Abstract
Background
Frailty is an indicator of health status in old age. Its frequency has been described mainly for North America; comparable data from other countries are lacking. Here we report on the prevalence of frailty in 10 European countries included in a population-based survey.
Methods
Cross-sectional analysis of 18,227 randomly selected community-dwelling individuals 50 years of age and older, enrolled in the Survey of Health, Aging and Retirement in Europe (SHARE) in 2004. Complete data for assessing a frailty phenotype (exhaustion, shrinking, weakness, slowness, and low physical activity) were available for 16,584 participants. Prevalences of frailty and prefrailty were estimated for individuals 50–64 years and 65 years of age and older from each country. The latter group was analyzed further after excluding disabled individuals. We estimated country effects in this subset using multivariate logistic regression models, controlling first for age, gender, and then demographics and education.
Results
The proportion of frailty (three to five criteria) or prefrailty (one to two criteria) was higher in southern than in northern Europe. International differences in the prevalences of frailty and prefrailty for 65 years and older group persisted after excluding the disabled. Demographic characteristics did not account for international differences; however, education was associated with frailty. Controlling for education, age and gender diminished the effects of residing in Italy and Spain.
Conclusions
A higher prevalence of frailty in southern countries is consistent with previous findings of a north–south gradient for other health indicators in SHARE. Our data suggest that socioeconomic factors like education contribute to these differences in frailty and prefrailty.
Keywords: Frailty, Aged, Middle-aged, Frail elderly, Epidemiology, Prevalence
MOST industrialized countries will need to adapt their health care systems to meet the challenges arising from population aging. This will require meaningful estimates of population health from epidemiological surveys. Functional impairments in the oldest age group have been discussed with regard to current and projected long-term care needs; however, this limits the view of the future needs of aging populations. In Europe, the large cohort of post–World War II baby boomers will reach retirement age over the next two decades. And although preventing an unfavorable evolution toward loss of autonomy in this generation is a public health priority, little is known regarding the proportion at risk for functional decline in middle age and beyond. Health indicators based on selected chronic conditions or unhealthy behaviors are difficult to interpret because multiple combinations of degenerative diseases result in considerable heterogeneity in the risk for functional loss and health care needs.
Thus, the geriatric concept of frailty (1–3) is of particular interest because frailty is likely to be a precursor of disability (4–6) and may be reversible in its early stages (7). The prevalence of frailty might summarize health and the needs for prevention in middle-aged and older populations (8,9). A major impediment to measuring frailty in population-based surveys is the lack of an operational definition. However, Fried and colleagues (4,10) identified a frailty phenotype that was predictive of adverse outcomes such as falls and fractures (11,12), mobility and functional declines (4,5,12), hospitalizations (4), nursing home admissions (5), and death (4–6,11–13). The prevalence of this phenotype has mainly been estimated for Northern America and scant data are available for Europe (14,15). The population of European countries could experience different levels of frailty due to cultural, regional, or political distinctions.
The purposes of this study were to quantify the prevalence of frailty in community-dwelling middle-aged and older Europeans participating in the Survey of Health, Aging and Retirement in Europe (SHARE) in 2004, compare this prevalence among the 10 countries included in this survey, and evaluate selected population characteristics as potential explanations for international differences observed in the 65 years and older (65+) subgroup.
METHODS
Data Source and Participants
SHARE is a multidisciplinary European Union research project (16) covering 10 countries in its 2004 first wave: Austria, Denmark, France, Germany, Greece, Italy, the Netherlands, Spain, Sweden, and Switzerland. Probability samples were selected in each country, using sampling techniques adapted to the local conditions (17). Baseline data were collected from 24,690 individuals living in households that included at least one member who was 50 years of age or older. All household members in this age category and their spouses of whatever age were eligible; some countries also included institutionalized individuals. The overall response rate was 61.8% (Table 1), varying across countries from 50.2% to 73.6%, except in Switzerland, which had a particularly low response rate (37.6%) (18).
Table 1.
