Abstract
Background
An increasing percentage of childhood cancer patients are surviving their disease, but there is limited research on late recurrence. We sought to estimate late recurrence rates for the most common pediatric cancers and to determine risk factors for late recurrence.
Methods
The incidence of late recurrences, or first recurrences that occurred more than 5 years after diagnosis, was analyzed for the most common pediatric cancers using data from the Childhood Cancer Survivor Study, a retrospective cohort of 5-year survivors of childhood and adolescent cancers who were diagnosed between 1970 and 1986. A total of 12 795 survivors with no history of recurrence within 5 years after their original cancer diagnosis were included in the analysis, with a total of 217 127 person-years of follow-up. Cumulative incidence of late recurrence at 5, 10, 15, and 20 years after diagnosis was calculated using death as a competing risk. Adjusted relative rates of late recurrence were obtained using multivariable Poisson regression. All statistical tests were two-sided.
Results
Overall, 5-year survivors of pediatric cancers experienced a cumulative incidence of recurrent disease of 4.4%, 5.6%, and 6.2% at 10, 15, and 20 years, respectively. Cumulative incidence varied by diagnosis: Survivors of Ewing sarcoma and astrocytoma had the highest 20-year cumulative incidences at 13.0% (95% confidence interval [CI] = 9.4 to 16.5) and 14.4% (95% CI = 12.3 to 16.6), respectively. In multivariable analysis, the greatest risk factors for late recurrence included diagnosis, combination treatment with chemotherapy and radiation, earlier treatment era, and fewer years since diagnosis (P < .001 for all).
Conclusion
Late recurrence is a risk for some pediatric cancers. By understanding diagnosis-specific risks, patients, families, and their medical providers can be better informed of the probability of cure.
CONTEXT AND CAVEATS
Prior knowledge
Pediatric patients are increasingly surviving cancer, but more information was needed about incidence and risk factors for late recurrence.
Study design
Using data from the Childhood Cancer Study for 12 795 five-year survivors of the most common pediatric cancers, cumulative incidence of late recurrence at 5, 10, 15, and 20 years after diagnosis was calculated using death as a competing risk.
Contribution
Overall, 4.4% of pediatric cancers recurred by 10 years and 6.2% by 20 years after diagnosis. However, both Ewing sarcoma and astrocytoma recurred in more than 13% of cancer survivors. Earlier treatment era and combination treatment with radiation and chemotherapy were associated with increased risk of late recurrence.
Implications
These data help identify which cancer survivors are at greatest risk for recurrence and hence should be followed up more closely. They also help identify which treatment modalities are associated with greater long-term risk.
Limitations
The database used lacked subgroup data regarding disease stage, histology, treatment details, and sites of recurrence, all of which could have been useful for making more specific conclusions and recommendations. Also, for many, cancers recurrence rates are likely to have diminished as treatments improved over time; however, treatment era was not taken into account in this study.
From the Editors
Substantial progress has been made since the 1960s in improving survival from childhood and adolescent cancer. Overall 5-year survival in the 1960s was estimated at 28%, whereas current 5-year survival rates approach 80% (1). Often, 5-year disease-free survival is used to denote a cure; however, recurrences more than 5 years from diagnosis do occur. In 2008, Mertens et al. (2) reported on very late mortality in 5-year survivors of childhood cancer from the Childhood Cancer Survivor Study (CCSS) and found that the leading cause of death for 5-year survivors was recurrence or progression of the original cancer until 20 years from diagnosis.
The current literature on late recurrence is mostly limited to case reports, case series, and long-term analyses of specific cancers treated through individual study groups or institutions (3–28). Although these reports are limited by sample size and length of observation, they have led to some recommendations for routine follow-up evaluations and treatment. For example, it is routine to continue annual imaging more than 5 years after treatment of bone sarcomas for early detection and intervention of late pulmonary relapse (13,29,30). For other diseases such as acute lymphoblastic leukemia and Hodgkin lymphoma, increased patient survival beyond 5 years has been accompanied by an increase in late recurrences (3,11,26,31). The timing and probability of “cure” for this pediatric population depend on several factors, including cancer type and treatment regimen.
As more pediatric cancer patients enter long-term follow-up as survivors of their cancer, it is important for both patients and physicians to appreciate the risk of recurrence. Knowledge of a survivor's risk is particularly important because of the inconsistencies seen with the length of follow-up and surveillance completed for childhood cancer survivors. Although “cure” is difficult to define in this patient population, many survivors may not have formal oncology follow-up once they are considered “cured” by their primary oncology physician (32). Moreover, young adult survivors of childhood cancer eventually age-out of pediatric care and have uncertain medical follow-up into adulthood.
The CCSS is the largest cohort of adult survivors of childhood cancer under direct surveillance in the United States and is uniquely able to assess the outcome of late recurrence across diagnoses (33). The primary objectives of this analysis were to assess the risk of relapse 5 years or more after original diagnosis and to determine patient and cancer characteristics that affect risk of late relapse. Understanding diagnosis-specific risks of recurrence can aid the formulation of evidence-based recommendations for long-term disease surveillance and help inform patients and families of the probability of a late relapse.
Patients and Methods
Study Population and Design
The CCSS cohort characteristics and study design have been previously described in detail (34). In brief, CCSS is a retrospective cohort of 14 359 five-year survivors of childhood cancer who were younger than 21 years at diagnosis and diagnosed between 1970 and 1986 at participating institutions (see Appendix 1). The following types of cancer are included in the cohort: leukemia, central nervous system (CNS) tumors, Hodgkin lymphoma, non-Hodgkin lymphoma, malignant kidney tumors, neuroblastoma, soft tissue sarcoma, or bone tumors. These diagnoses represent 80% of cancer cases in this age group (33).
CCSS data include self-report questionnaires and medical record abstraction from the treating institution as previously described (34). Primary diagnosis and detailed treatment data (including chemotherapy, radiation therapy, and surgeries) with initiation and end dates were abstracted from the medical records of the treating institution using a standard medical record abstraction form. Study questionnaires and medical record abstraction forms can be found at www.stjude.org/ccss. Recruitment of eligible subjects by participating institutions began in August 1994 according to a uniform CCSS protocol. The protocol and contact documents were reviewed and approved by the institutional review board at each institution. Written informed consent was received from all participants 18 years or older and from a parent or guardian of participants younger than 18 years.
Late Recurrence
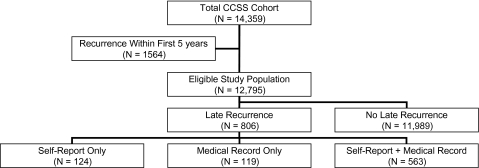
The study population for this analysis consisted of all 12 795 CCSS participants who had not experienced a recurrence of their original disease within the first 5 years following diagnosis (Figure 1). Subjects with an early recurrence (n = 1564) were excluded from this analysis because our focus was on late recurrences only. A late recurrence was defined as a relapse of the primary cancer that occurred beyond the 5-year anniversary of the original cancer diagnosis. Late recurrences were determined through self-report, medical record–based treatment data and/or mortality data. Self-reported recurrences occurring before December 31, 2002, were determined from three CCSS surveys administered before 2005 (Baseline; Follow-up 2000, and Follow-up 2003). Participants were asked to report the occurrence and date of any relapse or new cancer since their original diagnosis or since the completion of their previous CCSS questionnaire. All positive responses were independently reviewed by a single pediatric oncologist, and any reported outcomes that were suggestive of a new cancer were confirmed by pathology report. Only relapses of the primary cancer and not second malignant neoplasms were considered for this analysis.
Figure 1.
Categorization of recurrence status in the Childhood Cancer Survivor Study (CCSS).
