Abstract
Background
Calcium and vitamin D (CaD) supplementation trials including the Women's Health Initiative (WHI) trial of CaD have shown nonsignificant reductions in total mortality. This report examines intervention effects on total and cause-specific mortality by age and adherence.
Methods
The WHI CaD trial was a randomized, double-blind, placebo-controlled trial that enrolled 36,282 postmenopausal women aged 51–82 years from 40 U.S. clinical centers. Women were assigned to 1,000 mg of elemental calcium carbonate and 400 IU of vitamin D3 daily or placebo with average follow-up of 7.0 years.
Results
The hazard ratio (HR) for total mortality was 0.91 (95% confidence interval [CI], 0.83–1.01) with 744 deaths in women randomized to CaD versus 807 deaths in the placebo group. HRs were in the direction of reduced risk but nonsignificant for stroke and cancer mortality, but near unity for coronary heart disease and other causes of death. HRs for total mortality were 0.89 in the 29,942 women younger than 70 years (95% CI, 0.79–1.01) and 0.95 in the 6,340 women aged 70 and older (95% CI, 0.80–1.12; p value for age interaction = .10). No statistically significant interactions were observed for any baseline characteristics. Treatment effects did not vary significantly by season.
Conclusions
In the WHI CaD trial, supplementation did not have a statistically significant effect on mortality rates but the findings support the possibility that these supplements may reduce mortality rates in postmenopausal women. These data can neither support nor refute recommendations for higher dose vitamin D supplementation to reduce cancer or total mortality.
Keywords: Calcium, Vitamin D, Mortality, Cause-specific mortality, Women's Health Initiative
DESPITE predictions that some types of micronutrient supplementation might prevent cancer and cardiovascular disease (CVD) and reduce mortality, the results of large intervention trials of chemoprevention have been largely disappointing to date. Several randomized controlled trials have shown nonsignificant reductions in total mortality among women assigned to vitamin D3, calcium carbonate alone, or the combination of calcium plus vitamin D (1–4). Among the 36,282 women who participated in the Women's Health Initiative (WHI) calcium plus vitamin D (CaD) supplementation trial, the intention-to-treat hazard ratio (HR) for total mortality was 0.91 (95% confidence interval [CI], 0.83–1.01) (1). A recent meta-analysis, in which WHI women contributed nearly two thirds of the data, reported a significant reduction in mortality among nine trials (4) based only on previously published data.
We report here the first thorough evaluation of the possible effects of CaD supplementation on total and cause-specific mortality in the WHI CaD trial. This report also assesses the impact of adherence to CaD supplementation on treatment effect estimates and determines whether HRs for total mortality differed according to levels of selected baseline characteristics, including age.
METHODS
Design Overview
The WHI CaD trial is a randomized, double-blind, placebo-controlled trial designed to determine whether supplementation with calcium (1,000 mg/d) plus vitamin D (400 IU/d) would prevent hip fracture (the primary outcome), other fractures, or colorectal cancer (designated secondary outcome). These findings have been previously published (1,5).
Setting and Participants
Between 1993 and 1998, postmenopausal women aged 50–79 years were enrolled in the WHI randomized clinical trials designed to assess the risks and benefits of hormone therapy (HT) and dietary modification (DM) (6). One year later, participants in either the HT or the DM trial were invited to enroll in the CaD trial. Exclusion criteria for the CaD trial included a predicted survival of less than 3 years; a history of renal calculi or hypercalcemia; or current use of oral corticosteroids or calcitriol, or daily use of 600 IU or more of supplemental vitamin D (7). Personal use of calcium supplements up to 1,000 mg/d or vitamin D up to 600 IU/d was permitted. The protocol and consent forms were approved by the institutional review board at each participating institution. All women provided written informed consent.
Randomization and Intervention
Details regarding execution of the trial have been described previously (1,6). Briefly, using a permuted-block algorithm stratified by clinical center and age, 18,176 women were randomly assigned to receive one tablet of 500 mg of elemental calcium as calcium carbonate combined with 200 IU of vitamin D3 (GlaxoSmithKline. Pittsburgh, PA) twice daily, totaling 1,000 mg of elemental calcium and 400 IU of vitamin D. A total of 18,106 women were randomized to receive an identical-appearing placebo tablet twice daily. Blinding of the study was achieved by bottle labeling.
Outcomes and Follow-Up
Participants were contacted semiannually thereafter to ascertain self-reported medical history updates. When they could not be contacted, information on vital status was sought from previously identified proxy informants, National Death Index searches, and obituary notices. Regardless of adherence, participants were followed up until they died, were lost to follow-up, requested no further contact, or until the study ended. Ninety-seven percent of participants were followed to study completion. Causes of death were determined based on available medical records, autopsy reports, and the death certificate in a blinded fashion by local and central physician adjudicators. For 352 deaths (22.7%), the death certificate was the only available source of information for determining cause of death. Ninety-six percent of reported deaths were centrally adjudicated, 2.5% were locally adjudicated, and 1.5% could not be adjudicated.
