Abstract
The effects of exercise training on the nitric oxide synthase (NOS) isoform profile in aging fast-twitch (white gastrocnemius [WG]) and slow-twitch (soleus [SOL]) muscle have not been investigated. Six-month and 27-month male Fischer-344 rats were divided into the following groups: young sedentary (YS), young treadmill exercise trained for 12 weeks, old sedentary (OS), and old exercise trained (OE). Inducible NOS (iNOS) protein expression and activity were significantly higher in OS compared with YS, whereas exercise training significantly reduced iNOS protein and activity levels in the WG. Neuronal NOS protein expression decreased with aging in WG but was upregulated significantly with exercise training in OE for both WG and SOL. Endothelial NOS (eNOS) protein levels were depressed in WG of old rats but were higher in OE than in OS. eNOS was unaffected by aging or exercise in the SOL. Our results indicate that endurance exercise training attenuates age-induced alterations of NOS isoforms with a greater response in fast-twitch compared with slow-twitch muscle.
Keywords: Aging, Skeletal muscle, Exercise, Nitric oxide synthase, nNOS, eNOS, iNOS
AGING in skeletal muscle is characterized by a progressive decline in mass and force generation along with increased susceptibility to injury, oxidative stress, and inflammation (1–9). Indeed, Baldwin and colleagues (9) reported that anti-inflammatory therapy attenuated muscle injury, strength loss, and soreness following eccentric exercise in older individuals. Two expanding branches of Denham Harman's “free radical theory of aging” (10) are the “inflammatory hypothesis of aging” (11,12) and the “iNOS theory of aging” (13). These theories are largely based on direct relationships between chronic elevation of iNOS (i.e., inducible nitric oxide synthase) and other inflammatory proteins with the age-related decline in function of the brain, kidney, and heart (11–13).
Production of nitric oxide (•NO) as well as presence of NOS isoforms in skeletal muscle were first identified by Balon and Nadler (14) and Kobzik and colleagues (15), respectively. Production of •NO at low levels by the Ca2+-dependent constitutive isofoms neuronal NOS (nNOS) and endothelial NOS (eNOS) is involved in regulating skeletal muscle contractions, blood flow, glucose uptake, and metabolism (8,16,17). The nNOS isoform is expressed at higher levels in fast-twitch muscle fibers when compared with slow-twitch fibers and is integrated into the cytoskeleton where it is bound to dystrophin within the dystroglycan complex (17,18). The eNOS isoform is associated with mitochondria in mammalian muscle (17). The iNOS isoform is Ca2+ independent and normally expressed at low levels in skeletal muscle (17). However, iNOS can be dramatically increased by proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1-beta (IL-1ß) (17,19), and the transcription factor nuclear factor-kappaB (NF-ΚB) (20,21). Although •NO is integrated into physiological function at lower levels, higher concentrations, longer duration, critical location, and isoform shift can contribute to formidable pathology (17). The biphasic and dual nature of •NO may explain its ability to act in both protection and pathology.
A series of studies indicate that overproduction of •NO and iNOS may be involved in muscle wasting, or cachexia, often observed with chronic heart failure, muscular dystrophy, cancer, acquired immunodeficiency syndrome (AIDS), sepsis, inflammatory myopathies, and muscle crush injuries (20,22–24). Consistent with the earlier findings, NOS inhibitors have been shown to reduce skeletal muscle inflammation and necrosis (25). Muscle wasting is a hallmark of aging, particularly in fast-twitch muscle fibers (26,27). However, the mechanisms are poorly understood. In addition, nNOS protein expression in skeletal muscle has been shown to decrease with extreme disuse (28–30).
Elevation of iNOS and upstream inflammatory proteins such as TNF-α, IL-1ß, and NF-ΚB have been linked with aging in tissues such as the brain, kidney, and heart (12,13). Aging also exacerbated the inflammatory response to lipopolysaccharide (LPS) (12,31). In addition, Chung and colleagues (12) found that age-related increases of the proinflammatory mediator cytokines, iNOS, and NF-ΚB in kidney, heart, and brain were reversed with caloric restriction, a consistent model of increased longevity. Furthermore, Kayo and colleagues (32) reported an age-related selective upregulation of skeletal muscle messenger RNA (mRNA) involved in inflammation including TNF-α, IL-1ß, cytokine receptors, and NF-ΚB. However, the effect of aging on iNOS protein and activity levels in skeletal muscle is unknown.