Cancer treatments 5 years or more after the original diagnosis for all eligible study subjects, regardless of whether they had self-reported a late recurrence, were reviewed by one of the study investigators (K. Wasilewski-Masker). Medical record data reported by the CCSS institution were analyzed for greater than 6-month gaps in treatment or reinitiation of therapy that was consistent with the treatment of recurrent cancer. Surgeries that occurred 5 or more years after diagnosis were also counted, along with chemotherapy or radiation, as validation of recurrence status. Mortality status was obtained as previously reported by notification from a family member or by a listing in the National Death Index up until December 31, 2002 (35). In brief, if the subject had survived 5 years but subsequently died, a family member, usually a parent, was asked for information that included whether the death was because of the original cancer. Information from the original cancer diagnosis, death certificate, and from the parent's interview was reviewed to categorize whether the cause of death was recurrent or progressive disease. Patients were categorized as having had “no recurrence” when neither they nor their families reported a recurrence, and we had no other evidence of recurrence from treatment data or a death certificate.
Statistical Analysis
Descriptive data that included sex, race and/or ethnicity, primary diagnosis, treatment before recurrence, vital status, age at diagnosis, age at last follow-up, and years of follow-up since entry into the cohort were summarized for the 806 subjects with a late recurrence and compared with data for the 11 989 subjects with no recurrence.
Cumulative incidence of the first late recurrence was estimated by taking death as a competing risk and censoring at either the completion date for the last questionnaire or December 31, 2002, whichever was earlier (36). Multivariable Poisson regression was used to estimate the effects of multiple factors on the rate of late recurrence, and specifically focused on effects of primary diagnosis groups, using log person-years as an offset (37). The proportionality assumption of hazard functions was assessed graphically. Adjustment variables for the multivariate model included sex, race (non-Hispanic white, non-Hispanic black, Hispanic, and other), treatment modalities (chemotherapy and radiation, chemotherapy only, radiation only, and no chemotherapy or radiation), age at diagnosis (0–4, 5–9, 10–14, and 15–20 years), treatment era (1970–1972, 1973–1975, 1976–1978, 1979–1981, 1982–1984, and 1985–1986), and years since diagnosis (5–9, 10–14, 15–19, 20–24, and ≥25). Person-years at risk for late recurrence started at the 5-year anniversary from the original cancer diagnosis and ended at the earliest of late recurrence, death, December 31, 2002, or date of completion of the last questionnaire. All treatment exposures within the first 5 years from the original diagnosis were considered.
Statistical analyses were conducted using SAS Version 9.1 and R Version 2.5.1. All statistical inferences were two-sided.
Results
The study population included a total of 217 127 person-years at risk for late recurrence beginning at cohort entry (Table 1). The median age of the 12 795 eligible subjects was 8.3 years (range 0–20 years) at original diagnosis and 26 years (range 5.9–53.8 years) at follow-up, with a maximum of 34.6 years (median 21.6 years) of follow-up from initial diagnosis. Among the 12 795 subjects eligible for this analysis, 806 experienced a late recurrence (≥5 years from diagnosis). The majority of late recurrences (69.1%) occurred from 5 to 10 years after diagnosis, but late recurrences ranged from 5 to 29.0 years after the original diagnosis. At follow-up, 92.9% of the childhood cancer survivors with no recurrence were alive compared with 49.1% of those who had had a late recurrence.
Table 1.
Characteristics of patients with late recurrences compared with those with no history of recurrence in the Childhood Cancer Survivor Study*
| Characteristic | Eligible study population (N = 12 795) | No recurrence (n = 11 989) | Late recurrence (n = 806) |
|||||
| Total late recurrences | 5–9 y | 10–14 y | 15–19 y | 20–24 y | 25–29 y | |||
| Cases at risk (person-years at risk) | 12 795 (63 850.5) | 12 638 (61 160.4) | 11 681 (50 763.8) | 7844 (28 504.3) | 3714 (12 848.5) | |||
| Sex, No. (%)† | ||||||||
| Male | 6805 (53.2) | 6349 (53.0) | 456 (56.6) | 317 (56.9) | 87 (55.4) | 32 (56.1) | 18 (62.1) | 2 (33.3) |
| Female | 5990 (46.8) | 5640 (47.0) | 350 (43.4) | 240 (43.1) | 70 (44.6) | 25 (43.9) | 11 (37.9) | 4 (66.7) |
| Race and/or ethnicity, No. (%) | ||||||||
| White, non-Hispanic | 10 013 (82.5) | 9945 (83.3) | 678 (84.3) | 470 (84.7) | 134 (85.4) | 44 (77.2) | 25 (86.2) | 5 (83.3) |
| Black, non-Hispanic | 647 (5.3) | 618 (5.2) | 29 (3.6) | 15 (2.7) | 6 (3.8) | 6 (10.5) | 1 (3.4) | 1 (16.7) |
| Hispanic | 609 (5.0) | 569 (4.8) | 40 (5.0) | 28 (5.0) | 6 (3.8) | 5 (8.8) | 1 (3.4) | 0 (0.0) |
| Other | 868 (7.2) | 811 (6.8) | 57 (7.1) | 42 (7.6) | 11 (7.0) | 2 (3.5) | 2 (6.9) | 0 (0.0) |
| Diagnosis group, No. (%)† | ||||||||