Study pills were discontinued if women reported kidney stones, hypercalcemia, dialysis, or the use of calcitriol or daily supplements of more than 1,000 IU of vitamin D during semiannual contacts. During the study, adherence was assessed by weighing returned pill bottles.
An independent data and safety monitoring board reviewed the trial data semiannually. Closeout visits occurred as planned between October 1, 2004, and March 31, 2005, with final outcomes assessed before the treatment assignment was revealed. WHI investigators and National Institutes of Health sponsors all contributed to the study design and execution.
Nested Case-Control Study of Vitamin D Levels
Baseline serum measurements of 25-hydroxyvitamin D were available for 323 women who died and 1,962 living controls in the CaD trial from previous nested case-control studies (1,5). Levels of 25-hydroxyvitamin D were measured using the DiaSorin Liaison chemiluminescent immunoassay system at Diasorin headquarters (Stillwater, MN) as previously described (1,5).
Statistical Analysis
Primary analyses used time-to-event methods, according to the intention-to-treat principle. Mortality rates were compared in the two groups with the use of HRs (with 95 percent CIs) and stratified logrank tests from Cox proportional hazards models (8), stratified according to baseline age and treatment assignment in the HT and DM trials.
In addition, HRs were calculated by categories of causes of death (cardiovascular, cancer, other, and for CVD, coronary heart disease (CHD), and cerebrovascular cause of death separately). Because mortality rates rise markedly with age, treatment effects were examined separately for women younger and older than 70 years, the cut point prespecified in the protocol for defining older women. Analyses according to adherence to study medication were also conducted, with participants censored 6 months after first detection of becoming less than 80% adherent. Inverse probability weighting methods were used to estimate full-adherence HRs adjusting for covariates found to be associated with nonadherence (1,9).
Potential differential effects across categories of important risk factors for mortality were also tested individually with the use of a likelihood ratio test for interaction between the risk factor and the treatment assignment after including both as main effects. Twenty-three subgroup comparisons were tested; thus, about one test would be expected to be statistically significant at the .05 level by chance alone. All reported p values are two sided and were not adjusted for multiplicity.
Odds ratios for CaD supplementation were estimated using logistic regression across tertiles of serum 25-hydroxyvitamin D. The model stratified by randomization assignment in another WHI trial and age group, and adjusted for age, ethnicity, and latitude of clinical center. An interaction p value was calculated for treatment by continuous serum level.
RESULTS
Baseline characteristics were well balanced between the treatment groups (Table 1). Twenty-nine percent of study participants were taking at least 500 mg/d of supplemental calcium on their own. Low calcium intakes (<800 mg/d) were noted in approximately one third of study participants. The mean (SD) duration of follow-up was 7.0 (1.4) years. At time of closeout, 1,551 deaths had occurred, 744 in the CaD intervention group and 807 in the placebo group. The final HR for total mortality was 0.91 (95% CI, 0.83–1.01) (Table 2). CaD HRs were in the direction of reduced risk but nonsignificant for stroke and cancer mortality, whereas HRs were close to unity for CHD and other causes of death.
Table 1.
Characteristics of WHI CaD Participants at WHI Screening by Randomization Arm
| CaD (N = 18,176) |
Placebo (N = 18,106) |
||||
| N | Mean (SD) or % | N | Mean (SD) or % | p Value | |
| Age at screening, y | |||||
| Mean (SD) | 18,176 | 62.4 (7.0) | 18,106 | 62.4 (6.9) | .97 |
| 50–54 | 2,594 | 14.3 | 2,563 | 14.2 | |
| 55–59 | 4,134 | 22.7 | 4,131 | 22.8 | |