Overall Response Rate in SHARE Wave 1 (2004), Working Sample Size and Age Distribution
| Overall Response Rate (%) | Working Sample Size (N) | Weighted* Proportion 50–64 y of Age (%) | Weighted* Mean (SD) Age in 65+ Category (y) | |
| Sweden | 50.2 | 2,050 | 51.5 | 75.2 (7.6) |
| Denmark | 63.2 | 1,572 | 58.7 | 74.7 (6.9) |
| The Netherlands | 61.3 | 2,221 | 59.3 | 73.9 (6.4) |
| Germany | 63.4 | 2,278 | 50.6 | 73.8 (6.9) |
| Austria | 58.1 | 1,829 | 53.2 | 74.2 (6.7) |
| Switzerland | 37.6 | 938 | 55.2 | 74.5 (7.2) |
| France | 73.6 | 1,651 | 51.5 | 74.8 (6.9) |
| Italy | 55.1 | 1,971 | 48.7 | 74.2 (6.8) |
| Spain | 53.3 | 1,762 | 47.3 | 75.0 (7.1) |
| Greece | 61.4 | 1,955 | 50.4 | 73.8 (6.9) |
| All 10 countries | 61.8 | 18,227 | 50.7 | 74.3 (6.9) |
Notes: SHARE = Survey of Health, Aging and Retirement in Europe.
Accounting for the primary sampling unit, the strata, and the calibrated individual weight.
After excluding 5,431 individuals from additional samples drawn in some countries for a supplementary survey, 674 spouses younger than 50 years, 295 individuals living in institutions, 61 with insufficient information on sampling characteristics, and 2 nonevaluable individuals, 18,227 community-dwelling individuals were eligible for analysis.
Data Collection and Measurements
The survey was based on standardized computer-assisted face-to-face personal interviews conducted by trained (19) and supervised interviewers using a common questionnaire translated from a generic English version (available online at http://www.share-project.org/) (20). Interviews were supplemented by measurements of handgrip strength in all participants and a 2.5-m walking test in participants 75 years of age and older.
Variables Definition
Frailty and prefrailty were defined on the basis of the five dimensions in a phenotype described by Fried and associates (4). However, operationalization of these dimensions required adaptation to our survey contents. Exhaustion was identified as a positive response to the question, “In the last month, have you had too little energy to do things you wanted to do? (yes/no).” The shrinking criterion was fulfilled by reporting a “diminution in desire for food” in response to the question, “What has your appetite been like” or, in the case of an uncodable response to this question, by responding “less” to the following question: “So have you been eating more or less than usual?.” Weakness was derived from the highest of four consecutive dynamometer measurements of handgrip strength (two from each hand), applying gender and body mass index cutoffs set by Fried and associates. Because SHARE measured walking speed only in individuals 75 years of age and older, slowness was defined using mobility questions, after previous analysis showed a strong relationship between low speed and positive answers to either of the following two items: “Because of a health problem, do you have difficulty [expected to last more than 3 months] walking 100 meters” or “… climbing one flight of stairs without resting” (21). The low activity criterion was fulfilled in participants responding “one to three times a month” or “hardly ever or never” to the question, “How often do you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or going for a walk?.” One point was allocated for each fulfilled criterion; individuals with zero points were classified as nonfrail, with one or two points as prefrail and with three to five points as frail. Participants with health-related difficulties in one or more of the five Katz basic activities of daily living (eating, bathing or showering, dressing, transferring, using the toilet) for at least 3 months were considered disabled.
Statistical Analysis
A cross-sectional analysis of SHARE wave 1 (baseline) was performed with Stata 9.0 version 2.0.1, which includes design weights for multistage sampling design and calibration to population totals within countries to reflect national populations (17). Population characteristics in middle (50–64 years) and old (65+ years) ages were first described overall and in each country by computing the prevalence estimates with 95% confidence intervals (CIs). International differences in the prevalences of frailty and prefrailty were then explored in the subset of older individuals (65+ years) without disability using multivariate logistic regression models, adjusting first only for gender and age (in years) and then for both demographics and education (number of years) as an indicator of socioeconomic status; the outcome was frailty in Model 1, and either prefrailty or frailty in Model 2. Germany was chosen as the reference country, based on both statistical (large sample size) and geographical (central location in Europe) considerations. Adjusted odds ratios and 95% CIs were calculated for country effects. The significance level was set at p < .01 to account for multiple comparisons during interpretation. Results are presented for countries sorted from north to south based on their mean latitude.