| Acute lymphoblastic leukemia | 3521 (27.6) | 3294 (27.5) | 227 (28.2) | 186 (33.4) | 28 (17.8) | 8 (14.0) | 5 (17.2) | 0 (0.0) |
| Acute myeloid leukemia | 312 (2.4) | 293 (2.4) | 19 (2.4) | 12 (2.2) | 5 (3.2) | 2 (3.5) | 0 (0.0) | 0 (0.0) |
| Other leukemia | 280 (2.2) | 253 (2.1) | 27 (3.3) | 23 (4.1) | 3 (1.9) | 0 (0.0) | 1 (3.4) | 0 (0.0) |
| Astrocytomas | 1064 (8.3) | 908 (7.6) | 156 (19.4) | 90 (16.2) | 37 (23.6) | 19 (33.3) | 9 (31.0) | 1 (16.7) |
| Medulloblastoma, PNET | 347 (2.7) | 313 (2.6) | 34 (4.2) | 26 (4.7) | 5 (3.2) | 1 (1.8) | 2 (6.9) | 0 (0.0) |
| Other CNS tumors | 283 (2.2) | 259 (2.2) | 24 (3.0) | 11 (2.0) | 8 (5.1) | 2 (3.5) | 1 (3.4) | 2 (33.3) |
| Hodgkin lymphoma | 1730 (13.5) | 1616 (13.5) | 114 (14.1) | 75 (13.5) | 22 (14.0) | 13 (22.8) | 3 (10.3) | 1 (16.7) |
| Non-Hodgkin lymphoma | 1011 (7.9) | 986 (8.2) | 25 (3.1) | 15 (2.7) | 6 (3.8) | 3 (5.3) | 1 (3.4) | 0 (0.0) |
| Kidney tumors | 1196 (9.3) | 1185 (9.9) | 11 (1.4) | 7 (1.3) | 3 (1.9) | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Neuroblastoma | 901(7.0) | 876 (7.3) | 25 (3.1) | 14 (2.5) | 7 (4.5) | 2 (3.5) | 1 (3.4) | 1 (16.7) |
| Soft tissue sarcoma | 1155 (9.0) | 1083 (9.0) | 72 (8.9) | 44 (7.9) | 20 (12.7) | 3 (5.3) | 4 (13.8) | 1 (16.7) |
| Ewing sarcoma | 352 (2.8) | 307 (2.6) | 45 (5.6) | 33 (5.9) | 10 (6.4) | 2 (3.5) | 0 (0.0) | 0 (0.0) |
| Osteosarcoma | 596 (4.7) | 572 (4.8) | 24 (3.0) | 18 (3.2) | 3 (1.9) | 1 (1.8) | 2 (6.9) | 0 (0.0) |
| Other bone tumors | 47 (0.4) | 44 (0.4) | 3 (0.4) | 3 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment, No. (%)† | ||||||||
| Chemotherapy + radiation | 5854 (52.4) | 5434 (52.0) | 420 (58.7) | 313 (62.6) | 68 (48.2) | 26 (55.3) | 13 (56.5) | 0 (0.0) |
| Chemotherapy only | 2979 (26.7) | 2826 (27.0) | 153 (21.4) | 117 (23.4) | 30 (21.3) | 4 (8.5) | 2 (8.7) | 0 (0.0) |
| Radiation only | 1446 (13.0) | 1361 (13.0) | 85 (11.9) | 38 (7.6) | 26 (18.4) | 13 (27.7) | 5 (21.7) | 3 (75.0) |
| No chemotherapy or radiation | 887 (7.9) | 830 (7.9) | 57 (8.0) | 32 (6.4) | 17 (12.1) | 4 (8.5) | 3 (13.0) | 1 (25.0) |
| Age at diagnosis, No. (%), y† | ||||||||
| 0–4 | 5170 (40.4) | 4889 (40.8) | 281 (34.9) | 202 (36.3) | 55 (35.0) | 13 (22.8) | 9 (31.0) | 2 (33.3) |
| 5–9 | 2843 (22.2) | 2667 (22.2) | 176 (21.8) | 121 (21.7) | 35 (22.3) | 13 (22.8) | 5 (17.2) | 2 (33.3) |
| 10–14 | 2600 (20.3) | 2424 (20.2) | 176 (21.8) | 110 (19.7) | 36 (22.9) | 20 (35.1) | 8 (27.6) | 2 (33.3) |
| 15–20 | 2182 (17.1) | 2009 (16.8) | 173 (21.5) | 124 (22.3) | 31 (19.7) | 11 (19.3) | 7 (24.1) | 0 (0.0) |
| Age at last follow-up, No. (%), y† | ||||||||
| <20 | 1438 (11.2) | 1237 (10.3) | 201 (24.9) | 164 (29.4) | 34 (21.7) | 3 (5.3) | 0 (0.0) | 0 (0.0) |
| 20–29 | 5214 (40.8) | 4891 (40.8) | 323 (40.1) | 237 (42.5) | 60 (38.2) | 18 (31.6) | 7 (24.1) | 1 (16.7) |
| 30–39 | 4583 (35.8) | 4388 (36.6) | 195 (24.2) | 108 (19.4) | 45 (28.7) | 26 (45.6) | 13 (44.8) | 3 (50.0) |
| 40–49 | 1496 (11.7) | 1415 (11.8) | 81 (10.0) | 43 (7.7) | 18 (11.5) | 9 (15.8) | 9 (31.0) | 2 (33.3) |
| ≥50 | 64 (0.5) | 58 (0.5) | 6 (0.7) | 5 (0.9) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Years of follow-up, No. (%)† | ||||||||
| 5–9 | 157 (1.2) | 141 (1.2) | 16 (2.0) | 16 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 10–14 | 957 (7.5) | 848 (7.1) | 109 (13.5) | 92 (16.5) | 17 (10.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 15–19 | 3835 (30.0) | 3594 (30.0) | 241 (29.9) | 162 (29.1) | 60 (38.2) | 19 (33.3) | 0 (0.0) | 0 (0.0) |
| 20–24 | 4132 (32.3) | 3899 (32.5) | 233 (28.9) | 155 (27.8) | 43 (27.4) | 22 (38.6) | 13 (44.8) | 0 (0.0) |
| 25–29 | 2704 (21.1) | 2552 (21.3) | 152 (18.9) | 96 (17.2) | 28 (17.8) | 11 (19.3) | 14 (48.3) | 3 (50.0) |
| 30–35 | 1010 (7.9) | 955 (8.0) | 55 (6.8) | 36 (6.5) | 9 (5.7) | 5 (8.8) | 2 (6.9) | 3 (50.0) |
| Median | 21.6 | 22.0 | 21.0 | 20.5 | 21.0 | 22.4 | 25.8 | 30.5 |
| Range | 5.0–34.6 | 5.0–34.6 | 8.3–34.1 | 8.3–33.9 | 11.4–33.5 | 15.0–33.3 | 20.2–32.9 | 26.4–34.1 |
| Mortality status, No. (%)† | ||||||||
| Alive | 11 531 (90.1) | 11 135(92.9) | 396 (49.1) | 259 (46.5) | 84 (53.5) | 34 (59.6) | 14 (48.3) | 5 (83.3) |
| Dead | 1264 (9.9) | 854 (7.1) | 410 (50.9) | 298 (53.5) | 73 (46.5) | 23 (40.4) | 15 (51.7) | 1 (16.7) |
CNS = central nervous system; PNET = primitive neuroectodermal tumor.
P < .001 for the comparison of demographical variables among no recurrence and late recurrence, using the χ2 test.
Cumulative Incidence
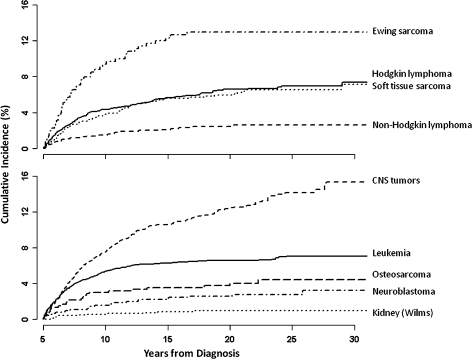
Estimates of cumulative incidence of late recurrence were 4.4% at 10 years, 5.6% at 15 years, and 6.2% at 20 years for all childhood cancer survivors, regardless of cancer type. A slightly higher cumulative incidence of late recurrences was seen in male survivors compared with female survivors and in all other racial and/or ethnic groups compared with blacks (Table 2). Subjects with Ewing sarcoma (13.0% at 20 years, 95% confidence interval [CI] = 9.4% to 16.5%) and astrocytoma (14.4% at 20 years, 95% CI = 12.3% to 16.6%) were at highest risk for late recurrence (Table 2 and Figure 2). Survivors of kidney tumors were at the lowest risk for recurrence of a childhood cancer (0.9% at 20 years, 95% CI = 0.4% to 1.5%; Table 2 and Figure 2).
Table 2.