| 60–69 | 8,275 | 45.5 | 8,245 | 45.5 | |
| 70–79 | 3,173 | 17.5 | 3,167 | 17.5 | |
| Race/ethnicity | .45 | ||||
| White | 15,047 | 82.8 | 15,106 | 83.4 | |
| Black | 1,682 | 9.3 | 1,635 | 9.0 | |
| Hispanic | 789 | 4.3 | 718 | 4.0 | |
| American Indian/Native American | 77 | 0.4 | 72 | 0.4 | |
| Asian/Pacific Islander | 369 | 2.0 | 353 | 1.9 | |
| Unknown | 212 | 1.2 | 222 | 1.2 | |
| Education at screening | .98 | ||||
| High school or less | 4,286 | 23.6 | 4,289 | 23.7 | |
| Some school after high school | 5,301 | 29.2 | 5,248 | 29.0 | |
| College graduate | 1,862 | 10.2 | 1,856 | 10.3 | |
| Graduate school | 4,693 | 25.8 | 4,687 | 25.9 | |
| Hormone use at annual visit 1 | .23 | ||||
| Never used | 5,814 | 32.0 | 5,690 | 31.4 | |
| Past user | 3,004 | 16.5 | 2,932 | 16.2 | |
| Current user | 9,358 | 51.5 | 9,484 | 52.4 | |
| Daily supplemental calcium use | |||||
| Mean (SD) | 18,176 | 322.6 (479.2) | 18,106 | 326.2 (482.4) | .48 |
| None, mg | 8,009 | 44.1 | 7,871 | 43.5 | |
| <500 | 4,975 | 27.4 | 4,922 | 27.2 | |
| >500 | 5,192 | 28.6 | 5,313 | 29.3 | |
| Daily total calcium (supplements + diet), mg | |||||
| Mean (SD) | 17,821 | 1,148.4 (654.2) | 17,753 | 1,154.3 (658.0) | .40 |
| <800 | 6,104 | 33.6 | 6,003 | 33.2 | |
| 800 to <1,200 | 4,715 | 25.9 | 4,655 | 25.7 | |
| ≥1,200 | 7,002 | 38.5 | 7,095 | 39.2 | |
| Daily vitamin D (supplements + diet), IU | |||||
| Mean (SD) | 17,821 | 365.3 (265.5) | 17,753 | 367.9 (266.0) | .36 |
| <200 | 6,827 | 37.6 | 6,671 | 36.8 | |
| 200 to <400 | 3,379 | 18.6 | 3,423 | 18.9 | |
| 400 to <600 | 4,188 | 23.0 | 4,295 | 23.7 | |
| ≥600 | 3,427 | 18.9 | 3,364 | 18.6 | |
| History of hypertension | .88 | ||||
| Never hypertensive | 6,944 | 38.2 | 6,925 | 38.2 | |
| Untreated hypertensive | 6,686 | 36.8 | 6,692 | 37.0 | |
| Treated hypertensive | 4,546 | 25.0 | 4,489 | 24.8 | |
| Systolic blood pressure | 18,176 | 127.4 (17 .1) | 18,106 | 127.5 (17.2) | .48 |
| Diastolic blood pressure | 18,175 | 75.9 (9.1) | 18,101 | 75.9 (9.0) | .56 |
| Smoking | .50 | ||||
| Never smoked | 9,428 | 52.1 | 9,325 | 51.3 | |
| Past smoker | 7,133 | 39.4 | 7,255 | 39.9 | |
| Current smoker | 1,356 | 7.5 | 1,405 | 7.7 | |
| Alcohol use | .88 | ||||
| Nondrinker | 1,863 | 10.2 | 1,891 | 10.4 | |
| Past drinker | 3,192 | 17.6 | 3,209 | 17.7 | |
| <1 drink per mo | 2,529 | 13.9 | 2,520 | 13.9 | |
| <1 drink per wk | 3,863 | 21.3 | 3,758 | 20.8 | |
| 1 to <7 drinks per wk | 4,683 | 25.8 | 4,706 | 26.0 | |
| ≥7 drinks per wk | 1,910 | 10.5 | 1,900 | 10.5 | |
| Physical activity | |||||
| Mean (SD) METs/wk | 16,546 | 10.7 (12.7) | 16,448 | 10.6 (12.4) | .60 |
| 0–3.00 | 5,517 | 30.4 | 5,478 | 30.3 | |
| >3.00 to <11.75 | 5,463 | 30.1 | 5,477 | 30.2 | |
| ≥11.75 | 5,566 | 30.6 | 5,493 | 30.3 | |
| Body mass index, kg/m2 | |||||
| Mean (SD) | 18,084 | 29.1 (5.9) | 18,011 | 29.0 (5.9) | .24 |
| <25 | 4,745 | 26.1 | 4,833 | 26.7 | |
| 25–29.9 | 6,472 | 35.6 | 6,483 | 35.8 | |
| ≥30 | 6,867 | 37.8 | 6,695 | 37.0 | |
| No. of CVD risk factors | .23 | ||||
| None | 5,544 | 30.5 | 5,579 | 30.8 | |
| 1–2 | 12,337 | 67.9 | 12,195 | 67.4 | |
| >3 | 295 | 1.6 | 332 | 1.8 | |
| History of CVD | 1,173 | 6.5 | 1,221 | 6.7 | .27 |
| History of treated diabetes | 885 | 4.9 | 875 | 4.8 | .87 |
| DM trial arm | .30 | ||||
| Placebo | 7,827 | 43.1 | 7,738 | 42.7 | |
| Intervention | 4,767 | 26.2 | 4,878 | 26.9 | |
| Not randomized | 5,582 | 30.7 | 5,490 | 30.3 | |
| HT trial arm | .74 | ||||
| Placebo | 4,015 | 22.1 | 3,957 | 21.9 | |
| Active | 4,039 | 22.2 | 4,078 | 22.5 | |
| Not randomized | 10,122 | 55.7 | 10,071 | 55.6 | |
| No. of chronic conditions at screening | 18,176 | 1.2 (0.9) | 18,106 | 1.2 (0.9) | .93 |
| Depressive symptoms score | 18,135 | 2.30 (2.55) | 18,057 | 2.33 (2.59) | .18 |
| Physical function score | 18,127 | 81.5 (20.1) | 18,061 | 81.4 (20.3) | .50 |
Note: CaD = calcium and vitamin D; CVD = cardiovascular disease; DM = dietary modification; HT = hormone therapy; MET = metabolic equivalent; WHI = Women's Health Initiative.
