Discussion
In the present study, neonates demonstrated physiological differences that were not only correlated with early life experience such as feeding and circumcision but also were associated with measures of maternal mental health history. Neonates born to mothers with a history of depression and maternal life stressors were associated with lower neonatal HRV, a measure of physiological regulation and potential resilience to stress. In this sample of healthy African American neonates, obstetrical experiences were not associated with significant differences in HRV. Although there was a low rate of breast-feeding and a high rate of circumcision in this urban sample, we replicated previous studies that have shown that breast- versus bottle-feeding results in higher HRV (DiPietro et al., 1987) and that circumcision results in lower heart rate (Davis & Emory, 1995). In the final regression analysis, past maternal depression history, feeding method, and circumcision contributed to unique variance in neonatal HRV.
Our most striking finding, although modest due to sample size, was decreased HRV in neonates born to mothers who fulfilled the criteria for past major depression. This parallels results found by another research group who reported association between depressive symptoms during pregnancy and neonatal HRV in a sample of predominantly Hispanic women (Field et al., 2004). These results suggest that maternal psychological functioning during pregnancy affects the developing autonomic nervous system. A few studies on fetal reactivity have provided further support for this hypothesis. For example, Allister, Lester, Carr, and Liu (2001) demonstrated that fetuses of untreated, depressed mothers show elevated resting heart rates when compared to fetuses of women without depression. Other data have demonstrated that during maternal cognitive testing, fetuses of depressed women show greater increases in heart rate than do fetuses of women with anxiety disorders (Monk et al., 2004), suggesting that there may be some specificity of effects of depressed mood, or perhaps depressed mood in the contexts of other psychosocial stressors. Further hypothesis testing needs to be conducted in samples with a sufficient number of different psychological profiles (e.g., depression, anxiety, and comorbid depression and anxiety) and in low- as well as high-stress psychosocial contexts to better specify the key components of this effect.
The results of the present study are limited for several reasons. First, women were assessed during their hospitalization for labor and delivery. The perinatal period may not be the ideal time to assess maternal mental health because of the potential impact (positive or negative) of delivery on women's psychological state. Our results may be influenced by recall bias during the perinatal period. Second, current or past anxiety disorders were not assessed. These and other comorbid psychiatric disorders also may influence neonatal HRV. Moreover, it would be better to examine the influence of maternal mental health on neonatal HRV with a sample having similar postnatal conditions. This population had a high, but variable, rate of formula-feeding and circumcision. Due to the challenges of recruiting participants and collecting all the data within a short hospitalization, the cardiac measures were not ascertained at ideal times for males (e.g., before circumcision) nor was it within the scope of this study to have additional measures of infant state (video, eye movements, and/or EEG monitoring).
Clinical Implications
Few studies have examined associations of HRV and pre-, peri-, and postnatal factors in the same group of neonates. To our knowledge, this is the first study to examine these relationships in a sample of healthy neonates born to African American women. Given its high incidence, depression screening should become a regular part of prenatal care because it may impact infant physiological state and outcome. This is especially important in minority populations with socioeconomic challenges that increase risk for depression. Treatment of maternal depression with therapy or medications may alter fetal or neonatal heart rate or HRV. Further longitudinal research needs to be conducted because HRV is a measurable physiological variable that may be associated with infant behavioral outcomes.
Given its potential as an early intervention, breast-feeding may be particularly important for infants of women with a history of maternal depression. In a group of predominantly European American mothers with depression that was measured at 1 and 3 months' postpartum, infants who had stable breast-feeding patterns were less likely to have reactive temperaments and or show electrocortical asymmetries associated with negative emotional states (Jones, McFall, & Diego, 2004). Breast-fed infants may have increased HRV due to greater maternal contact (Dunn & Richards, 1977), differences of substances in colostrum, and/or the level of neonate hunger or arousal during the first few days of milk production. Future studies need to be done because our sample is too small to examine HRV and interactions between maternal mental health history and feeding method. Cardiac measures may be a useful way to monitor interventions that have a goal of promoting maternal and infant mental health, especially in high-risk populations.
Contributor Information
Suma Jacob, University of Illinois at Chicago.
Michelle Byrne, University of Chicago.
Kate Keenan, University of Chicago.