A change in the balance or ratio between iNOS and other isoforms has been proposed to occur with aging (8,10). In this model, aging increases iNOS/nNOS ratio, implying a shift from a function of contractile to inflammatory role. Unfortunately, only limited data exist describing age-related changes in iNOS, nNOS, and eNOS levels in skeletal muscle. Capanni and colleagues (33) reported elevated nNOS protein levels in the gracilis muscle of old Wistar rats. These findings appear to be inconsistent with data from disuse models that indicate a suppression of nNOS expression (29). In addition, Richmonds and colleagues (34) found lower nNOS activity in the soleus (SOL) and extendor digitorum longus (EDL) muscles of old Fischer-344 rats compared with young adults. These discrepancies may be due to differences in rat strain or muscle used, as the gracilis is a small helper muscle, involved in hip adduction and knee flexion and not significant in locomotion.
Exercise training reduces the risk for injury and inflammation against mechanical and oxidant perturbations while increasing antioxidant enzyme activities and protective heat shock proteins (35,36). An initial report by Hambrecht and colleagues (23) demonstrated that iNOS and NF-ΚB upregulation from heart failure was reversed in skeletal muscle by exercise training. Thus, we postulate that exercise training would reduce iNOS and upregulate constitutive NOS in skeletal muscle in old rats. Therefore, the purpose of this investigation was to test the hypotheses that (a) iNOS protein expression and activity in fast-twitch rat skeletal muscle would be higher in old rats than young, whereas nNOS and eNOS protein and constitutive NOS activity levels would be lower in muscle from old rats; (b) 12 weeks of treadmill exercise training would attenuate age-induced alterations in iNOS, nNOS, and eNOS in skeletal muscle; and (c) age and exercise differences would be larger in white fast-twitch muscle (e.g., white gastrocnemius [WG]) compared with red slow-twitch muscle (e.g., SOL).
METHODS
Animals
We used 3-month-old and 24-month-old Fischer-344 rats as our young adult and old groups, respectively, at the initiation of our study. Fischer-344 rats are a common National Institutes of Health (NIH) aging model and have an average life span of 702 days. Animals have been purchased from the NIH colony and cared for at the Comparative Biology facility at Texas A&M University in accordance with NIH and the University Laboratory Animal Care Committee standards. Rats were housed in a temperature-controlled (23 ± 2°C) room with a 12:12-hour light–dark cycle. Water and rat chow were provided ad libitum. One half of the rats in each age group ran for 12 weeks (5 d/wk) on a motorized treadmill for 60 min/d, and the other half served as sedentary age-matched controls: young sedentary controls (YS; n = 10), young trained (YE; n = 10), old sedentary controls (OS; n = 10), and old trained (OE; n = 10). Two of the rats died of natural causes in the OS group but none in the remaining groups. After 12 weeks of exercise training, the young and old rats in each age group were euthanized. At the time of sacrifice, rats were 6 and 27 months of age.
Exercise Training Protocol
Rats in the exercise groups ran on a motor-driven treadmill at 15 m/min up a 15° incline, 1 h/d, 5 d/wk for 12 weeks. Rats in the exercise groups were gradually conditioned to perform this level of exercise over the first 5 weeks of the 12-week training program. The intensity was designed to correspond to 75% of VO2max (maximal aerobic capacity) in the old group (37). This exercise regimen has previously been shown to elevate citrate synthase activity, a marker of mitochondrial content, following chronic exercise (35).
Tissue Preparation and Experimental Design
Rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) following the exercise training period in young and old exercise trained and sedentary groups. Animals in the exercise training groups were anesthetized 48 hours following the last bout of exercise training to avoid influence of the last acute exercise bout. Preparation of hind-limb muscles followed the procedure described by Lawler and colleagues (38). The SOL and gastrocnemius muscles were quickly extracted, weighed, and placed in ice-cold phosphate-buffered saline (PBS; pH 7.4). The white and red portions of the gastrocnemius were separated. The SOL (90% type I fibers), WG (92% type IIb), and red gastrocnemius (35% type Ila, 51% type I) muscles (39) were then frozen in liquid nitrogen and stored at –80°C until analyses.
Homogenization Procedure
Gastrocnemius and SOL samples were minced into fine pieces and homogenized (20:1 w/v) in ice-cold (4°C) lysis buffer solution (pH 7.40) containing the following: 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 350 mM NaCl, 20% glycerol, 1% Igepal-CA630, 1 mM MgCl2, 0.1 mM dithiothreitol (DTT), 0.5 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM ethylene glycol tetraacetic acid (EGTA), and protease inhibitor cocktail (Roche, Penzberg, Germany). Minced muscle samples were homogenized (20:1 w/v) using a ground glass on ground glass homogenizer (Bellco Biotechnology, Vineland, NJ) at 4°C and then twice centrifuged (4°C) for 10 minutes at 10,000 × g. Total protein was determined using the Bradford technique. SOL samples were prepared for measurement of nicotinamide adenine dinucleotide phosphate (NADP)-specific isocitrate dehydrogenase and lipid peroxidation in 100 mM Tris buffer (pH 7.4) with 0.13 mM butylated hydroxytoluene added to minimize oxidation of lipids and proteins during the homogenization procedure.