Table 2.
Effect of Calcium With Vitamin D Supplementation on Total, CVD, Cancer, and Other Mortality, According to Randomly Assigned Group
| CaD |
Placebo |
||
| Cases (annualized %) | Cases (annualized %) | HR (95% CI) | |
| Follow-up time, y (mean ± SD) | 7.0 ± 1.4 | 7.0 ± 1.4 | |
| Total mortality | 744 (0.58) | 807 (0.63) | 0.91 (0.83–1.01) |
| CVD death | 226 (0.18) | 244 (0.19) | 0.92 (0.77–1.10) |
| CHD death | 130 (0.10) | 128 (0.10) | 1.01 (0.79–1.29) |
| Cerebrovascular death | 54 (0.04) | 60 (0.05) | 0.89 (0.62–1.29) |
| Cancer death | 344 (0.27) | 382 (0.30) | 0.89 (0.77–1.03) |
| Other/unknown death | 174 (0.14) | 181 (0.14) | 0.95 (0.77–1.17) |
| Participants younger than 70 years | |||
| Follow-up time, y (mean ± SD) | 7.1 ± 1.4 | 7.1 ± 1.4 | |
| Total mortality | 466 (0.44) | 517 (0.49) | 0.89 (0.79–1.01) |
| CVD death | 115 (0.11) | 135 (0.13) | 0.85 (0.66–1.08) |
| CHD death | 70 (0.07) | 70 (0.07) | 0.99 (0.71–1.38) |
| Cerebrovascular death | 21 (0.02) | 33 (0.03) | 0.62 (0.36–1.08) |
| Cancer death | 245 (0.23) | 268 (0.25) | 0.91 (0.76–1.08) |
| Other/unknown death | 106 (0.10) | 114 (0.11) | 0.92 (0.70–1.20) |
| Participants 70 or older | |||
| Follow-up time, y (mean ± SD) | 6.7 ± 1.4 | 6.7 ± 1.5 | |
| Total mortality | 278 (1.30) | 290 (1.37) | 0.95 (0.80–1.12) |
| CVD death | 111 (0.52) | 109 (0.51) | 1.01 (0.78–1.32) |
| CHD death | 60 (0.28) | 58 (0.27) | 1.02 (0.71–1.47) |
| Cerebrovascular death | 33 (0.15) | 27 (0.13) | 1.20 (0.72–2.01) |
| Cancer death | 99 (0.46) | 114 (0.54) | 0.86 (0.65–1.12) |
| Other/unknown death | 68 (0.32) | 67 (0.32) | 1.01 (0.72–1.42) |
Note: CaD = calcium and vitamin D; CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio.
Intention-to-treat HRs by age at baseline (<70 vs ≥70 years) suggested lower HRs among younger women for total, stroke, and other causes of death. The HRs for total mortality were 0.89 in women younger than 70 (95% CI, 0.79–1.01) compared with 0.95 in women of ages 70 and older (95% CI, 0.80–1.12; p value for age interaction = .10). HRs for cancer death were similar across age categories.
At trial closure, 76% of women enrolled were still taking study medications and 59% were taking at least 80% of study pills. When participants were censored 6 months after first detection of adherence less than 80%, HRs were 0.87 for total mortality (95% CI, 0.73–1.04), 0.85 for cancer mortality (95% CI, 0.66–1.09), and 0.57 (95% CI, 0.30–1.09) for stroke mortality. HRs suggested greater benefit for adherent women younger than 70 years, as compared with older adherent women (data not shown). Tests for age interaction were statistically significant for total mortality (p = .02) but not for the cause-specific categories. Estimated full-adherence HRs based on the inverse probability weighting method were similar to those for adherent women and all CIs included 1.0.
Intention-to-treat HRs did not vary significantly for any of the 23 baseline risk factors examined for mortality. In addition to the subgroups shown in Table 3, no significant differences were observed by subgroups of education, alcohol intake, history of diabetes, depressive symptoms, physical function score, and disability in activities of daily living, or randomization in the dietary modification trial. In models adjusting for season as a time-dependent variable, risk of mortality was increased during winter months, with a HR of 1.14 (95% CI, 0.99–1.32). Treatment effects did not vary significantly by season at time of death (p value for interaction = .20), although HRs were observed to be reduced in winter (HR = 0.81; 95% CI, 0.66–0.98), summer (HR = 0.87; 95% CI, 0.71–1.06), and autumn (HR = 0.93; 95% CI, 0.77–1.13), but not in spring (HR = 1.10; 95% CI, 0.89–1.36).
Table 3.
