Abstract
Background
Aging is associated with a loss of muscle mass and increased body fat. The effects of diet-induced weight loss on muscle mass in older adults are not clear.
Purpose
This study examined the effects of diet-induced weight loss, alone and in combination with moderate aerobic exercise, on skeletal muscle mass in older adults.
Methods
Twenty-nine overweight to obese (body mass index = 31.8 ± 3.3 kg/m2) older (67.2 ± 4.2 years) men (n = 13) and women (n = 16) completed a 4-month intervention consisting of diet-induced weight loss alone (WL; n = 11) or with exercise (WL/EX; n = 18). The WL intervention consisted of a low-fat, 500–1,000 kcal/d caloric restriction. The WL/EX intervention included the WL intervention with the addition of aerobic exercise, moderate-intensity walking, three to five times per week for 35–45 minutes per session. Whole-body dual-energy x-ray absorptiometry, thigh computed tomography (CT), and percutaneous muscle biopsy were performed to assess changes in skeletal muscle mass at the whole-body, regional, and cellular level, respectively.
Results
Mixed analysis of variance demonstrated that both groups had similar decreases in bodyweight (WL, −9.2% ± 1.0%; WL/EX, −9.1% ± 1.0%) and whole-body fat mass (WL, −16.5%, WL/EX, −20.7%). However, whole-body fat-free mass decreased significantly (p < .05) in WL (−4.3% ± 1.2%) but not in WL/EX (−1.1% ± 1.0%). Thigh muscle cross-sectional area by CT decreased in both groups (WL, −5.2% ± 1.1%; WL/EX, −3.0% ± 1.0%) and was not statistically different between groups. Type I muscle fiber area decreased in WL (−19.2% ± 7.9%, p = .01) but remained unchanged in WL/EX (3.4% ± 7.5%). Similar patterns were observed in type II fibers (WL, −16.6% ± 4.0%; WL/EX, −0.2% ± 6.5%).
Conclusion
Diet-induced weight loss significantly decreased muscle mass in older adults. However, the addition of moderate aerobic exercise to intentional weight loss attenuated the loss of muscle mass.
Keywords: Weight loss, Aerobic exercise, Obese, Muscle
THE loss of muscle mass that occurs with age, known as sarcopenia (1,2), is associated with progressive muscle weakness (3), a greater risk for falls (4,5), and susceptibility to injury (6) and, consequently, can lead to loss of independence and physical mobility (7,8). Recent evidence indicates that obesity confounds the influence of sarcopenia on the loss of muscle function in older men and women (9,10). Therefore, preventing overweight and obesity while maintaining muscle mass is a clinically relevant goal particularly for older adults.
A conundrum is that traditional diet-induced weight loss programs in younger men and women result in not only loss of adipose tissue but also in a significant loss of lean body mass. This might suggest that diet-induced weight loss in older adults, who have an accelerated loss of muscle mass, may produce even greater declines in muscle mass (11). Although the exact cause of sarcopenia and accompanying loss of function are not completely understood, decreases in physical activity with age (12) may contribute to increases in body fat, decreases in muscle mass, and decreases in muscle quality (13–15). Resistance (16–23) and aerobic exercise (24) have the potential to reduce body fat and slow or prevent age-related changes in skeletal muscle, including the loss of muscle. However, although aerobic exercise does not typically increase muscle mass, it is not clear whether moderate-intensity aerobic exercise, primarily walking, can attenuate or prevent the loss of muscle mass that might occur with intentional, diet-induced weight loss in older adults. Therefore, the purpose of this study was twofold. The primary aim was to investigate the effects of diet-induced weight loss on changes in muscle mass in older adults. The secondary aim was to determine whether moderate aerobic exercise can attenuate the potentially adverse effects that weight loss alone might have on muscle mass.