Western Immunoblot Analysis
Protein expression of iNOS, nNOS, and eNOS was determined by western immunoblot analysis. Separating gel (375 mM Tris–HCl, pH 8.8; 0.4% sodium dodecyl sulfate (SDS); 10% acrylamide) and stacking gel (125 mM Tris–HCl, pH 6.8; 0.4% SDS; 10% acrylamide monomer) solutions were made and polymerization was then initiated by tetramethylethylenediamine (TEMED) and ammonium persulfate. Separating and stacking gels were then quickly poured into a Bio-Rad Protein III gel-box (Bio-Rad, Hercules, CA). Total protein was measured for each skeletal muscle homogenate. Then, 30 μg of protein from muscle homogenates was placed in sample buffer (Tris, pH 6.8 with 2% SDS; 30 mM DTT; 25% glycerol), loaded into the wells of the 10% polyacrylamide gels, and electrophoresed at 150 V. The gels were then transferred at 30 V overnight onto a nitrocellulose membrane (Bio-Rad). Membranes were blocked in 5% nonfat milk in PBS with 0.1% Tween-20 for 6 hours. After blocking, membranes were incubated at room temperature in PBS and the appropriate primary antibodies for 12 hours: iNOS (1:7,500) and nNOS (1:500) were purchased from BD Transduction Laboratories (Lexington, KY) and eNOS (1:200) from Santa Cruz Biotechnology (Santa Cruz, CA). Following three washings in PBS with 0.4% Tween-20, membranes were incubated with horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology) in PBS at room temperature for 90 minutes. Following three washes in PBS with 0.4% Tween-20, an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ) was used for visualization. Densitometry (as gray scale relative to background × area) was performed using a Kodak film cartridge and film, a scanner interfaced with a microcomputer, and the NIH Image Analysis 1.62 software program. To ensure equal loading of protein per lane, Ponceau-S staining was performed and assessed for each membrane, and the lane background reading was subtracted from each protein blot density. Membranes were reprobed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an additional load control marker. nNOS, eNOS, and iNOS levels were equated per microgram protein.
NOS Activity Assays
NOS activity was determined by measuring the conversion of [14C]arginine to [14C]citrulline (40). Frozen tissues were homogenized in 6:1 (w/v) HEPES buffer (pH 7.4, 10 mM HEPES, 0.1 mM EDTA, 1 mM DTT, 1 mg/mL phenylmethanesulphonylfluoride (PMSF), 0.32 mM sucrose, 10 μg/mL aprotinin, 10 μg/mL pepstatin A). Protein concentration was measured by the Bradford technique with bovine serum albumin (BSA) as standard (Pierce, Rockford, IL). The crude homogenates were assayed using a kit produced by Stratagene (La Jolla, CA). The isolated [14C]citrulline levels were quantified using a liquid scintillation counter. Enzyme activity is expressed in counts per microgram of total protein to quantify total NOS activity. To differentiate between iNOS activity, which is independent of Ca2+ and calmodulin, and constitutive NOS isoform activity, NOS activity was also measured in the presence of 1.5 mM EGTA and 1.5 mM EDTA in place of CaCl2 and calmodulin in the reaction buffer, respectively. A negative control for the NOS assay was determined in the presence of 1 mM L-NG-nitroarginine methyl ester (NAME) (nonspecific NOS inhibitor). Ca2+/calmodulin-dependent NOS activity (eNOS + nNOS) was calculated as the difference between activities measured in the presence of CaCl2 and that measured in the EDTA/EGTA buffer. Ca2+/calmodulin-independent NOS activity (iNOS) was calculated as the difference between samples assayed in the presence of EGTA/EDTA and in the presence of L-NAME.
Statistics
One-way analyses of variance with Fisher’s LSD used post-hoc were conducted to determine the existence of mean differences among YS, YE, OS, and OE groups. The level of significance was set at p < .05.