HRs Relating CaD to Risk of Total Mortality Stratified by Selected Baseline Characteristics
| CaD (N = 18,176) |
Placebo (N = 18,106) |
|||||
| No. of Deaths | % | No. of Deaths | % | HR (CI)* | p Value for Interaction† | |
| Ethnicity | .30 | |||||
| White | 607 | 0.57 | 679 | 0.64 | 0.89 (0.80–0.99) | |
| Black | 79 | 0.68 | 89 | 0.78 | 0.91 (0.67–1.23) | |
| Hispanic | 23 | 0.42 | 11 | 0.22 | 2.28 (1.07–4.87) | |
| American Indian | 5 | 0.93 | 4 | 0.79 | 0.84 (0.16–4.48) | |
| Asian/Pacific Islander | 18 | 0.73 | 12 | 0.51 | 1.60 (0.75–3.43) | |
| Other/Unknown | 12 | 0.83 | 12 | 0.81 | 0.90 (0.45–1.80) | |
| HT use at annual visit 1 | .14 | |||||
| Never used | 280 | 0.69 | 275 | 0.69 | 1.00 (0.84–1.18) | |
| Past user | 118 | 0.56 | 155 | 0.75 | 0.74 (0.58–0.94) | |
| Current user | 346 | 0.52 | 377 | 0.57 | 0.93 (0.80–1.07) | |
| Calcium supplementation, mg | .64 | |||||
| None | 366 | 0.64 | 369 | 0.66 | 0.97 (0.84–1.12) | |
| <500 | 199 | 0.57 | 226 | 0.66 | 0.86 (0.71–1.04) | |
| ≥500 | 179 | 0.50 | 212 | 0.58 | 0.87 (0.71–1.06) | |
| Baseline total calcium (supplements) | .20 | |||||
| <800 | 282 | 0.65 | 313 | 0.74 | 0.88 (0.75–1.04) | |
| 800 to <1,200 | 186 | 0.56 | 204 | 0.63 | 0.88 (0.72–1.08) | |
| ≥1,200 | 259 | 0.53 | 269 | 0.54 | 0.98 (0.83–1.17) | |
| Baseline total vitamin D (supplements) | .59 | |||||
| <200 | 275 | 0.56 | 303 | 0.64 | 0.88 (0.74–1.03) | |
| 200 to <400 | 144 | 0.60 | 150 | 0.62 | 0.98 (0.78–1.23) | |
| 400 to <600 | 170 | 0.58 | 189 | 0.63 | 0.92 (0.75–1.14) | |
| ≥600 | 138 | 0.58 | 144 | 0.62 | 0.93 (0.73–1.17) | |
| Latitude of clinical center | .70 | |||||
| Southern: <35° north | 232 | 0.60 | 255 | 0.67 | 0.89 (0.74–1.06) | |
| Middle: 35°–40° north | 209 | 0.60 | 238 | 0.69 | 0.86 (0.71–1.03) | |
| Northern: >40° north | 303 | 0.55 | 314 | 0.57 | 0.96 (0.82–1.13) | |
| Blood pressure | .39 | |||||
| Treated | 237 | 0.77 | 278 | 0.92 | 0.84 (0.70–1.00) | |
| ≥140/90 | 305 | 0.64 | 307 | 0.65 | 0.98 (0.84–1.15) | |
| Normal | 202 | 0.40 | 222 | 0.45 | 0.90 (0.75–1.09) | |
| Smoking | .20 | |||||
| Never smoked | 326 | 0.49 | 322 | 0.48 | 1.01 (0.86–1.17) | |
| Past smoker | 300 | 0.59 | 355 | 0.71 | 0.83 (0.71–0.97) | |
| Current smoker | 108 | 1.11 | 120 | 1.28 | 0.87 (0.67–1.13) | |
| Physical activity (METs/wk) | .06 | |||||
| ≤3.00 | 237 | 0.63 | 278 | 0.75 | 0.85 (0.71–1.01) | |
| >3.00 to <11.75 | 218 | 0.58 | 249 | 0.66 | 0.88 (0.73–1.06) | |
| ≥11.75 | 188 | 0.49 | 179 | 0.47 | 1.03 (0.84–1.26) | |
| No. of CVD risk factors | .59 | |||||
| None | 130 | 0.32 | 153 | 0.38 | 0.85 (0.67–1.08) | |
| 1–2 | 596 | 0.69 | 616 | 0.73 | 0.95 (0.85–1.07) | |
| ≥3 | 18 | 0.89 | 38 | 1.70 | 0.49 (0.27–0.87) | |
| Body mass index, kg/m2 | .49 | |||||
| <25 | 197 | 0.58 | 216 | 0.63 | 0.93 (0.76–1.12) | |
| 25 to <30 | 232 | 0.51 | 261 | 0.57 | 0.89 (0.74–1.06) | |
| ≥30 | 315 | 0.66 | 328 | 0.70 | 0.93 (0.80–1.09) | |
| History of CVD | .90 | |||||
| No | 645 | 0.54 | 694 | 0.58 | 0.92 (0.82–1.02) | |
| Yes | 99 | 1.25 | 113 | 1.38 | 0.91 (0.70–1.20) | |
| Number of chronic conditions‡ | .67 | |||||
| None | 95 | 0.32 | 107 | 0.36 | 0.86 (0.65–1.14) | |
| 1 | 300 | 0.52 | 298 | 0.52 | 1.00 (0.86–1.18) | |
| 2 | 244 | 0.72 | 283 | 0.84 | 0.86 (0.72–1.02) | |
| 3 or more | 105 | 1.49 | 119 | 1.73 | 0.87 (0.66–1.13) | |
| Self-reported health status | .13 | |||||
| Excellent | 84 | 0.39 | 79 | 0.36 | 1.14 (0.83–1.55) | |
| Very good | 296 | 0.52 | 305 | 0.54 | 0.93 (0.79–1.09) | |
| Good | 273 | 0.67 | 304 | 0.75 | 0.89 (0.76–1.05) | |
| Fair | 79 | 0.99 | 106 | 1.28 | 0.79 (0.59–1.06) | |
| Poor | 12 | 2.31 | 13 | 3.07 | 0.51 (0.18–1.43) | |
Notes: CaD = calcium and vitamin D; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; HT = hormone therapy; MET = metabolic equivalent.