RESULTS
Of 18,227 participants 50 years of age and older, 9.0% had missing information on one or two dimensions of frailty, leaving 16,584 participants for analysis. In the middle-aged population, 4.1% (95% CI 3.4–4.7) were frail and 37.4% (35.8–39.1) were prefrail. A sensitivity analysis conducted on 18,227 observations with imputation of zero for up to two missing values on frailty criteria produced slightly higher estimates of the prevalence of frailty and prefrailty, with differences never exceeding 1.7% in both age categories (data not shown, results available upon request). Women were more frequently frail and prefrail (5.2% [4.3–6.1] and 42.0% [40.0–44.0], respectively) than men (2.9% [2.0–3.8] and 32.7% [30.5–34.9], respectively; p < .001). In the 65+ group, 17.0% (15.3–18.7) were frail and 42.3% (40.5–44.1) prefrail, with frailty and prefrailty noted in 21.0% (18.6–23.3) and 42.7% (40.2–45.1), respectively, of women, and in 11.9% (10.3–13.6) and 41.9% (39.5–44.2), respectively, of men (p < .001).
Table 2 shows significant country differences in the prevalences of frailty criteria and frailty status. In both age categories, frailty and prefrailty were particularly frequent in Spain and, to a lesser extent, in Italy. All frailty criteria except low physical activity had a higher prevalence in Spain. Weakness, slowness, and low physical activity were frequent in Italy in the 65+ group. In contrast, Sweden and Switzerland were characterized by a low prevalence of frailty. Their profiles were particularly favorable in the dimensions of slowness and physical activity.
Table 2.
Prevalence of Frailty and Disability in Community-Dwelling Population, by Country and Age Category (weighted proportion and 95% confidence interval)
| 10 Countries | Sweden | Denmark | The Netherlands | Germany | Austria | Switzerland | France | Italy | Spain | Greece | |
| Age 50–64 y (n) | 9,074 | 1,038 | 877 | 1,261 | 1,146 | 849 | 470 | 803 | 958 | 733 | 939 |
| Frailty criteria | |||||||||||
| Exhaustion | 27.3 (25.8–29.9) | 31.6 (28.5–34.6) | 30.8 (27.6–34.1) | 23.6 (20.7–26.5) | 20.4 (17.3–23.6) | 20.5 (17.6–23.5) | 25.4 (21.3–29.4) | 30.0 (26.0–33.9) | 29.8 (25.7–34.0) | 38.4 (34.3–42.4) | 22.0 (19.2–24.8) |
| Shrinking | 5.2 (4.6–5.9) | 5.2 (3.7–6.7) | 5.9 (4.1–7.7) | 4.1 (2.3–6.0) | 3.4 (2.2–4.7) | 5.9 (4.2–7.6) | 5.2 (3.1–7.3) | 6.7 (5.0–8.4) | 4.4 (2.9–5.9) | 8.9 (6.7–11.0) | 4.2 (2.8–5.5) |
| Weakness | 6.1 (5.3–6.8) | 4.5 (3.1–5.9) | 3.1 (1.9–4.4) | 4.7 (3.6–5.8) | 3.9 (2.6–5.2) | 3.5 (2.2–4.7) | 2.5 (1.1–4.0) | 4.6 (3.0–6.3) | 7.2 (5.2–9.3) | 13.9 (11.1–16.6) | 5.7 (4.2–7.2) |
| Slowness | 6.7 (5.8–7.6) | 2.9 (1.8–4.0) | 5.5 (3.9–7.1) | 7.3 (5.5–9.1) | 5.9 (3.6–8.2) | 6.5 (4.5–8.5) | 3.9 (2.0–5.8) | 4.3 (2.7–5.9) | 8.8 (6.7–10.9) | 9.5 (7.0–11.9) | 8.6 (6.7–10.4) |