RESULTS
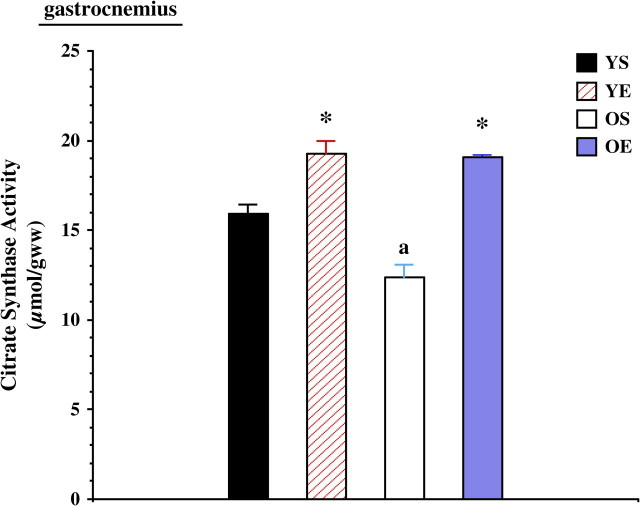
The effectiveness of the exercise training protocol was confirmed by increased heart/body mass ratios and increased citrate synthase activities in skeletal muscle. Heart mass/body mass was increased by exercise training in both young (+18.7%) and old (+16.7%) rats. Citrate synthase activity was significantly lower in the gastrocnemius in old rats, but exercise training significantly increased citrate synthase activity in both the old and the young groups (Figure 1). Exercise training also significantly increased SOL citrate synthase activity in both age groups (data not shown). Muscle mass/body mass ratios in the medial head of the gastrocnemius were 500.9 ± 23.0 mg/kg for YS, 514.8 ± 56.8 mg/kg for YE, 386.9 ± 30.6 mg/kg for OS, and 407.6 ± 19.5 mg/kg for OE. In the SOL muscle, muscle mass/body mass ratios were 421.7 ± 33.5 mg/kg for YS, 507.1 ± 87.3 mg/kg for YE, 360.3 ± 44.8 mg/kg for OS, and 418.3 ± 49.2 mg/kg for OE. Muscle mass decreased with age (p < .01) and trended upward (p = .13) with exercise training. In a parallel series of experiments (27), exercise training resulted in a partial improvement of muscle fiber cross-sectional area of the tibialis anterior muscle.
Figure 1.
Citrate synthase activities in the rat gastrocnemius from young (7 months) sedentary (YS), young exercise trained (YE), old (27 months) sedentary (OS), and old exercise trained (OE) groups. “*” indicates significantly different from the sedentary group. “a” indicates significantly different from young controls.
Protein levels of nNOS were not significantly altered in the WG of old rats (Figure 2a). However, exercise training significantly increased (p < .001) nNOS protein expression of WG by 136% in the young group and 279% in the old group (Figure 2a). Exercise training resulted in a significant increase (p < .05) in nNOS protein levels in the SOL of old (+98%) rats (Figure 2b). SOL nNOS levels trended to be higher in the YE group (+22%) compared with the YS, but this was not statistically significant (p = .09). In addition, the iNOS to nNOS ratio increased with aging by a substantial 181% in the gastrocnemius, whereas exercise training markedly decreased iNOS/nNOS ratio in both young (−89%) and old (−92%) age groups (data not shown).
Figure 2.
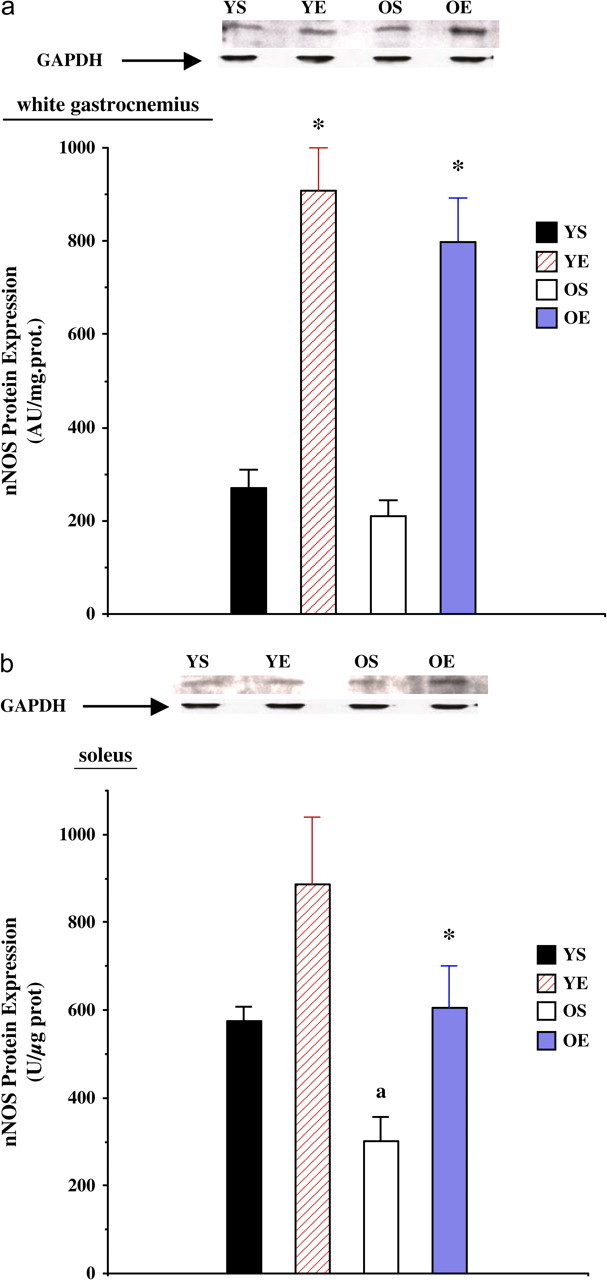
(a) Neuronal nitric oxide synthase (nNOS) protein expression in the white gastrocnemius from young (7 months) sedentary (YS), young exercise trained (YE), old (27 months) sedentary (OS), and old exercise trained (OE) groups. (b) nNOS protein expression in the soleus from YS, YE, OS, and OE groups. Representative nNOS and GAPDH control blots are included. “*” indicates significantly different from the sedentary group.