HRs and 95% CIs are derived from Cox proportional hazards models stratified by age and randomization in the WHI dietary modification or HT trial, or both.
p values are for the interaction between the subgroup variable and treatment assignment.
Number of chronic conditions includes self-report of coronary heart disease, congestive heart failure, treated diabetes, hypertension (treated or untreated), hip fracture, emphysema, cancer, or arthritis. HT at annual visit 1 includes randomized treatment assignment in the WHI HT trial and personal use of HT.
In the nested case-control study, CaD intervention effects did not vary significantly by baseline levels of serum 25-hydroxyvitamin D (p = .66), although the odds ratio was in the direction of benefit for women in the lowest tertile (Table 4). Compared with women in the highest tertile of serum 25-hydroxyvitamin D, there was a significantly increased risk for death for women in the middle and low tertiles. The odds ratio for serum 25-hydroxyvitamin D level analyzed as a continuous variable was 0.80 (95% CI, 0.67–0.95) for a difference of 29.9 nmol/L (the interquartile range).
Table 4.
ORs* for Total Mortality According to Tertile of Serum 25-Hydroxyvitamin D Level and CaD Study Group, as Determined in a Nested Case-Control Study
| Main Effect OR† (95% CI) | CaD, Cases/Controls | Placebo, Cases/Controls | OR (95% CI) | p Value for Interaction | |
| Tertile of serum 25-hydroxy vitamin D, nmol/L | .65 | ||||
| ≥52.5 | 1.00 | 53/404 | 50/425 | 1.04 (0.69–1.59) | |
| 35.4–52.4 | 1.42 (1.06–1.91) | 57/301 | 59/296 | 0.96 (0.64–1.45) | |
| <35.4 | 1.46 (1.07–1.99) | 47/270 | 57/266 | 0.79 (0.51–1.23) |
Notes: CaD = calcium and vitamin D; CI = confidence interval; OR = odds ratios.
ORs were derived from logistic regression models stratified by age group and randomization assignment in the hormone therapy or dietary modification trial, or both, and further adjusted for age, ethnicity (white, black, Hispanic, other), latitude of clinical center, and season of blood draw.
Serum 25-hydroxyvitamin D main effects models are also adjusted for CaD treatment assignment.
DISCUSSION
In this large randomized trial of women aged 50–79, CaD supplementation for an average of 7 years was associated with a nonsignificant reduction in the risk of death (9% with 95% CI, 17% reduction to a 1% increase in risk). The evidence was most consistent for a decrease in cancer mortality.
Among the 23 interactions tested, none was statistically significant. Age, as the strongest predictor of mortality in the general population, was of particular interest. Among the women younger than 70 years, CaD supplementation appeared to reduce risks of total, CVD, and cancer death. In older women, only cancer mortality appeared to be reduced. The interaction between CaD supplementation and age was not statistically significant for total mortality overall but was so among adherent women (p = .02).
Because the majority of statistical tests reported herein are not significant at the .05 level, a viable explanation for these results is chance alone. Among the younger women, there was a difference favoring intervention for reduced mortality within the first year, which is difficult to attribute to intervention. A trial designed to confirm this association would require more than 90,000 women aged 50 and older followed for 5 or more years to detect a statistically significant reduction in mortality rates of 9%–10%.
Nonetheless, previous randomized controlled trials, most of which enrolled only older women, have also shown nonsignificant reductions in total mortality. A recent meta-analysis of nine trials testing vitamin D supplementation reported an overall HR of 0.93 (95% CI, 0.87–0.99); however, this point estimate largely reflects the contribution of WHI women who contributed a majority of the person-time. Six of the remaining eight smaller trials showed nonsignificant reductions in total mortality (4). Cause-specific mortality was examined in only one previous trial and showed nonsignificant reductions in cardiovascular, total cancer, and colorectal cancer mortality with very wide CIs (3).