METHODS
Participants
Men and women aged 60–75 years who were overweight to moderately obese (body mass index [BMI] = 25.0–38.0 kg/m2) were recruited from the Pittsburgh metropolitan and surrounding areas. Participants were considered for this intentional weight loss intervention if they (a) had either impaired glucose tolerance (IGT; 2-hour oral glucose tolerance (OGTT) test ≥140 mg/dL), impaired fasting glucose (IFG; fasting glucose ≥100 but ≤126 mg/dL), or drug-naive type 2 diabetes mellitus (T2DM; fasting glucose ≥126 but ≤200 mg/dL and OGTT ≥200 mg/dL); (b) had no history of clinically significant cardiovascular disease; (c) had a resting systolic blood pressure of 150 mmHg or less and diastolic blood pressure of 95 mmHg or less; (d) were a nonsmoker; (e) was a stable weight (no gain or loss of >6 kg in 6 months); and (f) were sedentary (currently participating in aerobic exercise less than 2 d/wk). The study was approved by the University of Pittsburgh Institutional Review Board, and a written informed consent was obtained from each volunteer.
All the participants diagnosed as having IGT and IFG were randomized into one of the following two groups: weight loss (WL) or weight loss combined with exercise (WL/EX) for 4 months. All participants with T2DM were not randomized; they were enrolled into the WL/EX group for ethical reasons related to withholding treatment that would optimally include both weight loss and exercise.
Diet-Induced Weight Loss Intervention
The goal of the WL intervention was to produce an 8%–10% loss in total body weight. A caloric restriction of 500–1,000 kcal/d (<30% of calories from fat) was implemented based on the participant's baseline weight. Total caloric needs were determined by multiplying the participant's baseline weight (kg) by 25. From this, the dietitian made the required caloric adjustments to produce a negative energy intake resulting in a loss of 0.5–1 kg of body weight per week.
Weight Loss and Exercise Intervention
Participants in this intervention arm received both the nutrition intervention and an individualized aerobic exercise program. Therefore, the weight loss goal for this group was similar to the WL group. In addition, this group performed exercise nearly 5 days per week for about 45 minutes at a heart rate range of 65%–75% of maximal heart rate as determined during a maximal capacity aerobic test (25). Three sessions were supervised in our facility and two were unsupervised. The exercise program was progressive such that by the last 8 weeks, exercise sessions were performed at about 45 minutes and the intensity of the exercise was raised to 75% of their maximal heart rate. Participants wore a polar heart rate monitor (FS1; Polar Electro, Kempele, Finland) for each exercise session, and at the end of each session, an average heart rate, exercise duration, and rating of perceived exertion were recorded. Exercise sessions consisted of treadmill or outside brisk walking as the primary mode of exercise and cycling as a secondary mode. Each participant was issued a personal binder with exercise logs to record exercise session data (ie, heart rate, duration, and intensity), and the exercise logs were reviewed weekly for exercise adherence.
Body Weight and Lean Mass
Body weight was measured weekly using a Scale Tronix electronic scale (Tronix Inc., Wheaton, IL). Dual-energy x-ray absorptiometry (DXA; GE Lunar Prodigy scanner, Encore software 2005; General Electric, Milwaukee, WI) was used to measure changes in total and regional fat mass (FM) (26) and fat-free mass (FFM), including appendicular (lower and upper limb) lean mass.
Thigh Muscle Cross-Sectional Area
Computed tomography (CT) images were acquired using a GE Hi-speed multiple-slice scanner (General Electric) as previously described (27). Thigh and abdominal muscle cross-sectional area (CSA) was quantified using SliceOmatic software version 3 (Tomovision, Montréal, Canada). Skeletal muscle attenuation was measured for each participant as the mean attenuation value between 0 and 100 Hounsfield Units (HU) as a marker of skeletal muscle lipid content (27,28). Normal-density muscle (NDM), containing lower lipid content (35–100 HU) and low-density muscle (LDM), containing high lipid content (0–35 HU), were also quantified (28). Muscle CSA and muscle attenuation (28) were quantified for the quadriceps and hamstring muscles (29) and for the psoas major and the erector spinae muscles that stabilize the spinal column in the abdominal cavity.
Skeletal Muscle Fiber Size
Participants had a percutaneous biopsy of the left vastus lateralis at the Clinical Translation Research Center as previously described (30). Muscle specimens were trimmed of adipose and connective tissue, frozen in liquid nitrogen and stored at −80°C. Skeletal muscle fiber CSA was measured in type I (high oxidative), type IIA (aerobic or glycolytic), and type IIX (low oxidative) fibers using immunohistochemical staining (25,31). Images were obtained for analysis using a Leica DM 4000b light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Ten muscle fibers were randomly chosen for each fiber type (I, IIA, IIX) and the area was computed using manual planimetry to outline each muscle fiber (Northern Exposure imaging software; MVIA Inc., Monaca, PA). In a subset of six participants, fiber CSA in 50 type I and 50 type IIA fibers was measured, and the mean ± standard error difference compared with the mean fiber CSA measured in 10 fibers was less than 1.0% ± 1.0% for type I fibers and 3.3% ± 3.1% for type IIA fibers. Thus, a measure of fiber CSA in 10 fibers is representative of the sample obtained.