Protein expression of eNOS was significantly (p < .01) lower in the old (−67%) WG (Figure 3a). Exercise training resulted in higher eNOS protein levels (p < .05) in the WG from both young (+57%) and old (+88%) rats. In contrast, eNOS protein expression was not significantly altered as a function of age in the SOL muscle (Figure 3b). In addition, exercise training resulted in no effect on eNOS protein expression in the SOL.
Figure 3.
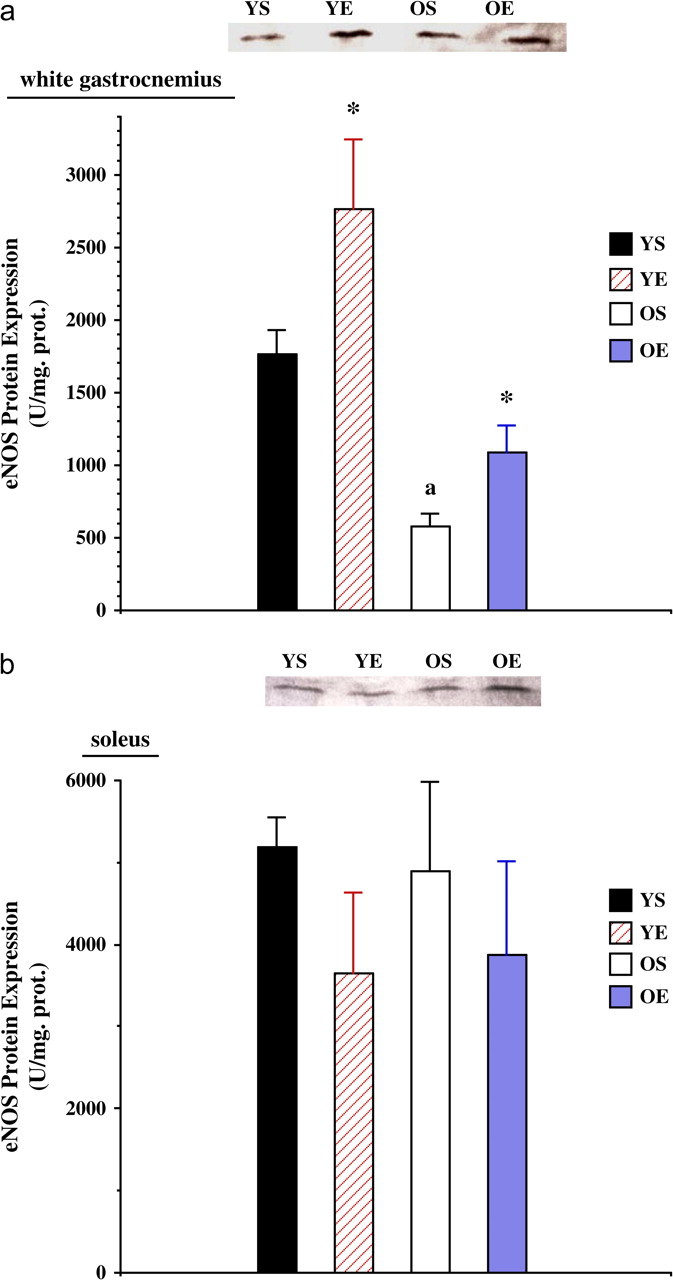
(a) Endothelial nitric oxide synthase (eNOS) protein expression in the white gastrocnemius from young (7 months) sedentary (YS), young exercise trained (YE), old (27 months) sedentary (OS), and old exercise trained (OE) groups. (b) eNOS protein expression in the soleus from YS, YE, OS, and OE groups. Representative eNOS blots are included. “*” indicates significantly different from the sedentary group.