If the results did not occur by chance, multiple pathways could be involved including any elements of the supplements tested: calcium, vitamin D3, or carbonate, or all. Calcium supplementation has been shown in small experimental studies to lower blood pressure (10) and reduce cholesterol levels (11–13). Observational epidemiological studies have shown an association of calcium intake with reduced stroke incidence and mortality (14–16) and inconsistent associations with reduced risk of coronary disease (16,17). Lower bone density has been associated with increased mortality risks (18,19). In the WHI CaD trial, the intervention group had statistically significant but small reductions in low-density lipoprotein, weight, and waist circumference, whereas blood pressure change was slightly increased compared with placebo (20). Although the intervention improved hipbone density by about 1%, it produced no significant differences in CHD or stroke incidence.
Vitamin D insufficiency has now been linked to a broad spectrum of human diseases from cancer to cardiovascular to autoimmune conditions (21). A recent ecological study showed consistently elevated death rates from cancer sites among U.S. women with less solar ultraviolet-B exposure (22). Vitamin D receptors are present in many cell types and 1,25-dihydroxyvitamin D has been shown to favor cell differentiation over proliferation and to inhibit potential for metastasis and invasiveness (23). Serum vitamin D levels were inversely associated with colon cancer risk in the Nurses' Health Study (24), in the WHI CaD trial (6), and with colon cancer mortality in National Health and Nutrition Examination Survey (25). Supplementation with 1,100 IU of vitamin D3 reduced the risk of cancer in a recent small trial of healthy postmenopausal women (26). Two recent prospective studies link levels of serum 25-hydroxyvitamin D below 44.4 nmol/L (27) or 25.2 nmol/L (28) to increased risk of total mortality.
The emerging optimism over vitamin D supplementation for the prevention of cancer (29) is occurring in the absence of confirmation from large prevention trials that can determine the benefits and any currently unknown risks. Proponents argue that the necessary levels of vitamin D supplementation to exert anticancer effects might be 1,500–2,000 IU/d based on selective findings from observational studies (30,31). The optimum dose of vitamin D for reducing cancer or mortality is unknown and, if it exists, can only be determined from future large randomized trials.
The trends toward reductions in risks of both cardiovascular and cancer mortality in this trial, if real, suggest mechanisms that may have broad physiological effects. Vitamin D supplementation has been shown experimentally to increase levels of the anti-inflammatory cytokine interleukin-10, while preventing increases in tumor necrosis factor alpha (32). Calcium and vitamin D supplementation both lower parathyroid hormone levels (PTH), and PTH levels have been associated with an increased risk of mortality in a vitamin D–deficient cohort of the very old age (33). Chronic metabolic acidosis increases with age and declining kidney function even when plasma measures of acid–base balance are normal. Administration of bicarbonate has been shown to reverse acidosis (34,35). Beneficial effects of CaD supplementation on cytokine profiles, PTH, or acidosis might be expected to produce greater benefits in older women, whereas the opposite trend was observed in this trial. However, it is also possible that mortality could be delayed by improvements in these parameters and eventually benefits diminish with disease progression in later life.
Strengths of this trial include its large size, diversity, long duration, excellent retention, and reasonably good adherence for an average of 7 years of follow-up. This study is limited by lack of statistical power for detecting intervention effects on mortality, as it was not designed for this purpose. We did not collect postintervention serum specimens for exploring potential intermediate effects of the intervention that might have influenced mortality. We cannot distinguish between effects of calcium, vitamin D, or carbonate because the intervention combined these ingredients. We do not know whether higher supplement doses of vitamin D would have produced different results.
In conclusion, the WHI CaD trial supports the hypothesis that CaD supplementation provides a modest reduction in rates of total and categories of cause-specific mortality, but the results are too imprecise to be definitive. Moreover, these data can neither support nor refute recommendations for higher dose vitamin D supplementation to reduce cancer or total mortality. This hypothesis merits further consideration by testing alternative underlying mechanisms and possibly by testing higher dose supplements in even larger randomized trials.
Figure 1.
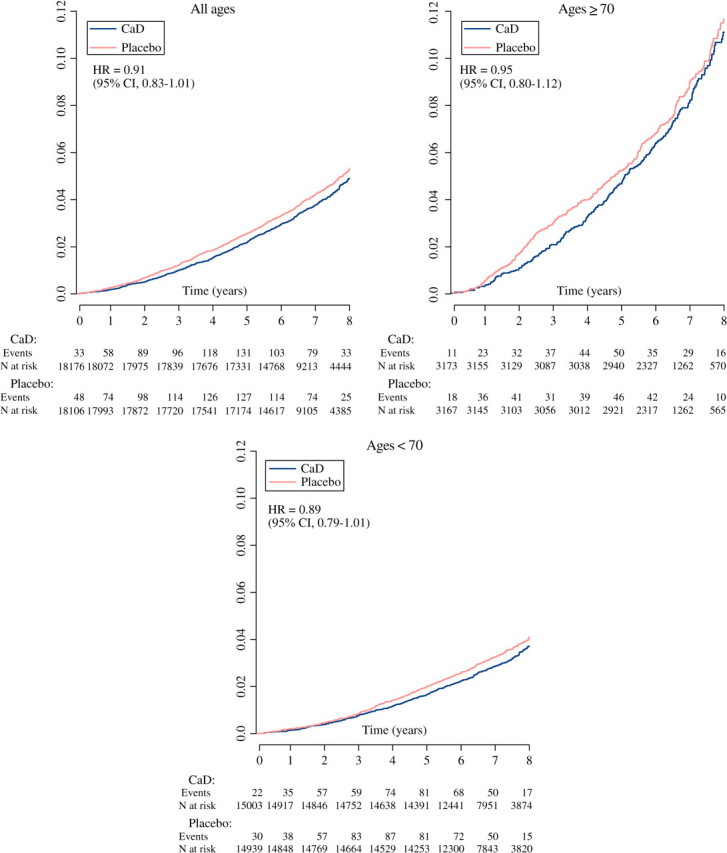
Cumulative mortality Kaplan–Meier curves by age group.