Statistical Analysis
Data are presented in terms of mean and standard error of the mean. One-way analyses of variance (ANOVAs) were performed to compare baseline characteristics between groups, including age, body weight, BMI, FM, FFM, and percent FM. Repeated measures ANOVAs were performed on the dependent variables as a function of group (WL and WL/EX) and time (pre- and postintervention). Pairwise comparisons using the Bonferroni adjustment for multiple comparisons were conducted on the within-subject factor to discriminate means when ANOVA yielded significant results. Statistical analyses were performed using SPSS version 15 (SPSS Inc., Chicago, IL) software, with statistical significance defined as p ≤ .05.
RESULTS
Participants
Twenty-five participants with IGT or IFG were randomized into WL (7 women, 4 men) and WL/EX (5 women, 4 men) intervention groups. Another 9 participants (4 women, 5 men) with newly diagnosed T2DM were enrolled into WL/EX by default. The IGT and T2DM groups had similar baseline characteristics and responses to the WL/EX intervention (data not shown). Hence, their results were collapsed. Mean age was similar in both groups (WL, 68.4 ± 1.5 years; WL/EX, 66.4 ± 1.1 years). Five participants dropped out (1 WL, 4 WL/EX) of the study for medical or personal issues, and only those who completed the interventions were included in the analyses. Participants in the WL/EX intervention exercised an average of 3.7 ± 1.0 sessions per week (supervised 2.49 ± 1.0; unsupervised 1.29 ± 1.0) at an estimated 40 ± 2.3 minutes per session, with the primary mode being treadmill or outside walking (97% ± 2.5%).
Body Weight and Total Body Composition Changes
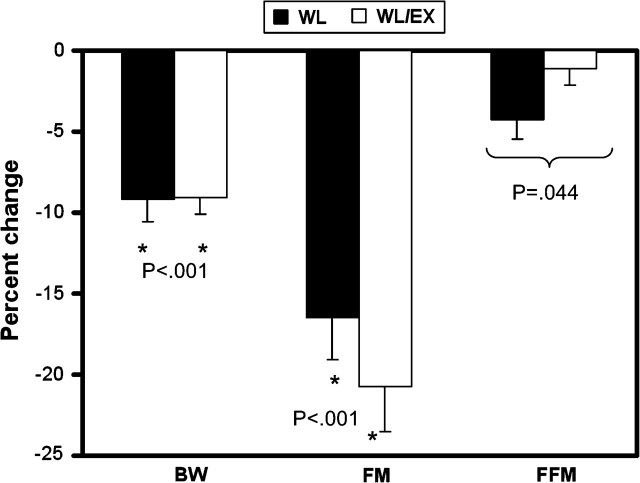
Body weight decreased significantly in both groups (WL, −9.1% ± 1.0%; WL/EX, −9.2% ± 1.0%). They also showed similar decreases in BMI and FM (Table 1). An interaction effect was observed for FFM, with the WL group having lost significantly more FFM than the WL/EX group (Table 1; Figure 1). Although both groups had similar decreases in body weight and FM, the WL group had a larger decrease in FFM.
Table 1.