Cumulative incidence of late recurrence in the Childhood Cancer Survivor Study at 10, 15, and 20 years after diagnosis, with death as a competing risk*
| Characteristic | Cumulative incidence (95% CI) |
P | ||
| 10 y | 15 y | 20 y | ||
| All patients | 4.4 (4.0 to 4.7) | 5.6 (5.2 to 6.0) | 6.2 (5.8 to 6.6) | |
| Sex | ||||
| Male | 4.7 (4.2 to 5.2) | 6.0 (5.4 to 6.6) | 6.6 (6.0 to 7.2) | .037 |
| Female | 4.0 (3.5 to 4.5) | 5.2 (4.6 to 5.8) | 5.7 (5.1 to 6.3) | |
| Race and/or ethnicity | ||||
| White, non-Hispanic | 4.4 (4.0 to 4.8) | 5.7 (5.3 to 6.2) | 6.2 (5.8 to 6.7) | .33 |
| Black, non-Hispanic | 2.3 (1.2 to 3.5) | 3.3 (1.9 to 4.7) | 4.6 (2.9 to 6.4) | |
| Hispanic | 4.6 (2.9 to 6.3) | 5.7 (3.8 to 7.5) | 6.8 (4.7 to 8.8) | |
| Other | 4.8 (3.4 to 6.3) | 6.2 (4.6 to 7.8) | 6.5 (4.8 to 8.2) | |
| Diagnosis group | ||||
| Acute lymphoblastic leukemia | 5.3 (4.5 to 6.0) | 6.1 (5.3 to 6.9) | 6.4 (5.6 to 7.2) | <.001 |
| Acute myeloid leukemia | 3.8 (1.7 to 6.0) | 5.5 (2.9 to 8.0) | 6.2 (3.5 to 8.9) | |
| Other leukemia | 8.2 (5.0 to 11.5) | 9.4 (5.9 to 12.8) | 9.4 (5.9 to 12.8) | |
| Astrocytomas | 8.5 (6.8 to 10.1) | 12.1 (10.1 to 14.0) | 14.4 (12.3 to 16.6) | |
| Medulloblastoma, PNET | 7.5 (4.7 to 10.3) | 9.0 (6.0 to 12.0) | 9.3 (6.2 to 12.3) | |
| Other CNS tumors | 3.9 (1.6 to 6.2) | 6.9 (3.9 to 9.9) | 8.0 (4.7 to 11.4) | |
| Hodgkin lymphoma | 4.3 (3.4 to 5.3) | 5.7 (4.6 to 6.7) | 6.6 (5.4 to 7.8) | |
| Non-Hodgkin lymphoma | 1.5 (0.7 to 2.2) | 2.1 (1.2 to 3.0) | 2.4 (1.5 to 3.4) | |
| Kidney tumors | 0.6 (0.2 to 1.0) | 0.8 (0.3 to 1.4) | 0.9 (0.4 to 1.5) | |
| Neuroblastoma | 1.6 (0.7 to 2.4) | 2.4 (1.4 to 3.4) | 2.6 (1.6 to 3.7) | |
| Soft tissue sarcoma | 3.8 (2.7 to 4.9) | 5.6 (4.3 to 6.9) | 5.9 (4.6 to 7.3) | |
| Ewing sarcoma | 9.4 (6.3 to 12.4) | 12.3 (8.9 to 15.8) | 13.0 (9.4 to 16.5) | |
| Osteosarcoma | 3.0 (1.6 to 4.4) | 3.5 (2.1 to 5.0) | 3.8 (2.2 to 5.3) | |
| Other bone tumors | 6.4 (0.0 to 13.4) | 6.4 (0.0 to 13.4) | 6.4 (0.0 to 13.4) | |
| Treatment | ||||
| Chemotherapy + radiation | 5.4 (4.8 to 5.9) | 6.5 (5.9 to 7.2) | 7.1 (6.4 to 7.7) | <.001 |
| Chemotherapy only | 3.9 (3.2 to 4.6) | 5.0 (4.2 to 5.8) | 5.2 (4.4 to 6.0) | |
| Radiation only | 2.6 (1.8 to 3.5) | 4.5 (3.4 to 5.5) | 5.5 (4.3 to 6.7) | |
| No chemotherapy or radiation | 3.6 (2.4 to 4.8) | 5.6 (4.1 to 7.1) | 6.2 (4.6 to 7.8) | |
| Age at diagnosis, y | ||||
| 0–4 | 3.9 (3.4 to 4.4) | 5.0 (4.4 to 5.6) | 5.3 (4.7 to 5.9) | <.001 |
| 5–9 | 4.3 (3.5 to 5.0) | 5.5 (4.7 to 6.4) | 6.1 (5.2 to 7.0) | |
| 10–14 | 4.2 (3.5 to 5.0) | 5.7 (4.8 to 6.6) | 6.6 (5.6 to 7.6) | |
| 15–20 | 5.7 (4.7 to 6.7) | 7.1 (6.1 to 8.2) | 7.8 (6.6 to 8.9) | |
CI = confidence interval; CNS = central nervous system; PNET = primitive neuroectodermal tumor. Cumulative incidence curves were compared using the method proposed by Gray (38).
Figure 2.
Cumulative incidence of first late recurrence by diagnosis using death as a competing risk. CNS = central nervous system. Soft tissue sarcoma includes rhabdomyosarcoma and other (soft tissue sarcoma). Leukemia includes acute lymphoblastic leukemia, acute myeloid leukemia, and other leukemias. CNS tumors include astrocytoma, medulloblastoma or primitive neuroectodermal tumor, and other CNS tumors. Kidney (Wilms) includes all kidney tumors.
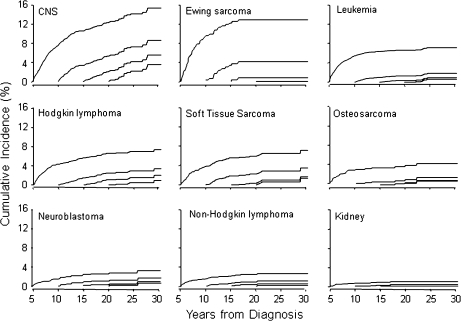
Cumulative incidence of late recurrence among childhood cancer survivors who had already experienced recurrence-free survival for 5, 10, 15, or 20 years from original diagnosis progressively declined in all diagnostic groups over time (Figure 3). However, survivors of CNS malignancies remained at a relatively high risk of recurrence even after 15–20 years of recurrence-free survival. For Hodgkin lymphoma, soft tissue sarcoma, and Ewing sarcoma patients who had survived without recurrence for 10 years, there was a greater than 3% incidence of subsequent recurrence. For most pediatric cancers, relapse after 15 years of recurrence-free survival is rare.
Figure 3.
Conditional cumulative recurrence curves, conditioned on recurrence-free survival at 5, 10, 15, and 20 years since the original diagnosis, taking death as a competing risk. x-axis = years from diagnosis. y-axis = cumulative incidence (%). CNS = central nervous system. Soft tissue sarcoma includes rhabdomyosarcoma and other (soft tissue sarcoma). Leukemia includes acute lymphoblastic leukemia, acute myeloid leukemia, and other leukemias. CNS tumors include astrocytoma, medulloblastoma or primitive neuroectodermal tumor, and other CNS tumors. Kidney (Wilms) includes all kidney tumors.
Multivariable Analysis
Multivariable Poisson regression analysis that included diagnosis group, sex, race and/or ethnicity, treatment modality, age at diagnosis, treatment era, and years since diagnosis as covariates was used to determine independent risk factors for late recurrence (Table 3). The greatest risk factors for late recurrence included a diagnosis of Ewing sarcoma or a CNS tumor, combination treatment with chemotherapy and radiation, earlier treatment era, and fewer years since diagnosis (P < .001 for all). Survivors of Ewing sarcoma (adjusted rate ratio [RR] = 1.7, 95% CI = 1.2 to 2.4) and CNS tumors (astrocytomas: RR = 4.5, 95% CI = 3.4 to 5.9; medulloblastoma or primitive neuroectodermal tumors: RR = 2.4, 95% CI = 1.6 to 3.5; other CNS tumors: RR = 2.3, 95% CI = 1.4 to 3.7) were at a higher risk for late recurrence than childhood acute lymphoblastic leukemia survivors. Survivors of kidney tumors (Wilms) and non-Hodgkin lymphoma were at statistically significant lower risk (RR = 0.2, 95% CI = 0.1 to 0.3 and RR = 0.3, 95% CI = 0.2 to 0.5, respectively) compared with survivors of acute lymphoblastic leukemia. Males had a 1.2-fold (95% CI = 1.0 to 1.4) higher relative risk compared with females for the recurrence of childhood cancers overall. Risk of late recurrence was somewhat higher in those who were diagnosed at older ages as compared with those who were diagnosed when younger. As expected, the risk of late recurrence decreased with increasing time since diagnosis and later treatment era.