The activity of nNOS + eNOS (constitutive NOS) was significantly lower (−36%) with age (p < .001) in the WG (Figure 4a). Exercise training resulted in a significant increase (+26%) in nNOS + eNOS activity in the WG of the old group (p < .05), with little change in the young group. However, nNOS + eNOS activity was not affected by age differences or exercise training in the SOL (Figure 4b).
Figure 4.
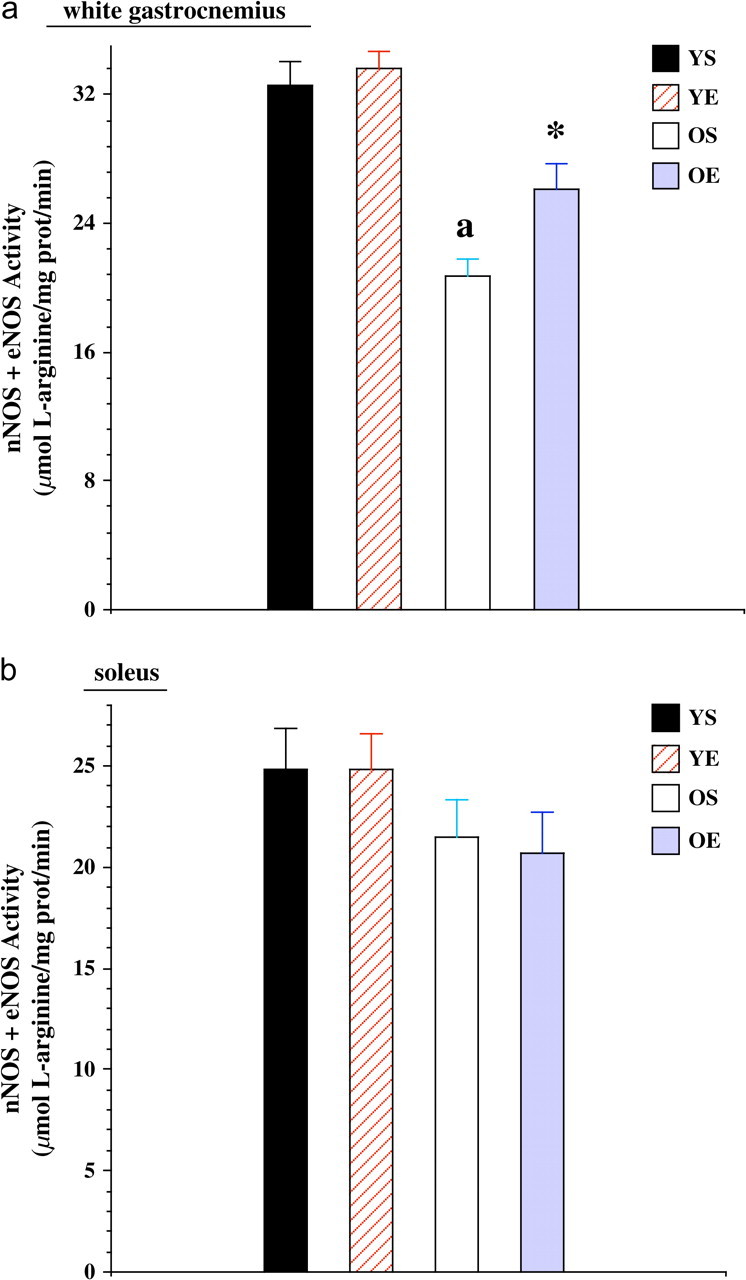
(a) Neuronal nitric oxide synthase (nNOS) + endothelial nitric oxide synthase (eNOS) activity in the white gastrocnemius from young (7 months) sedentary (YS), young exercise trained (YE), old (27 months) sedentary (OS), and old exercise trained (OE) groups. “*” indicates significantly different from the sedentary group. “a” indicates significantly different from young controls. (b) nNOS + eNOS activity in the soleus from YS, YE, OS, and OE groups.
Protein expression of iNOS, assessed by western immunoblot analysis, in the gastrocnemius was 118% greater (p < .05) in old rats than in the young rats (Figure 5a). Exercise training decreased iNOS protein levels (p < .05) by 62.6% in the YE group when compared with young controls. In addition, gastrocnemius iNOS protein expression was also reduced by 67.8% in the OE group when compared with the OS group (p < .01). We were not able to detect iNOS protein expression in the SOL.
Figure 5.