| Low activity | 13.8 (12.3–15.3) | 4.5 (3.1–5.8) | 6.3 (4.6–8.1) | 8.8 (6.7–10.8) | 9.6 (7.0–12.3) | 13.6 (10.7–16.5) | 11.2 (8.1–14.3) | 14.8 (12.1–17.5) | 24.7 (19.6–29.7) | 11.8 (8.8–14.7) | 10.9 (8.9–13.0) |
| Frailty status | |||||||||||
| Prefrailty | 37.4 (35.8–39.1) | 35.9 (32.8–39.1) | 34.9 (31.5–38.3) | 30.8 (27.1–34.5) | 30.9 (27.3–34.5) | 30.4 (26.7–34.2) | 36.1 (31.4–40.7) | 40.3 (36.6–44.0) | 43.5 (39.4–47.7) | 44.9 (40.7–49.0) | 33.5 (30.3–36.8) |
| Frailty | 4.1 (3.4–4.7) | 1.9 (1.1–2.8) | 3.0 (1.8–4.2) | 3.5 (2.4–4.7) | 2.6 (1.5–3.8) | 3.9 (2.6–5.2) | 1.3 (0.3–2.2) | 3.2 (1.9–4.5) | 5.9 (4.1–7.7) | 7.5 (5.5–9.5) | 2.8 (1.7–3.9) |
| Disability | |||||||||||
| BADL* difficulty | 4.5 (3.9–5.2) | 4.7 (3.3–6.1) | 5.8 (4.2–7.4) | 3.7 (2.4–5.0) | 4.3 (2.8–5.8) | 4.4 (2.8–6.1) | 3.4 (1.6–5.2) | 5.2 (3.5–6.9) | 3.9 (2.4–5.4) | 6.0 (4.1–8.0) | 2.6 (1.5–3.6) |
| Age 65+ y (n) | 7,510 | 873 | 635 | 830 | 933 | 707 | 412 | 687 | 833 | 816 | 784 |
| Frailty criteria | |||||||||||
| Exhaustion | 36.7 (34.7–38.7) | 39.1 (35.5–42.7) | 32.9 (29.0–36.8) | 28.9 (24.5–33.3) | 30.0 (25.5–34.4) | 27.6 (23.3–31.9) | 28.0 (23.6–32.5) | 36.4 (32.6–40.3) | 38.4 (33.3–43.6) | 54.5 (50.1–59.0) | 29.8 (26.5–33.1) |
| Shrinking | 11.1 (9.8–12.4) | 8.8 (6.7–10.9) | 8.4 (6.1–10.6) | 6.8 (5.2–8.4) | 8.0 (5.2–10.9) | 6.9 (4.7–9.1) | 5.0 (2.9–7.1) | 9.5 (7.1–11.9) | 13.4 (10.0–16.8) | 19.2 (15.9–22.5) | 10.8 (8.7–13.0) |
| Weakness | 26.3 (24.4–28.3) | 17.9 (15.0–20.7) | 16.9 (13.7–20.0) | 17.8 (14.2–21.4) | 15.1 (12.0–18.2) | 13.6 (10.4–16.8) | 16.7 (13.0–20.5) | 22.7 (19.2–26.2) | 38.1 (32.0–44.1) | 44.0 (39.8–48.2) | 27.3 (24.1–30.6) |
| Slowness | 22.7 (20.9–24.6) | 12.7 (10.2–15.2) | 19.5 (16.2–22.8) | 19.9 (16.7–23.0) | 18.7 (15.0–22.4) | 18.9 (14.7–23.1) | 10.3 (7.2–13.4) | 20.7 (17.1–24.3) | 28.1 (23.1–33.2) | 29.3 (25.2–33.4) | 27.6 (24.4–30.8) |
| Low activity | 21.3 (19.2–23.4) | 10.3 (8.0–12.5) | 14.1 (11.2–17.1) | 16.9 (13.5–20.3) | 15.7 (12.4–19.0) | 24.8 (19.1–30.5) | 15.5 (11.7–19.3) | 22.6 (19.0–26.2) | 30.1 (23.2–37.0) | 22.9 (19.1–26.7) | 17.3 (14.6–20.0) |
| Frailty status | |||||||||||
| Prefrailty | 42.3 (40.5–44.1) | 45.3 (41.8–48.9) | 38.4 (34.5–42.3) | 38.5 (34.4–42.7) | 34.6 (31.1–38.1) | 40.7 (36.5–45.0) | 46.5 (41.5–51.4) | 43.6 (39.6–47.6) | 45.6 (40.7–50.5) | 50.9 (46.8–55.1) | 44.9 (41.4–48.4) |
| Frailty | 17.0 (15.3–18.7) | 8.6 (6.5–10.8) | 12.4 (9.6–15.1) | 11.3 (9.0–13.5) | 12.1 (8.8–15.3) | 10.8 (8.0–13.5) | 5.8 (3.5–8.1) | 15.0 (12.2–17.8) | 23.0 (18.0–28.0) | 27.3 (23.5–31.0) | 14.7 (12.2–17.3) |
| Disability | |||||||||||
| BADL* difficulty | 13.5 (12.2–14.7) | 11.1 (8.7–13.5) | 11.8 (9.1–14.5) | 9.0 (7.1–10.9) | 11.4 (8.6–14.1) | 8.4 (6.1–10.7) | 9.9 (7.0–12.7) | 16.1 (13.1–19.2) | 16.2 (13.0–19.3) | 14.1 (11.0–17.2) | 9.5 (7.4–11.6) |
Note:*Basic activities of daily living: bathing or showering, dressing, eating, getting in and out of bed, using the toilet.