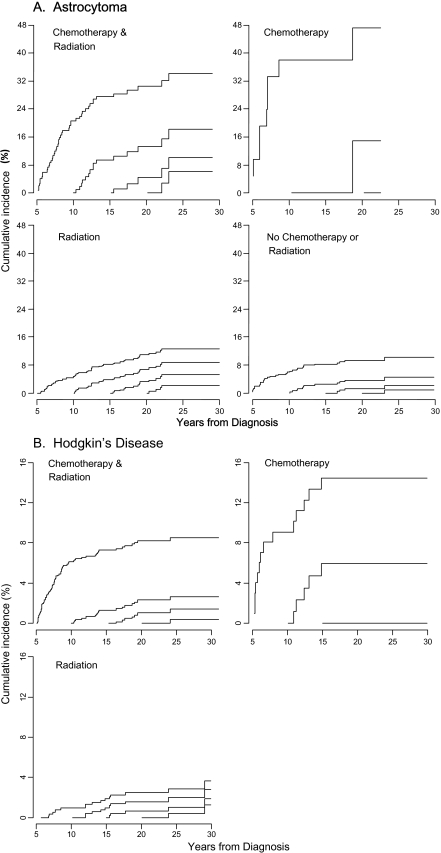
Multivariable Poisson regression using all variables in Table 3 except diagnosis was next used to assess the effect of treatment on late recurrence in each of the diagnosis groups. Treatment effect was statistically significant for only astrocytomas and Hodgkin lymphoma with cumulative incidence of late recurrence greatest for patients who were treated with chemotherapy only for both diagnoses (Figure 4). The interaction between treatment and era was tested and was not significant (P = .24 for astrocytomas and P = .27 for Hodgkin lymphoma), thus treatment effect did not differ by treatment era.
Figure 4.
Treatment effect on conditional cumulative recurrence for astrocytomas (A) and Hodgkin lymphoma (B), conditioned on recurrence-free survival of 5, 10, 15, and 20 years since the original diagnosis, taking death as a competing risk. Treatment effect is statistically significant for astrocytomas and Hodgkin disease only. Other diagnosis groups are not shown. x-axis = years from diagnosis. y-axis = cumulative incidence (%).
Discussion
Five-year disease-free survival is often interpreted to mean “cure.” However, this study of 5-year disease-free survivors of the most common forms of childhood cancer diagnosed between 1970 and 1986 demonstrated a 6.2% cumulative incidence for a recurrence from 5 to 20 years after the primary diagnosis. Although the majority of recurrences occurred 5–10 years after diagnosis, late recurrences occurred up to 29 years after diagnosis. Survivors of Ewing sarcoma and astrocytoma were at highest risk of a late recurrence, whereas survivors of kidney tumors were at lowest risk.
The CCSS cohort provides a unique opportunity to study outcomes, including late recurrence, among long-term survivors of childhood cancer. The large number of adult survivors of childhood cancer who are under direct and active surveillance in CCSS, coupled with the extended length of follow-up, provides one of the largest and most comprehensively characterized cohorts available for the study of late recurrence rates. Determination of the rates and patterns of late recurrences for specific types of childhood cancer is important to provide guidance to patients, who need to know the probability of recurrence after having survived recurrence-free for their particular diagnosis, and to providers to help inform recommendations on long-term disease surveillance.
It has been suggested that “cure” be defined as the point at which the chance of mortality from the original cancer is equal to that of death from any cause in the general population (32). Although one can question the basis for this proposed definition, the fact that recurrence confers subsequent increased risk for early death is well documented and supported by this study. Recurrence or progression of the primary cancer was the most common cause of mortality among childhood cancer survivors from 5 to 20 years after diagnosis in an earlier analysis of late mortality in the CCSS cohort (2). Both in our analysis of late recurrence and in the previous analysis of late mortality, survivors of brain tumors and Ewing sarcoma were at highest risk and survivors of renal tumors were at lowest risk (2). Additionally, at the time of follow-up, only 49.1% of those with a late recurrence were alive compared with 92.9% of survivors with no recurrence.
Families and providers often want to know when the risk for recurrences becomes so low that they may consider using the term “cured.” Although these data demonstrate that most disease-free survivors will be “cured” 5 years from diagnosis, there remain survivors among all diagnoses at risk for late recurrence. It is likely impossible to say when an individual survivor is “cured.” What can be confirmed by this analysis are those at higher and lower risk for a late recurrence, which can help direct surveillance practices.
Although surveillance neuroimaging is often used to detect recurrence of brain tumors, the optimal length and cost-effectiveness of such surveillance are not known (39). Our analysis confirms a risk of recurrence of pediatric cancers up to 25 years after the original diagnosis, which may help to validate long-term surveillance practices. For example, because it is well appreciated that late recurrence can occur among Ewing sarcoma survivors (9,12,16,24,40–42), the European Society of Medical Oncology currently recommends that survivors be followed annually with radiographic scans between 5 and 10 years after treatment (30). The approximately 9.4% rate that we observed for a first recurrence of Ewing sarcoma between 5 and 10 years after the primary diagnosis validates European Society of Medical Oncology recommendations. Extended follow-up (range 5–35 years) in our study indicates that although the cumulative incidence of a late recurrence among Ewing sarcoma survivors increases to approximately 13% at 20 years, recurrence among those patients who have survived at least 15 years is low. Therefore, it may be advisable to extend surveillance for Ewing sarcoma survivors to 15 years. Conversely, our findings confirm that long-term radiological follow-up for recurrence of Wilms tumor is not justified (43). In this era of increasing health-care costs, it is important to determine which survivors are at low risk of late recurrence, for whom long-term surveillance is not warranted.
Our analysis was limited by the lack of subgroup data needed to make more specific recommendations with regard to risk and surveillance. The medical record abstraction data for the CCSS do not contain detailed information, such as disease stage, histology, or biology. Subgroups of pediatric cancers that differ based on these features may also differ with respect to risk of late recurrence. For example, although late recurrence was rare for neuroblastoma and non-Hodgkin lymphoma, caution should be taken in making generalized recommendations based on these low recurrence rates, given the heterogeneity of these diagnoses.
In addition, the medical record abstraction that we used was limited to dates of recurrences and did not include detailed data, such as sites of recurrence. The relatively low late recurrence rate that we observed among osteosarcoma survivors, with few recurrences reported beyond 10 years, was unexpected. Previously published reports have led to recommendations for prolonged screening up to 10 years after completion of therapy for pulmonary relapse in survivors of osteosarcoma (6,13,29,44). Within the CCSS cohort, late pulmonary relapse could have been underestimated if it was not self-reported and was treated with surgery alone.
Our study could be limited by reliance on self-reported and available medical record information, which could result in underascertainment of late recurrences overall. Medical records may be incomplete if treatment for a late recurrence occurred at another institution. Also, self-reported data are not infallible: Although the majority of childhood cancer survivors have general knowledge about their cancer diagnosis and treatment, they lack specific knowledge (45), which could particularly affect the retrospective collection of information on recurrence status and dates. Because the CCSS has a rigorous process for validation of second malignant neoplasms (46), it is less likely that overreporting could occur due to misclassification of a second malignant neoplasm as recurrent cancer.