Body Composition and Regional Changes in Muscle Mass as Measured From DXA and Computed Tomography
| WL, N = 11 |
WL/EX, N = 18 |
Between Group | Within Group |
||||
| Pre | Post | Pre | Post | WL | WL/EX | ||
| Body composition | |||||||
| BMI (kg/m2) | 32.9 ± 1.0 | 28.8 ± 1.0 | 31.9 ± 1.0 | 28.8 ± 1.0 | .438 | .001 | .001 |
| FFM (kg) | 47.3 ± 2.5 | 45.3 ± 2.5 | 51.3 ± 2.7 | 50.7 ± 2.6 | .044 | .007 | .075 |
| FM (kg) | 38.4 ± 2.0 | 32.2 ± 2.1 | 35.6 ± 1.9 | 28.6 ± 2.0 | .528 | .001 | .001 |
| % FM | 43.6 ± 1.8 | 40.1 ± 2.2 | 39.9 ± 1.8 | 34.7 ± 2.1 | .205 | .001 | .001 |
| Appendicular skeletal muscle mass (kg) | |||||||
| Upper limb | 5.0 ± .43 | 5.6 ± 3.7 | 5.7 ± .42 | 5.6 ± .38 | .352 | .707 | .305 |
| Lower limb | 15.7 ± 1.0 | 15.2 ± 1.0 | 17.1 ± 1.0 | 16.8 ± 1.0 | .397 | .030 | .153 |
| Trunk | 22.6 ± 1.1 | 21.6 ± 1.2 | 24.8 ± 1.3 | 24.5 ± 1.1 | .191 | .031 | .352 |
| Thigh muscle mass (cm2) | |||||||
| CSA | 108.4 ± 5.5 | 102.8 ± 5.3 | 124.7 ± 7.6 | 120.3 ± 6.9 | .525 | .001 | .003 |
| NDM | 81.1 ± 5.6 | 74.5 ± 4.7 | 93.4 ± 6.5 | 90.9 ± 6.6 | .021* | .002 | .114 |
| LDM | 27.3 ± 2.1 | 28.2 ± 2.7 | 31.3 ± 2.6 | 29.3 ± 2.4 | .074 | .499 | .047 |
| Quadriceps | 56.3 ± 3.3 | 53.8 ± 3.1 | 60.2 ± 3.9 | 58.4 ± 3.6 | .469 | .007 | .022 |
| Hamstrings | 50.6 ± 2.7 | 48.5 ± 2.7 | 63.2 ± 3.7 | 60.8 ± 3.5 | .863 | .018 | .030 |
| Abdominal muscle mass (cm2) | |||||||
| Psoas major | 12.1 ± 1.1 | 12.3 ± 1.1 | 14.4 ± 1.0 | 14.2 ± 1.0 | .135 | .359 | .230 |
| Erector spinae | 15.4 ± 1.1 | 15.1 ± 1.0 | 16.2 ± 1.3 | 15.9 ± 1.1 | .852 | .566 | .656 |
Notes: Values are means ± SE. CSA = cross-sectional area; DXA = dual-energy x-ray absorptiometry; FM = fat mass; FFM = fat-free mass; LDM = low-density muscle; NDM = normal density muscle; WL = weight loss; WL/EX = weight loss combined with exercise. Bold values indicate significance.
After a natural logarithmic transformation was performed, a significant between-group change was found.
Figure 1.
Percent change in body weight (BW), and whole-body fat mass (FM) and fat-free mass (FFM) as measured by dual-energy x-ray absorptiometry. BW and FM decreased similarly in both the weight loss (WL) and weight loss combined with exercise (WL/EX) groups (“*” significant within-group effect). A significant interaction effect was observed for FFM, with the WL group showing a decrease in FFM, whereas the WL/EX group remained relatively unchanged.
Regional Muscle Mass Changes
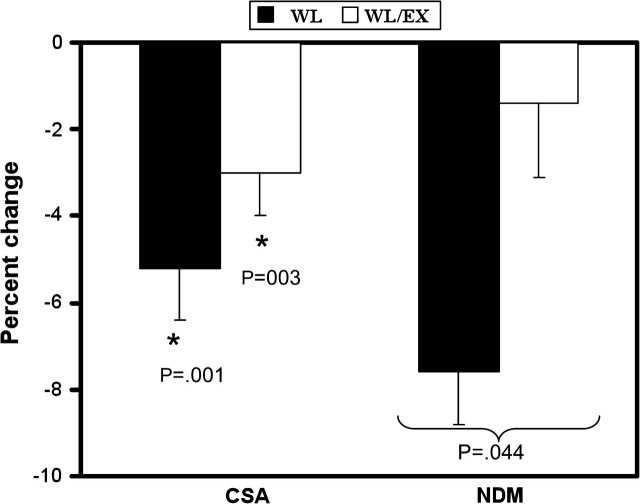
Within-group post hoc analysis revealed that lower limb and trunk FFM decreased significantly within the WL group but not in the WL/EX group (Table 1). No changes were observed for upper limb FFM for either group. Also no changes were observed for the erector spinae or psoas major muscles of the abdomen. Thigh muscle CSA determined by CT decreased significantly in both groups (Table 1; Figure 2), and a similar decrease was observed for quadriceps CSA and hamstring CSA for both groups, suggesting that both muscle areas displayed similar decreases. We also examined changes in NDM and LDM, the latter being muscle containing greater lipid content. An interaction effect was observed for NDM, with the WL group having lost significantly more NDM muscle mass than the WL/EX group (Table 1; Figure 2). We did not find significant changes in LDM of the thigh.