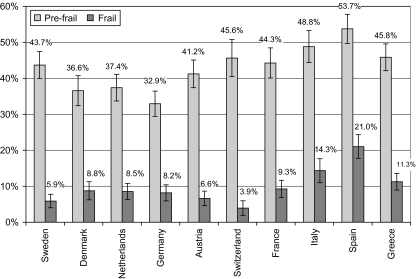
Table 2 also shows differences in the prevalence of disability for those in the 65+ group, ranging from 8.4% in Austria to more than 16.2% in Italy. Figure 1 indicates a high prevalence of frailty in southern countries (Spain, Italy, France, and Greece). A high frequency of disability was also recorded for Spain, Italy, and France (Table 2). However, in the nondisabled population, frailty was again found to be more common in southern Europe (21.0% in Spain, 14.3% in Italy, 11.3% in Greece, 9.3% in France), whereas its prevalence was lower than 9.0% in all other countries (Figure 2). Prefrailty was also most frequent in Spain, Italy, and Greece.
Figure 1.
Percentage of the 65 years and older community-dwelling population classified as prefrail and frail by country (weighted results).
Figure 2.
Percentage of the 65 years and older community-dwelling population without disability classified as prefrail and frail by country (weighted results).
Table 3 (upper) shows unadjusted country effects. In adjusted multivariate analysis controlling for demographics only, these effects did not change substantially (data not shown, available upon request): Frailty remained more frequent in Spain and Italy and less frequent in Switzerland as compared with Germany (Model 1). In contrast, the four southernmost European countries included in SHARE had a higher age- and gender-adjusted proportion with signs of prefrailty or frailty (Model 2). However, the estimates of country effects changed markedly after simultaneously adjusting for gender, age, and education (Table 3, lower panel). In both models, women were more likely to be frail than men and increasing age positively influenced the probability of frailty, whereas additional years of education had a negative effect on this probability. After adjusting for demographics and education, only Sweden and Switzerland still had a significantly different prevalence of frailty than the reference country at p < .01 (Model 1). The combined prevalence of prefrailty and frailty (Model 2) was not lower in Sweden and Switzerland but was significantly higher in Spain and Italy.
Table 3.
Country Effects on Frailty in the 65 Years and Older Community-Dwelling Population Without Disability (weighted results)
| Model 1 Outcome: Frail |
Model 2 Outcome: Prefrail or Frail |
|||
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Unadjusted | ||||
| Sweden | 0.70 (0.45–1.10) | .126 | 1.41 (1.14–1.74) | .002 |
| Denmark | 1.08 (0.70–1.66) | .738 | 1.19 (0.94–1.49) | .144 |
| The Netherlands | 1.05 (0.69–1.58) | .822 | 1.22 (0.98–1.51) | .073 |
| Austria | 0.80 (0.52–1.24) | .316 | 1.32 (1.06–1.64) | .014 |
| Switzerland | 0.46 (0.25–0.85) | .013 | 1.41 (1.09–1.82) | .009 |
| France | 1.14 (0.76–1.73) | .521 | 1.65 (1.31–2.07) | .000 |
| Italy | 1.88 (1.26–2.81) | .002 | 2.46 (1.94–3.12) | .000 |
| Spain | 2.99 (2.09–4.27) | .000 | 4.24 (3.34–5.39) | .000 |
| Greece | 1.42 (0.98–2.08) | .066 | 1.91 (1.54–2.36) | .000 |
| Adjusted for | ||||
| Gender (women) | 1.58 (1.23–2.04) | .000 | 1.25 (1.07–1.45) | .005 |
| Age, y | 1.08 (1.06–1.10) | .000 | 1.08 (1.07–1.10) | .000 |
| Education, y | 0.91 (0.88–0.94) | .000 | 0.93 (0.91–0.95) | .000 |
| Sweden | 0.44 (0.27–0.70) | .001 | 0.97 (0.77–1.23) | .830 |
| Denmark | 0.80 (0.51–1.25) | .329 | 0.97 (0.76–1.23) | .791 |
| The Netherlands | 0.76 (0.49–1.17) | .212 | 0.95 (0.75–1.20) | .661 |
| Austria | 0.64 (0.41–1.01) | .055 | 1.11 (0.88–1.40) | .367 |
| Switzerland | 0.31 (0.16–0.60) | .000 | 1.16 (0.88–1.53) | .297 |
| France | 0.58 (0.35–0.96) | .033 | 1.06 (0.82–1.38) | .654 |
| Italy | 0.99 (0.59–1.65) | .961 | 1.59 (1.19–2.13) | .002 |
| Spain | 1.28 (0.78–2.10) | .333 | 2.32 (1.72–3.13) | .000 |
| Greece | 0.72 (0.44–1.17) | .187 | 1.22 (0.94–1.58) | .134 |
Note: CI = confidence interval; OR = odds ratio. Germany is the reference country.