Also, it is important to interpret results from the CCSS cohort with the understanding that survivors were diagnosed between 1970 and 1986. Though long-term follow-up is needed to establish late recurrence rates, survival and treatment approaches are continually changing for more recently diagnosed patients. For example, in the treatment era of this report (ie, among those patients diagnosed between 1970 and 1986), only a small proportion of children survived high-risk neuroblastoma (47,48). Thus, the majority of neuroblastoma survivors in this cohort likely had low- or intermediate-risk disease. As treatment has intensified for high-risk neuroblastoma and other childhood cancers with a poor prognosis, late recurrence will likely be increasing as greater disease control is achieved in the nearer term (15,49,50). However, with improved survival and implementation of more efficacious treatment regimens for pediatric cancers on the whole, the risk of late recurrence may be reduced overall (33,47).
Lastly, late, as opposed to early, recurrence rates may be higher for survivors in the cohort that was treated in the era before magnetic resonance imaging and in some cases the era before computed tomography, for whom detection may have been delayed compared with more recently diagnosed patients. For example, the incidence of brain tumors has increased over the past 30 years, but this is likely because of improved imaging techniques (51). Diagnosis of recurrences of CNS tumors and other malignancies that rely on magnetic resonance imaging for optimal imaging is likely to be similarly improved.
Results from the CCSS cohort, while historical, are helpful to serve as a basis for discussions with patients and their parents on the diagnosis-specific risk of late recurrence. Although they are not common, late recurrences do occur in childhood cancer. For certain diagnoses such as Ewing sarcoma and CNS tumors, patients remain at substantial clinical risk for recurrence beyond 10 years from diagnosis. Because the risk for a late recurrence extends into young adulthood for many survivors, it is essential to maintain insurance coverage and to transition care to adult providers when appropriate for continued follow-up. Future research is needed to determine the risk of recurrence in subgroups of survivors and the cost-effectiveness of long-term disease surveillance in at-risk patient groups because these factors need to be considered in long-term follow-up and transitional care plans.
Funding
Department of Health and Human Services (U24-CA 55727 to L.L.R.); American Lebanese Syrian Associated Charities to St Jude Children's Research Hospital; Children's Oncology Group (Aflac Young Investigator Award in Adolescent and Young Adult Oncology to K.W.-M.).
Appendix 1
The Childhood Cancer Survivor Study (CCSS) is a collaborative multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived 5 or more years after diagnosis of childhood cancer.
CCSS is a retrospectively ascertained cohort of 20 346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (National Cancer Institute U24 CA55727) awarded to St Jude Children's Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14 000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and use the CCSS resource, visit www.stjude.org/ccss
CCSS Institutions and Investigators
| St Jude Children's Research Hospital, Memphis, TN | Leslie L. Robison, PhD*†, Melissa Hudson, MD†‡, Greg Armstrong, MD, MSCE†, Daniel M. Green, MD† |
| Children's Healthcare of Atlanta/Emory University, Atlanta, GA | Lillian Meacham, MD‡, Ann Mertens, PhD† |
| Children's Hospitals and Clinics of Minnesota Minneapolis, St Paul, MN | Joanna Perkins, MD, MS‡ |
| Children's Hospital and Medical Center, Seattle, WA | Douglas Hawkins, MD‡, Eric Chow, MD, MPH† |
| Children's Hospital, Denver, CO | Brian Greffe, MD‡ |
| Children's Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH‡ |
| Children's Hospital, Oklahoma City, OK | John Mulvihill, MD†‡ |
| Children's Hospital of Orange County, Orange, CA | Leonard Sender, MD‡ |
| Children's Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg, MD‡, Anna Meadows, MD† |
| Children's Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak, MD‡ |
| Children's National Medical Center, Washington, DC | Gregory Reaman, MD‡, Roger Packer, MD† |
| Cincinnati Children's Hospital Medical Center, Cincinnati, OH | Stella Davies, MD, PhD†‡ |
| City of Hope Medical Center, Los Angeles, CA | Smita Bhatia, MD†‡ |
| Cook Children's Medical Center, Ft Worth, TX | Paul Bowman, MD, MPH‡ |
| Dana-Farber Cancer Institute/Children's Hospital, Boston, MA | Lisa Diller, MD†‡ |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD†‡ |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg, MBChB‡, Paul C. Nathan, MD†‡ |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD†‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD‡ |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD†‡, Kevin Oeffinger, MD† |
| Miller Children's Hospital, Long Beach, CA | Jerry Finklestein, MD‡ |
| National Cancer Institute, Bethesda, MD | Roy Wu, PhD†, Nita Seibel, MD†, Preetha Rajaraman, PhD† |
| Nationwide Children's Hospital, Columbus, OH | Amanda Termuhlen, MD‡, Sue Hammond, MD† |
| Northwestern University, Chicago, IL | Kimberley Dilley, MD, MPH‡ |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD‡ |
| Roswell Park Cancer Institute, Buffalo, NY | Martin Brecher, MD‡ |
| St Louis Children's Hospital, St Louis, MO | Robert Hayashi, MD‡ |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD‡, Sarah S. Donaldson, MD† |
| Texas Children's Hospital, Houston, TX | Zoann Dreyer, MD‡ |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH‡ |
| University of Alberta, Edmonton, AB | Yutaka Yasui, PhD†‡ |
| University of California-Los Angeles, CA | Jacqueline Casillas, MD, MSHS‡, Lonnie Zeltzer, MD† |
| University of California-San Francisco, CA | Robert Goldsby, MD‡ |
| University of Chicago, Chicago, IL | Tara Henderson, MD, MPH‡ |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD‡ |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH†‡ |
| University of Southern California, Los Angeles, CA | Dennis Deapen, DrPH†‡ |
| UT-Southwestern Medical Center, Dallas, TX | Daniel Bowers, MD‡ |
| U.T.M.D. Anderson Cancer Center, Houston, TX | Louise Strong, MD†‡, Marilyn Stovall, MPH, PhD† |
Project Principal Investigator (U24 CA55727).
Member CCSS Steering Committee.
Institutional Principal Investigator.
Table 3.