Figure 2.
Percent changes in thigh muscle cross-sectional area (CSA) and thigh normal-density muscle (NDM) area as measured by computed tomography. Thigh CSA significantly decreased for both groups (“*” significant within-group effect). A significant interaction effect was found for NDM after a logarithmic transformation was performed, with the weight loss (WL) group showing a decrease in NDM, whereas the weight loss combined with exercise (WL/EX) group remained relatively unchanged.
Skeletal Muscle Fiber Area
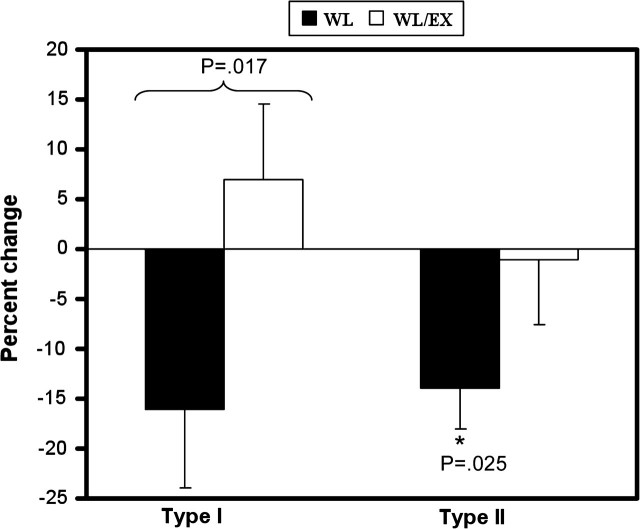
An interaction effect was observed for CSA of the type I fibers, with the WL group having lost significantly more CSA than the WL/EX group (Table 2; Figure 3). A significant within-group effect was found for type II (IIA and IIX) muscle fiber CSA and post hoc analysis revealed that type II muscle fiber area decreased significantly within the WL group but remained unchanged in the WL/EX group (Table 2; Figure 3). An analysis of specific type II fibers subgroups indicated that neither type IIA fiber CSA nor type IIX fiber CSA was significantly altered (Table 2). Similarly, the proportion of all three fiber types did not change for either group.
Table 2.
Changes in Muscle Fiber Size and Type as Measured From Percutaneous Muscle Biopsy
| WL, N = 11 |
WL/EX, N = 18 |
Between Group | Within Group |
||||
| Pre | Post | Pre | Post | WL | WL/EX | ||
| Fiber area (μm2) | |||||||
| Type I | 6,913 ± 706 | 5,584 ± 422 | 6,430 ± 533 | 6,648 ± 537 | .017 | .031 | .597 |
| Type II | 5,816 ± 799 | 4,749 ± 429 | 5,864 ± 421 | 5,561 ± 384 | .231 | .025 | .442 |
| Type IIA | 6,678 ± 888 | 5,646 ± 531 | 6,320 ± 424 | 5,833 ± 379 | .457 | .121 | .270 |
| Type IIX | 4,307 ± 603 | 3,514 ± 364 | 4,595 ± 399 | 5,060 ± 613 | .090 | .166 | .349 |
| Fiber percentage (%) | |||||||
| Type I | 51.9 ± 12.2 | 48.6 ± 9.9 | 50.8 ± 11.9 | 50.3 ± 12.7 | .641 | .545 | .597 |
| Type IIA | 39.6 ± 11.2 | 42.6 ± 8.5 | 41.6 ± 10.1 | 44.8 ± 11.4 | .958 | .536 | .422 |
| Type IIX | 8.3 ± 5.9 | 9.9 ± 9.7 | 9.1 ± 8.6 | 6.5 ± 8.1 | .245 | .633 | .963 |
Note: WL = weight loss; WL/EX = weight loss combined with exercise. Values are means ± SE, p < .05. Bold values indicate significance.