DISCUSSION
Data from SHARE showed that signs of frailty were not rare in the middle-aged and were frequent in the older-aged community-dwelling population in Europe. The data also showed between-country differences in the distribution of a frailty phenotype. This survey was based on a large probability sample that included 50- to 64-year-old individuals, a group for which few studies provide estimates of frailty, and it relied on a standardized instrument, thus permitting international comparisons.
The prevalence of frailty in SHARE was higher than expected from reports of the landmark Cardiovascular Health Study (65+ years: men 4.9%, women 7.3% frail) (4), the Invecchiare in Chianti study (65+ years: 8.8% frail) (14), the Osteoporotic Fractures in Men study (65+ years: 4% frail) (13), the Women's Health and Aging Studies (70–79 years: 11.3% frail) (5), the Women's Health Initiative study (65–79 years: 16.3% frail) (6), or the Study of Osteoporotic Fractures (69+ years: 16.3% frail) (11,12). However, other studies found higher estimates, such as the Hispanic Established Populations for the Epidemiologic Studies of the Elderly study (70+ years: 20%) (22) and the Massachusetts Male Aging Study (70–79 years: 11.0%; 80–86 years: 36.5%) (23). Variations across studies may be due to methodological differences that preclude direct comparison of the results. One variation is the protocol for excluding individuals with health conditions potentially related to frailty. SHARE did not exclude individuals on the basis of selected diseases, which could have resulted in higher proportions of frailty. A different operationalization of the dimensions of the frailty phenotype identified by Fried and associates could be another reason for variations in the estimated prevalence of prefrailty and frailty. Criteria in SHARE were not identical to those defined in the Cardiovascular Health Study, except for weakness (4), and may be less specific, leading to higher prevalence estimates particularly for exhaustion, which was common in the SHARE population. The longitudinal design of SHARE will permit verification of the predictive validity of frailty criteria assessed in this survey. A third methodological difference is the treatment of missing information. In the Cardiovascular Health Study, participants with missing information for less than two frailty components were considered evaluable, whereas SHARE data were analyzed only for participants with complete data for all components. The sensitivity analysis conducted on SHARE data showed that imputation tends to decrease the estimated proportion of nonfrail slightly; however, this effect was negligible.
Variations between European countries in the frequency of frailty are consistent with previous findings of a north–south gradient characterizing other health indicators in SHARE (16). Lower rates of institutionalization of older disabled persons in southern countries may be one explanation for a higher prevalence of frailty in their communities because frailty is strongly associated with disability. Organization for Economic Co-operation and Development's statistics (24) indicated that there were 14.8 long-term care beds per 1,000 inhabitants 65 years of age and older in Italy and 77.9 in Sweden in 2003; however, we found that France ranked high in both long-term care bed density and frailty prevalence. Moreover, higher levels of frailty were also observed in the older nondisabled population and in the middle-aged population of southern countries. In contrast, a lower frequency of frailty was noted in Switzerland and Sweden. Low participation may explain the favorable health indicators found for Switzerland, based on the assumption that the fittest are more likely to participate in surveys. However, similar participation rates in Sweden, Spain, and Italy make this explanation less likely to account for the differences in frailty prevalence among these three countries. Like most other studies, we found a higher prevalence of frailty in women and in older-aged people. However, in between countries differences persisted after controlling for age and gender. Differences could also result from cultural characteristics influencing the perception of health or from misunderstandings due to language differences despite considerable efforts invested in translation. However, although this explanation cannot be completely discarded, we found that objectively measured low grip strength contributed to the higher prevalence of frailty in Spain and Italy.