Rate ratios for risk factors for late recurrence, using Poisson regression*
| Variable | Unadjusted |
Adjusted |
||
| RR (95% CI) | P | RR (95% CI) | P | |
| Sex | ||||
| Male | 1.2 (1.0 to 1.4) | .018 | 1.2 (1.0 to 1.4) | .021 |
| Female (referent) | 1.0 | 1.0 | ||
| Race | ||||
| Black, non-Hispanic | 0.8 (0.5 to 1.1) | .20 | 0.9 (0.6 to 1.5) | .77 |
| Hispanic | 1.1 (0.8 to 1.5) | .49 | 1.2 (0.9 to 1.7) | .28 |
| Other | 1.1 (0.8 to 1.4) | .52 | 1.0 (0.8 to 1.4) | .87 |
| White, non-Hispanic (referent) | 1.0 | 1.0 | ||
| Diagnosis group | ||||
| Acute myeloid leukemia | 1.0 (0.6 to 1.6) | .96 | 0.9 (0.5 to 1.5) | .63 |
| Other leukemia | 1.5 (1.0 to 2.3) | .035 | 1.4 (0.9 to 2.1) | .15 |
| Astrocytomas | 2.4 (2.0 to 3.0) | <.001 | 4.5 (3.4 to 5.9) | <.001 |
| Medulloblastoma, PNET | 1.6 (1.1 to 2.3) | .014 | 2.4 (1.6 to 3.5) | <.001 |
| Other CNS tumors | 1.4 (0.9 to 2.2) | .10 | 2.3 (1.4 to 3.7) | .001 |
| Hodgkin lymphoma | 1.0 (0.8 to 1.2) | .73 | 1.0 (0.7 to 1.3) | .93 |
| Non-Hodgkin lymphoma | 0.4 (0.2 to 0.6) | <.001 | 0.3 (0.2 to 0.5) | <.001 |
| Kidney tumors | 0.1 (0.1 to 0.2) | <.001 | 0.2 (0.1 to 0.3) | <.001 |
| Neuroblastoma | 0.4 (0.3 to 0.6) | <.001 | 0.5 (0.3 to 0.8) | .003 |
| Soft tissue sarcoma | 0.9 (0.7 to 1.2) | .45 | 0.9 (0.7 to 1.2) | .63 |
| Ewing sarcoma | 2.1 (1.5 to 2.8) | <.001 | 1.7 (1.2 to 2.4) | .003 |
| Osteosarcoma | 0.6 (0.4 to 0.9) | .016 | 0.5 (0.3 to 0.8) | .008 |
| Other bone tumors | 0.9 (0.3 to 2.8) | .84 | 1.1 (0.3 to 4.4) | .93 |
| Acute lymphoblastic leukemia (referent) | 1.0 | 1.0 | ||
| Treatment | ||||
| Chemotherapy + radiation | 1.1 (0.8 to 1.5) | .49 | 2.6 (1.9 to 3.7) | <.001 |
| Chemotherapy only | 0.9 (0.6 to 1.2) | .37 | 2.6 (1.8 to 3.8) | <.001 |
| Radiation only | 0.8 (0.6 to 1.2) | .33 | 0.9 (0.6 to 1.3) | .57 |
| No chemotherapy or radiation (referent) | 1.0 | 1.0 | ||
| Age at diagnosis, y | ||||
| 0–4 (referent) | 1.0 | 1.0 | ||
| 5–9 | 1.1 (0.9 to 1.4) | .19 | 1.0 (0.8 to 1.2) | .87 |
| 10–14 | 1.2 (1.0 to1.5) | .024 | 1.0 (0.8 to 1.3) | .77 |
| 15–20 | 1.5 (1.2 to 1.8) | <.001 | 1.5 (1.2 to1.9) | .001 |
| Years since diagnosis | ||||
| 5–9 (referent) | 1.0 | 1.0 | ||
| 10–14 | 0.3 (0.3 to 0.4) | <.001 | 0.3 (0.3 to 0.4) | <.001 |
| 15–19 | 0.1 (0.1 to 0.2) | <.001 | 0.1 (0.1 to 0.2) | <.001 |
| 20–24 | 0.1 (0.1 to 0.2) | <.001 | 0.1 (0.1 to 0.2) | <.001 |
| ≥25 | 0.1 (0.0 to 0.1) | <.001 | 0.0 (0.0 to 0.1) | <.001 |
| Year of diagnosis | ||||
| 70–72 | 0.9 (0.7 to 1.2) | .62 | 2.1 (1.6 to 2.8) | <.001 |
| 73–75 | 0.9 (0.7 to 1.1) | .24 | 1.6 (1.2 to 2.1) | <.001 |
| 76–78 | 0.7 (0.6 to 0.9) | .014 | 1.3 (1.0 to 1.7) | .07 |
| 79–81 | 0.8 (0.6 to 1.0) | .031 | 1.2 (0.9 to 1.6) | .19 |
| 82–84 | 0.9 (0.7 to 1.2) | .51 | 1.2 (0.9 to 1.5) | .20 |
| 85–86 (referent) | 1.0 | 1.0 | ||
CI = confidence interval; CNS = central nervous system; PNET = primitive neuroectodermal tumor.
Footnotes
The funding sources had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Smith M, Gloeckler Ries L. Childhood Cancer: Incidence, Survival and Mortality in Principles and Practice of Pediatric Oncology. Philadelphia, PA: JB Lippincott Raven; 2002. [Google Scholar]
- 2.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brierley JD, Rathmell AJ, Gospodarowicz MK, Sutcliffe SB, Pintillie M. Late relapse after treatment for clinical stage I and II Hodgkin's disease. Cancer. 1997;79(7):1422–1427. [PubMed] [Google Scholar]
- 4.Cotterill SJ, Pearson AD, Pritchard J, Kohler JA, Foot AB. Late relapse and prognosis for neuroblastoma patients surviving 5 years or more: a report from the European Neuroblastoma Study Group “Survey”. Med Pediatr Oncol. 2001;36(1):235–238. doi: 10.1002/1096-911X(20010101)36:1<235::AID-MPO1057>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Dannecker G, Leidig E, Treuner J, Niethammer D. Late recurrence of neuroblastoma: a reason for prolonged follow-up? Am J Pediatr Hematol Oncol. 1983;5(3):271–274. [PubMed] [Google Scholar]
- 6.Ferrari S, Briccoli A, Mercuri M, et al. Late relapse in osteosarcoma. J Pediatr Hematol Oncol. 2006;28(7):418–422. doi: 10.1097/01.mph.0000212944.82361.1d. [DOI] [PubMed] [Google Scholar]
- 7.Fiorillo A, Migliorati R, Fiore M, Palombini L. Late recurrence of disseminated neuroblastoma after 3 years of continuous remission. J Pediatr. 1984;104(1):161–162. doi: 10.1016/s0022-3476(84)80625-3. [DOI] [PubMed] [Google Scholar]
- 8.Hata Y, Sasaki F, Naito H, Takahashi H, Namieno T, Uchino J. Late recurrence in neuroblastoma. J Pediatr Surg. 1991;26(12):1417–1419. doi: 10.1016/0022-3468(91)91053-2. [DOI] [PubMed] [Google Scholar]
- 9.Kinsella TJ, Miser JS, Waller B, et al. Long-term follow-up of Ewing's sarcoma of bone treated with combined modality therapy. Int J Radiat Oncol Biol Phys. 1991;20(3):389–395. doi: 10.1016/0360-3016(91)90047-8. [DOI] [PubMed] [Google Scholar]
- 10.Richards MJ, Joo P, Gilbert EF. The rare problem of late recurrence in neuroblastoma. Cancer. 1976;38(4):1847–1852. doi: 10.1002/1097-0142(197610)38:4<1847::aid-cncr2820380464>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Shihabi S, Deutsch M, Jacobs SA. Very late relapse of Hodgkin's disease: a report of five patients. Am J Clin Oncol. 2001;24(6):576–578. doi: 10.1097/00000421-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Thariat J, Italiano A, Peyrade F, et al. Very late local relapse of Ewing's sarcoma of the head and neck treated with aggressive multimodal therapy. Sarcoma. 2008;2008:854141. doi: 10.1155/2008/854141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss SJ, McTiernan A, Whelan JS. Late relapse of osteosarcoma: implications for follow-up and screening. Pediatr Blood Cancer. 2004;43(6):692–697. doi: 10.1002/pbc.20154. [DOI] [PubMed] [Google Scholar]
- 14.Cervera A, Kingston JE, Malpas JS. Late recurrence of neuroblastoma: a report of five cases and review of the literature. Pediatr Hematol Oncol. 1990;7(4):311–322. doi: 10.3109/08880019009033408. [DOI] [PubMed] [Google Scholar]
- 15.Escobar MA, Grosfeld JL, Powell RL, et al. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg. 2006;41(2):377–381. doi: 10.1016/j.jpedsurg.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Gasparini M, Lombardi F, Ballerini E, et al. Long-term outcome of patients with monostotic Ewing's sarcoma treated with combined modality. Med Pediatr Oncol. 1994;23(5):406–412. doi: 10.1002/mpo.2950230504. [DOI] [PubMed] [Google Scholar]
- 17.Hauben EI, Bielack S, Grimer R, et al. Clinico-histologic parameters of osteosarcoma patients with late relapse. Eur J Cancer. 2006;42(4):460–466. doi: 10.1016/j.ejca.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Ishikawa K, Toyoda Y, et al. Late recurrence of neuroblastoma stage 4S with unusual clinicopathologic findings. J Pediatr Surg. 2001;36(6):953–955. doi: 10.1053/jpsu.2001.24002. [DOI] [PubMed] [Google Scholar]