Figure 3.
Percent change in thigh muscle fiber area as measured by percutaneous muscle biopsy. Type I muscle fiber area decreased in the weight loss (WL) group and remained unchanged in the weight loss combined with exercise (WL/EX) group (significant interaction effect). A significant within-group change was observed for the WL group for type II muscle fiber area, whereas the WL/EX group remained relatively unchanged (“*” significant within group effect).
DISCUSSION
A primary outcome of this study was that intentional diet-induced weight loss resulted in a significant loss of muscle mass concomitant to the loss of body fat in older overweight to obese adults. These findings are consistent with previous data in younger (14,29) and older individuals (32) demonstrating a loss of muscle mass following caloric restriction. This suggests that diet-induced weight loss in older adults, who are more prone to sarcopenia, may accelerate the loss of muscle mass (11). The potentially negative effects of intentional weight loss on the loss of muscle mass is particularly relevant to older adults who are becoming more obese, while at the same time losing muscle mass (33,34).
A second important finding in this study was that moderate aerobic exercise attenuated the loss of lean muscle mass induced by energy restriction. A novel aspect of this study was the use of different but complimentary methods to assess the effects of weight loss and exercise on muscle mass. Examination of whole body composition changes using DXA revealed that both the WL and the WL/EX groups lost a similar amount of weight and body fat. However, there was a fourfold greater loss of FFM with WL alone compared with WL/EX. This attenuated loss of muscle mass did not translate to changes in thigh muscle CSA on CT; the loss of total thigh, quadriceps, and hamstring muscle CSA was not different between WL and WL/EX groups. The reasons for this apparent discrepancy are not clear. It is possible that including more participants would have increased our ability to detect significant between-group differences in the loss of muscle mass on CT. Another novel finding was the ability of exercise to reduce the loss of NDM assessed by CT, which represents a measure of “leaner” muscle containing lower lipid concentrations (35). We interpret these findings to reflect a significantly greater maintenance of leaner muscle with the addition of exercise to weight loss; more of the muscle that is lost with weight loss alone is that containing higher amounts of lipid.
Another key finding of this study was that weight loss reduced the size of individual muscle fibers and that moderate exercise attenuated this loss in muscle fiber size. The size of type I, high-oxidative, muscle fibers was decreased by nearly 20% by weight loss alone. This loss of type I fiber CSA was completely prevented by the addition of moderate aerobic exercise. Weight loss also elicited a significant decrease in the type II fiber CSA. Although we did not detect a significant between-group difference in the change in type II fiber CSA, possibly owing to inadequate sample size, there was no within-group decrease in the CSA of type II fibers in those who performed exercise. There was no change observed in either of the specific type II fiber types (IIA or IIX). This may be related to a diminished statistical power for the nonsignificant change for the type IIA and IIX muscle fibers. This decrease in specific myofiber size due to energy restriction–induced weight loss in older adults is a novel and important extension of previous studies examining changes in lean body mass or muscle mass at the whole-body or tissue level in younger individuals (14,29,32,36).
This study was not without limitations. We did not have a control group, although every participant served as his or her own control in this repeated measures design. Another limitation was the relatively small number of participants, which precluded us from determining meaningful gender differences in these responses. It is also possible that the effects of weight loss and moderate exercise differ between overweight and obese individuals. Examination of the effects of the combination of resistance training and diet-induced weight loss as well as protein intake is warranted. Moreover, the inclusion of a muscular strength or power test may have provided insight into changes of muscle function that may accompany decreased muscle mass with weight loss. Future studies should be directed at these important related questions.
In summary, weight loss induced by energy restriction decreases muscle mass in older adults. However, the addition of moderate aerobic exercise to a weight loss program has a powerful effect to preserve muscle mass. These results were consistently observed using a variety of methods, including whole body composition, direct measure of muscle mass using imaging, and muscle cell size assessed by muscle biopsy. The implications of these novel findings are that diet-induced weight loss alone may have adverse effects on skeletal muscle in older adults at risk for the development of muscle weakness and disability. Therefore, this study advocates a program of increased physical activity in older adults who are trying to lose weight.