Although demographic characteristics did not explain international differences in frailty, we found a strong relationship between education and frailty, and an attenuation of country effects after adjusting for this factor. This illustrates the need to integrate nonmedical factors when studying the epidemiology of frailty. Although caution should be exercised when interpreting our results due to the cross-sectional study design, our data also suggest that education may protect from frailty at an individual level. This converges with recent reports of a negative relationship between the level of education and the frequency of cognitive disorders in later life. Future studies should examine the extent to which differences in frailty between European countries mirror differences in the prevalence of cognitive impairments.
Acknowledgments
The SHARE 2004 data collection was funded by the European Commission (project QLK6-CT-2001-00360), with contributions from the U.S. National Institute on Aging (U01 AG09740-13S2, P01 AG005842, P01 AG08291, P30 AG12815, Y1-AG-4553-01, and OGHA 04-064) and the National Science Funds in Austria (FWF) and Switzerland (Bundesamt für Bildung und Wissenschaft/OFES/UFES). B.S.-E. is coinvestigator of the SHARE project.
References
- 1.Bortz WM., II The physics of frailty. J Am Geriatr Soc. 1993;41:1004–1008. [PubMed] [Google Scholar]
- 2.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(suppl 3):3–29. [PubMed] [Google Scholar]
- 3.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: towards a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the Women's Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61:M262–M266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Fugate Woods N, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative observational study. J Am Geriatr Soc. 2005;53:1221–1230. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 7.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 8.Vellas B, Gillette-Guyonnet S, Nourashemi F, et al. Falls, frailty and osteoporosis in the elderly: a public health problem. Rev Med Interne. 2000;21:608–613. doi: 10.1016/s0248-8663(00)80006-5. [DOI] [PubMed] [Google Scholar]
- 9.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 10.Fried LP, Ferrucci L, Darer J, Williamson JD, Andersen G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the Study of Osteoporotic Fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 12.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for the prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 13.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puts MTE, Lips P, Deeg DJH. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53:40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 16.Börsch-Supan A, Brugiavini A, Jürges H, Mackenbach J, Sigrist J, Weber G. First Results From the Survey of Health, Ageing and Retirement in Europe. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. pp. 8–27. [Google Scholar]
- 17.Klevmarken A, Swensson B, Hesselius P. The SHARE sampling procedures and calibrated design weights. In: Börsch-Supan A, Jürges H, editors. The Survey of Health, Aging and Retirement in Europe: Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. pp. 28–69. [Google Scholar]
- 18.De Luca G, Paracchi F. Survey participation in the first wave of SHARE. In: Börsch-Supan A, Jürges H, editors. The Survey of Health, Aging and Retirement in Europe: Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. pp. 88–104. [Google Scholar]
- 19.Alcser KH, Benson G. The SHARE train-the-trainer program. In: Börsch-Supan A, Jürges H, editors. The Survey of Health, Aging and Retirement in Europe: Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. pp. 70–74. [Google Scholar]
- 20.Karkness J. SHARE translation procedures and translation assessment. In: Börsch-Supan A, Jürges H, editors. The Survey of Health, Aging and Retirement in Europe: Methodology. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2005. pp. 24–27. [Google Scholar]
- 21.Cuénoud P. Prevalence of the Frailty Phenotype in Community-Dwelling Population Aged 50 and Over in the Survey of Health, Ageing and Retirement in Europe [master's dissertation] Lausanne, Switzerland: Institute of Health Economics and Management, University of Lausanne; 2007. [Google Scholar]
- 22.Ottenbacher KJ, Ostir GV, Kristen Peek M, Al Snih S, Raji MA, Markides KS. Frailty in older Mexican Americans. J Am Geriatr Soc. 2005;53:1524–1531. doi: 10.1111/j.1532-5415.2005.53511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr BA, Bhasin S, Kupelian V, Araujo AB, O'Donnell AB, McKinlay JB. Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc. 2007;55:548–555. doi: 10.1111/j.1532-5415.2007.01121.x. [DOI] [PubMed] [Google Scholar]
- 24.Organisation for Economic Co-operation and Development. OECD Health Data 2007: Statistics and Indicators for 30 Countries [CD-ROM] Paris, France: OECD; 2007. [Google Scholar]