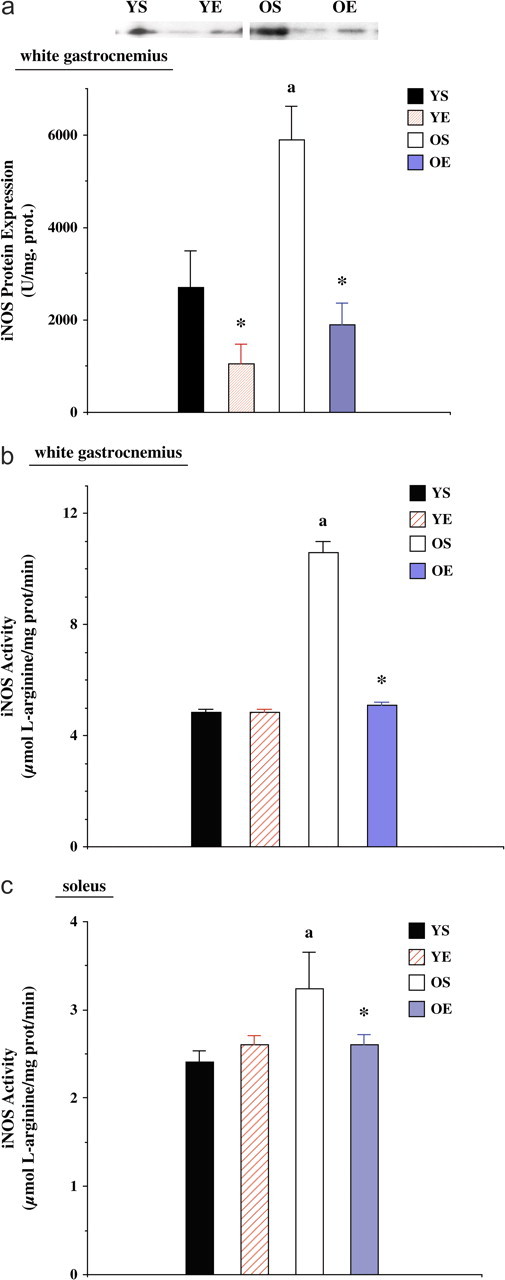
(a) Inducible nitric oxide synthase (iNOS) protein expression in the white gastrocnemius from young (7 months) sedentary (YS), young exercise trained (YE), old (27 months) sedentary (OS), and old exercise trained (OE) groups. Representative protein blots pictured above the graphic. (b) iNOS activity in the white gastrocnemius from YS, YE, OS, and OE groups. (c) iNOS activity in the soleus from YS, YE, OS, and OE groups. “*” indicates significantly different from the sedentary group. “a” indicates significantly different from young controls.
We also measured alterations in skeletal muscle iNOS activity caused by aging and exercise training. iNOS activity in the WG was dramatically increased (+119%) in OS compared with YS group (p < .05; Figure 5b). Exercise training resulted in a significantly lower iNOS activity (−52%) in the old age group (p < .05) but not in the WG samples from the young adult group. Old rats had significantly elevated (p < .05) NOS activity (+34%) in the SOL muscle (Figure 5c). In addition, exercise training significantly decreased SOL iNOS activity (−20%) in old rats to levels similar to that found in young rats.
DISCUSSION
Our study resulted in a number of novel and important findings. (a) Exercise training also increased nNOS protein expression in the aging WG and SOL muscles. (b) Exercise training also elevated eNOS protein levels in the WG but not in the SOL of old rats. (c) Exercise increased activity levels of constitutive NOS (nNOS + eNOS) in aging WG but not in SOL. (d) Increased age resulted in an upregulation of both iNOS protein expression and activity in rat WG, compared with young controls, and activity only in the SOL. (e) Conversely, 12 weeks of treadmill exercise training reduced iNOS protein expression and activity in skeletal muscle. Thus, we propose that exercise training appears to mitigate age-induced alterations in the NOS profile of skeletal muscle but is influenced by NOS isoforms and muscle fiber type.
Our results indicate that nNOS and eNOS protein and activity levels were lower in the WG of old rats. In contrast, exercise training resulted in an increase in nNOS and eNOS protein expression as well as nNOS + eNOS levels in the WG of senescent rats. Previous studies demonstrated that exercise and mechanical stress increased nNOS and eNOS levels in skeletal muscle of young rats (16,29). In addition, Koh and (28) Tidball demonstrated in a series of NOS inhibitor experiments that constitutive NOS appears to be critical in regrowth and recovery upon reloading. NOS inhibition also attenuated increases in VEGF mRNA by exercise (41). However, the current study is the first to show that inducibility of nNOS and eNOS in fast-twitch muscle to treadmill exercise training is still retained with advancing age.