Acknowledgments
We thank Krista Clark for her expert direction of the diet-induced weight loss program and Steve Anthony for his assistance with the exercise intervention. We also thank the American Diabetes Association (Clinical Research Award and National Institute on Aging R01, A620128), the Clinical Translation Research Center IP30DK46204), and the Obesity and Nutritional Research Center (5-M01RR00056) for their support.
References
- 1.Holloszy JO. The biology of aging. Mayo Clin Proc. 2000;75(suppl):S3–S8. discussion S8–9. [PubMed] [Google Scholar]
- 2.Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 3.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 4.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age Ageing. 1999;28(6):513–518. doi: 10.1093/ageing/28.6.513. [DOI] [PubMed] [Google Scholar]
- 5.Wickham C, Cooper C, Margetts BM, Barker DJ. Muscle strength, activity, housing and the risk of falls in elderly people. Age Ageing. 1989;18(1):47–51. doi: 10.1093/ageing/18.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–67. doi: 10.1093/gerona/50a.special_issue.64. Spec No. [DOI] [PubMed] [Google Scholar]
- 7.Bendall MJ, Bassey EJ, Pearson MB. Factors affecting walking speed of elderly people. Age Ageing. 1989;18(5):327–332. doi: 10.1093/ageing/18.5.327. [DOI] [PubMed] [Google Scholar]
- 8.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337(18):1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 10.Zoico E, Di Francesco V, Guralnik JM, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28(2):234–241. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–878. doi: 10.1093/ajcn/82.4.872. quiz 915–916. [DOI] [PubMed] [Google Scholar]
- 12.Harridge SR, Young A. Skeletal Muscle. London, UK: Wiley; 1998. [Google Scholar]
- 13.Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional age differences in body composition in persons 60+ years of age. J Gerontol A Biol Sci Med Sci. 1995;50(6):M307–M316. doi: 10.1093/gerona/50a.6.m307. [DOI] [PubMed] [Google Scholar]
- 14.Forbes GB. Longitudinal changes in adult fat-free mass: influence of body weight. Am J Clin Nutr. 1999;70(6):1025–1031. doi: 10.1093/ajcn/70.6.1025. [DOI] [PubMed] [Google Scholar]
- 15.Sugawara J, Miyachi M, Moreau KL, Dinenno FA, DeSouza CA, Tanaka H. Age-related reductions in appendicular skeletal muscle mass: association with habitual aerobic exercise status. Clin Physiol Funct Imaging. 2002;22(3):169–172. doi: 10.1046/j.1475-097x.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunstan DW, Daly RM, Owen N, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25(10):1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 17.Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 18.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50(4):655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 19.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 20.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64(3):1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 21.Hikida RS, Staron RS, Hagerman FC, et al. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol A Biol Sci Med Sci. 2000;55(7):B347–B354. doi: 10.1093/gerona/55.7.b347. [DOI] [PubMed] [Google Scholar]
- 22.Skelton DA, Young A, Greig CA, Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc. 1995;43(10):1081–1087. doi: 10.1111/j.1532-5415.1995.tb07004.x. [DOI] [PubMed] [Google Scholar]
- 23.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–1214. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 24.Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72(5):1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 25.Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287(5):E857–E862. doi: 10.1152/ajpendo.00459.2003. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman JM, Vermeulen A. Declining gonadal function in elderly men. Baillieres Clin Endocrinol Metab. 1997;11(2):289–309. doi: 10.1016/s0950-351x(97)80302-3. [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 30.Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14(1):101–102. [PubMed] [Google Scholar]
- 31.Dube JJ, Amati F, Stefanovic-Racic M, Toledo F, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62(8):866–871. doi: 10.1093/gerona/62.8.866. [DOI] [PubMed] [Google Scholar]
- 33.Lexell J. Ageing and human muscle: observations from Sweden. Can J Appl Physiol. 1993;18(1):2–18. doi: 10.1139/h93-002. [DOI] [PubMed] [Google Scholar]
- 34.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50 doi: 10.1093/gerona/50a.special_issue.11. Spec No: 11–16. [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 36.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102(2):634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]