- 19.Koksal Y, Akyuz C, Varan A, Atilla B, Gedikoglu G, Buyukpamukcu M. Late recurrence in primary region of parosteal osteosarcoma: a case report. Pediatr Hematol Oncol. 2008;25(1):83–88. doi: 10.1080/08880010701621010. [DOI] [PubMed] [Google Scholar]
- 20.Lau TW, Wong JW, Yip DK, Chien EP, Shek TW, Wong LL. Local recurrence of parosteal osteosarcoma adjacent to a prosthesis after 20 years: a case report. J Orthop Surg (Hong Kong) 2004;12(2):263–266. doi: 10.1177/230949900401200225. [DOI] [PubMed] [Google Scholar]
- 21.Le BH, Buchanan MR, Steer C, de Boer R. Late recurrence of osteosarcoma. Intern Med J. 2007;37(6):420–422. doi: 10.1111/j.1445-5994.2007.01346.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine PL, Berberich FR, Burke JS, Mott MG, Wilbur JR. Lymphoblastic lymphoma: late relapse in childhood. Med Pediatr Oncol. 1983;11(1):33–36. doi: 10.1002/mpo.2950110107. [DOI] [PubMed] [Google Scholar]
- 23.Lodha R, Jain Y, Pemde H, Bhargava M, Arya LS. Late relapse in a case of childhood acute lymphoblastic leukemia. Indian Pediatr. 1998;35(3):281–283. [PubMed] [Google Scholar]
- 24.McLean TW, Hertel C, Young ML, et al. Late events in pediatric patients with Ewing sarcoma/primitive neuroectodermal tumor of bone: the Dana-Farber Cancer Institute/Children's Hospital experience. J Pediatr Hematol Oncol. 1999;21(6):486–493. [PubMed] [Google Scholar]
- 25.Rose PS, Dickey ID, Wenger DE, Unni KK, Sim FH. Periosteal osteosarcoma: long-term outcome and risk of late recurrence. Clin Orthop Relat Res. 2006;453:314–317. doi: 10.1097/01.blo.0000229341.18974.95. [DOI] [PubMed] [Google Scholar]
- 26.Sadowitz PD, Smith SD, Shuster J, Wharam MD, Buchanan GR, Rivera GK. Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 1993;81(3):602–609. [PubMed] [Google Scholar]
- 27.Sung L, Anderson JR, Donaldson SS, Spunt SL, Crist WM, Pappo AS. Late events occurring five years or more after successful therapy for childhood rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Eur J Cancer. 2004;40(12):1878–1885. doi: 10.1016/j.ejca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Wysocki M, Balcar-Boron A, Pilecki O, Koltan S. Late relapse after presumed cure of childhood acute lymphoblastic leukemia. Acta Haematol Pol. 1993;24(4):381–384. [PubMed] [Google Scholar]
- 29.Saeter G. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2007;18(suppl 2) doi: 10.1093/annonc/mdm047. ii77–ii78. [DOI] [PubMed] [Google Scholar]
- 30.Saeter G. Ewing's sarcoma of bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2007;18(suppl 2) doi: 10.1093/annonc/mdm048. ii79–ii80. [DOI] [PubMed] [Google Scholar]
- 31.Rivera GK, Hudson MM, Liu Q, et al. Effectiveness of intensified rotational combination chemotherapy for late hematologic relapse of childhood acute lymphoblastic leukemia. Blood. 1996;88(3):831–837. [PubMed] [Google Scholar]
- 32.Haupt R, Spinetta JJ, Ban I, et al. Long term survivors of childhood cancer: cure and care. The Erice statement. Eur J Cancer. 2007;43(12):1778–1780. doi: 10.1016/j.ejca.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Ries L, Smith M, Gurney J, et al. NIH Publication. Bethesda, MD: National Institute of Health; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995, National Cancer Institute, SEER Program; pp. 99–4649. [Google Scholar]
- 34.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 35.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 36.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Breslow N, Day NE. The Design and Analysis of Cohort Studies (IARC Scientific Publication No. 82) Vol 2. Lyon: 1987. International Agency for Research on Cancer. [PubMed] [Google Scholar]
- 38.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 39.Korones DN, Butterfield R, Meyers SP, Constine LS. The role of surveillance magnetic resonance imaging (MRI) scanning in detecting recurrent brain tumors in asymptomatic children. J Neurooncol. 2001;53(1):33–38. doi: 10.1023/a:1011804404246. [DOI] [PubMed] [Google Scholar]
- 40.Bacci G, Ferrari S, Longhi A, et al. Therapy and survival after recurrence of Ewing's tumors: the Rizzoli experience in 195 patients treated with adjuvant and neoadjuvant chemotherapy from 1979 to 1997. Ann Oncol. 2003;14(11):1654–1659. doi: 10.1093/annonc/mdg457. [DOI] [PubMed] [Google Scholar]
- 41.Hayes FA, Thompson EI, Kumar M, Hustu HO. Long-term survival in patients with Ewing's sarcoma relapsing after completing therapy. Med Pediatr Oncol. 1987;15(5):254–256. doi: 10.1002/mpo.2950150506. [DOI] [PubMed] [Google Scholar]
- 42.Nilbert M, Saeter G, Elomaa I, Monge OR, Wiebe T, Alvegard TA. Ewing's sarcoma treatment in Scandinavia 1984-1990—ten-year results of the Scandinavian Sarcoma Group Protocol SSGIV. Acta Oncol. 1998;37(4):375–378. doi: 10.1080/028418698430601. [DOI] [PubMed] [Google Scholar]
- 43.Firoozi F, Kogan BA. Follow-up and management of recurrent Wilms’ tumor. Urol Clin North Am. 2003;30(4):869–879. doi: 10.1016/s0094-0143(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 44.Kempf-Bielack B, Bielack SS, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23(3):559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 45.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2002;287(14):1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 46.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93(8):618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 47.Matthay KK, O’Leary MC, Ramsay NK, et al. Role of myeloablative therapy in improved outcome for high risk neuroblastoma: review of recent Children's Cancer Group results. Eur J Cancer. 1995;31A(4):572–575. doi: 10.1016/0959-8049(95)00015-b. [DOI] [PubMed] [Google Scholar]
- 48.Philip T, Bernard JL, Zucker JM, et al. High-dose chemoradiotherapy with bone marrow transplantation as consolidation treatment in neuroblastoma: an unselected group of stage IV patients over 1 year of age. J Clin Oncol. 1987;5(2):266–271. doi: 10.1200/JCO.1987.5.2.266. [DOI] [PubMed] [Google Scholar]
- 49.Franks LM, Bollen A, Seeger RC, Stram DO, Matthay KK. Neuroblastoma in adults and adolescents: an indolent course with poor survival. Cancer. 1997;79(10):2028–2035. doi: 10.1002/(sici)1097-0142(19970515)79:10<2028::aid-cncr26>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 50.Imaizumi M, Watanabe A, Kikuta A, et al. Improved survival of children with advanced neuroblastoma treated by intensified therapy including myeloablative chemotherapy with stem cell transplantation: a retrospective analysis from the Tohoku Neuroblastoma Study Group. Tohoku J Exp Med. 2001;195(2):73–83. doi: 10.1620/tjem.195.73. [DOI] [PubMed] [Google Scholar]
- 51.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867–890. doi: 10.1016/j.ncl.2007.07.002. vii. [DOI] [PubMed] [Google Scholar]