Note: CaD = calcium and vitamin D; CI = confidence interval; HR = hazard ratio.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/.
Acknowledgments
The authors thank the WHI investigators, staff, and study participants for their outstanding dedication and commitment. A list of key investigators involved in this research follows: A full listing of WHI investigators can be found at the following Web site: http://www.whi.org. A.Z.L. affirms that everyone who significantly contributed to this work has been listed in the Acknowledgments. The WHI investigators and National Institutes of Health sponsors all contributed to the design and execution of the study. This work was supported by the National Heart, Lung, and Blood Institute of the U.S. Department of Health and Human Services. The active study drug and placebo were supplied by GlaxoSmithKline Consumer Healthcare (Pittsburgh). Clinical Trials Registration: ClinicalTrials.gov identifier: NCT00000611
References
- 1.Jackson RD, LaCroix AZ, Gass M, et al. for the Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 2.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166:869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomized double blind controlled trial. BMJ. 2003;326:1–6. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 5.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. For the Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 6.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RD, LaCroix AZ, Cauley JA, et al. The Women's Health Initiative Calcium-Vitamin D Trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(suppl 9):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR. Regression analysis and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 9.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–781. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 11.Groot PHE, Grose WFA, Dijkhuis-Stoffelsma R, Fernandes J, Ambagtsheer JJ. The effect of oral calcium carbonate administration on serum lipoproteins of children with familial hypercholesterolaemia (type II-A) Eur J Pediatr. 1980;135:81–84. doi: 10.1007/BF00445899. [DOI] [PubMed] [Google Scholar]
- 12.Denke MA, Fox MM, Schulte MC. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J Nutr. 1993;123:1047–1053. doi: 10.1093/jn/123.6.1047. [DOI] [PubMed] [Google Scholar]
- 13.Bell L, Halstenson CE, Halstenson CJ, Macres M, Keane WF. Cholesterol-lowering effects of calcium carbonate in patients with mild to moderate hypercholesterolemia. Arch Intern Med. 1992;152:2441–2444. [PubMed] [Google Scholar]
- 14.Abbott RD, Curb JD, Rodrigues BL, Sharp DS, Burchfiel CM, Yano K. Effect of dietary calcium and milk consumption on risk of thromboembolic stroke in older middle-aged men. Stroke. 1996;27:813–818. doi: 10.1161/01.str.27.5.813. [DOI] [PubMed] [Google Scholar]
- 15.Iso H, Stampfer MJ, Manson JE, et al. Prospective study of calcium, potassium and magnesium intake and risk of stroke in women. Stroke. 1999;30:1772–1779. doi: 10.1161/01.str.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 16.Umesawa M, Iso H, Date C, et al. For the JACC Study Group. Dietary intake of calcium in relation to mortality from cardiovascular disease: the JACC Study. Stroke. 2006;37:20–26. doi: 10.1161/01.STR.0000195155.21143.38. [DOI] [PubMed] [Google Scholar]
- 17.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, Vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149:151–161. doi: 10.1093/oxfordjournals.aje.a009781. [DOI] [PubMed] [Google Scholar]
- 18.Von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 19.Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 20.Hsia J, Heiss G, Ren H, et al. For the Women's Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 21.Grant WB. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog Biophys Mol Biol. 2006;92:65–79. doi: 10.1016/j.pbiomolbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer. 2006;6:264–272. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 25.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum Vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 26.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 27.Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 29.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective [Editorial] Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA. The 25-hydroxyvitamin D threshold for better health. J Steroid Biochem Mol Biol. 2007;103:614–619. doi: 10.1016/j.jsbmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 32.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin D status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab. 2004;89:5477–5481. doi: 10.1210/jc.2004-0307. [DOI] [PubMed] [Google Scholar]
- 34.Frassetto LA, Morris RC, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271(6 Pt 2):F1114–F1122. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 35.Frassetto L, Morris RC, Jr, Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab. 1997;82:254–259. doi: 10.1210/jcem.82.1.3663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