References
- Adams CD, Hillman N, Gaydos GR. Behavioral difficulties in toddlers: Impact of sociocultural and biological risk factors. Journal of Clinical Child Psychology. 1994;23(4):373–381. [Google Scholar]
- Allister L, Lester BM, Carr S, Liu J. The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation. Developmental Neuropsychology. 2001;20(3):639–651. doi: 10.1207/S15326942DN2003_6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Barnard KE, Johnson S, Booth CL, Bee H. Difficult life circumstances. Seattle, WA: NCAST; 1989. [Google Scholar]
- Butte NF, Smith EO, Garza C. Heart rates of breast-fed and formula-fed infants. Journal of Pediatric Gastroenterology & Nutrition. 1991;13(4):391–396. doi: 10.1097/00005176-199111000-00009. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28(2):103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Davis M, Emory E. Sex differences in neonatal stress reactivity. Child Development. 1995;66(1):14–27. doi: 10.1111/j.1467-8624.1995.tb00852.x. [DOI] [PubMed] [Google Scholar]
- DeGangi GA, DiPietro JA, Greenspan SI, Porges SW. Psychophysiological characteristics of the regulatory disordered infant. Infant Behavior and Development. 1991;14:37–50. [Google Scholar]
- DiPietro JA, Costigan KA, Pressman EK, Doussard-Roosevelt JA. Antenatal origins of individual differences in heart rate. Developmental Psychobiology. 2000;37(4):221–228. doi: 10.1002/1098-2302(2000)37:4<221::aid-dev2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Larson SK, Porges SW. Behavioral and heart rate pattern differences between breast-fed and bottle-fed neonates. Developmental Psychology. 1987;23:467–474. [Google Scholar]
- Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, et al. Socioeconomic status and psychiatric disorders: The causation-selection issue. Science. 1992;255:946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- Dunn JB, Richards MP. Observations on the developing relationship between mother and baby in the neonatal period. In: Schaffer HR, editor. Studies in mother–infant interaction. New York: Academic Press; 1977. pp. 427–455. [Google Scholar]
- Field T, Diego MA, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, et al. Prenatal depression effects on the fetus and the newborn. Infant Behavior & Development. 2004;27(2):216–229. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. Journal of Consulting and Clinical Psychology. 1989;57(2):269–274. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE, Ritter C, Lavin J, Hulsizer MR, Cameron RP. Depression prevalence and incidence among inner-city pregnant and postpartum women. Journal of Consulting and Clinical Psychology. 1995;63(3):445–453. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- Hout RL, Brennan P, Stowe ZN, Plotsky PM, Walker EF. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy but not postpartum. Annals of the New York Academy of Sciences. 2004;1032:234–236. doi: 10.1196/annals.1314.028. [DOI] [PubMed] [Google Scholar]
- Huiznick AC, Mulder EJH, Buitelaar JK. Prenatal stress and risk for psychology: Specific effects or induction of general susceptibility? Psychological Bulletin. 2004;130(1):115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- Jones AJ, McFall BA, Diego MG. Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: The mediating role of infant temperament. Biological Psychology. 2004;67:103–124. doi: 10.1016/j.biopsycho.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Longin E, Schaible T, Lenz T, Konig S. Short term heart rate variability in healthy neonates: Normative data and physiological observations. Early Human Development. 2005;81(8):663–671. doi: 10.1016/j.earlhumdev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lou HC, Hansen D, Nordentoft M, Pryds O, Jensen F, Nim J, et al. Prenatal stressors of human life affect fetal brain development. Developmental Medicine and Child Neurology. 1994;36(9):826–832. doi: 10.1111/j.1469-8749.1994.tb08192.x. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: Implications of glucocorticoid hormones. Neuroscience & Biobehavioral Reviews. 2003;27(1–2):119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Molfese VJ, Thomson B. Optimality versus complications: Assessing predictive values of perinatal scales. Child Development. 1985;56:810–823. [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, et al. Fetal heart rate reactivity differs by women's psychiatric status: An early marker for developmental risk? Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(3):283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry. 2005;58(3):211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Paine L, Strobino D, Witter F, Johnson T. Population differences affect nonstress test reactivity. Journal of Perinatology. 1991;11:41–45. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29(8):697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sameroff A, Seifer R, Zax M. Early development of children at risk for emotional disorder. Monographs of the Society for Research in Child Development. 1982;47(7 Serial No 199) [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thoppil J, Riutcel TL, Nalesnik SW. Early intervention for perinatal depression. American Journal of Obstetrics & Gynecology. 2005;192(5):1446–1448. doi: 10.1016/j.ajog.2004.12.073. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. Neuroscience & Biobehavioral Reviews. 2005;29(2):237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2004;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Glynn L, Sandman CA, Chicz-DeMet A, Hobel C. Racial/ethnic differences in maternal–placental stress physiology over the course of gestation. Journal of the Society for Gynecologic Investigation. 2002;19:198A. [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective study. American Journal of Obstetrics & Gynecology. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Annals of the New York Academy of Sciences. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Ramin SM, Rush AJ, Navarrete CA, Carmody T, March D, et al. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. American Journal of Psychiatry. 2001;158(11):1856–1863. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- Zayas LH, Cunningham J, McKee MD, Jankowski KRB. Depression and negative life events among pregnant African-American and Hispanic women. Women's Health Issues. 2002;12:16–22. doi: 10.1016/s1049-3867(01)00138-4. [DOI] [PubMed] [Google Scholar]