Skeletal muscle nNOS has its discrete localization to the sarcolemma in conjunction with a complex of transmembrane and cytoskeletal dystrophin. It is shown to play a role in the regulation of glucose uptake (42), contractile activity (15), and blood flow (43). However, little is known about the functional importance of nNOS localization in skeletal muscle. Thomas and colleagues (44) demonstrated that appropriate membrane targeting of nNOS by alpha-syntrophin is required for vasomodulation by skeletal muscle–derived •NO implicating for the potential gene therapy strategies to treat muscular dystrophy. In addition, recent study using muscle from transgenic mdx mice and Becker muscular dystrophies patients suggests that nNOS is a marker for complete restoration of the dystrophin-associated complex (45). eNOS is known to be an essential enzyme for vascular function and has been shown a specific localization in skeletal muscle mitochondria. •NO produced by eNOS is playing an important role in the regulation of skeletal muscle blood flow by interaction with other vasodilators (46–48).
Given the locations of nNOS and eNOS in the cytoskeleton and mitochondria, respectively, it is logical that exercise may promote upregulation of constitutive NOS through mechanical and metabolic stress. Previous reports indicate that exercise training increases nNOS and eNOS of the gastrocnemius and diaphragm of young adult rats (16,29,49,50). In addition, Tidball and colleagues (29) found that nNOS increased with loading, thus fortifying the hypothesis that changes in mechanical stress alter nNOS levels in skeletal muscle. Using an eNOS knockout model, Momken and colleagues (51) demonstrated the requirement of eNOS in protecting skeletal muscle mass and metabolic enzymes responding to exercise training. Recent study shows that eNOS(−/−) mice had significantly lower oxygen consumption and energy expenditure and, of importance, with defective mitochondria, as evidenced by a decreased beta-oxidation. In addition, impaired mitochondrial beta-oxidation was linked with an elevated free fatty acid and triglyceride in the gastrocnemius muscle (52). A series of NOS inhibition experiments by Criswell and colleagues (40,53) also indicated that hypertrophy and fiber-type response to overloading are NOS dependent, with the most likely candidate nNOS. Thus, constitutive NOS isoforms appear to be critical in adaptation of mass and fiber-type profile to mechanical and metabolic stress (29,40,53).
Our results show that although nNOS protein expression was inducible with exercise training in the SOL muscle from old rats, eNOS protein levels as well as eNOS + nNOS activity were unaltered. These data indicate a muscle fiber type–specific eNOS response to exercise training. Our data suggest that exercise training may be able to ameliorate loss of mass with aging, possibly through upregulation of nNOS and eNOS in fast-twitch muscle fibers, which are more susceptible to fiber atrophy and cell loss (26,27). It is possible that exercise-induced alterations in eNOS protein levels in the endothelium of arterioles affected our results in the SOL and gastrocnemius. However, Spier and colleagues (54) found using a similar exercise training protocol that arteriole eNOS levels were increased in SOL, inconsistent with our whole muscle data (Figure 4). It is also recently reported that 10–12 weeks of treadmill exercise training ameliorated the age-induced reduction of the acetylcholine-mediated vasodilatation in highly oxidative SOL muscle arterioles via increased eNOS protein expression (55). In addition, arterioles make up 1% or less of muscle mass and eNOS is primarily localized in association with mitochondria in skeletal muscle (56). Thus, arteriole eNOS did not suggest a significant influence on whole muscle eNOS levels, but did suggest differential inducibility of eNOS protein levels in the vasculature versus intramyocyte locations.
Growing evidence demonstrates that endothelium-dependent, •NO-mediated vasodilation is actually augmented by exercise training and increased endothelial •NO formation mostly by eNOS appears to have a role in the atherosclerotic disease (57,58). Gill and colleagues (59) also demonstrated a complete restoration of endothelium-dependent coronary relaxation during cardiac recovery from chronic heart failure by phosphorylation of eNOS prompting a restoration of •NO production. Interestingly, antioxidant supplementation including vitamin C, coenzyme Q, and alpha-tocopherol before exercise enhanced the contraction-induced increase in eNOS mRNA. This observation implies that reactive oxygen species (ROS) should be involved in the regulation of eNOS and suggests that intervention using antioxidant supplementation may be beneficial in certain diseases associated with impaired eNOS levels (60). Regarding the time course of the effect of exercise training on •NO vasodilator function, short-term exercise training appears to enhance eNOS and •NO bioactivity regulating shear stress homeostatically. After long-term training, structural adaptation occurs, possibly in part due to •NO-dependent structural changes in the vessels, resulting in an increase in lumen diameter (61,62).
As far as new aspects of the location of NOS are concerned, recent immunohistochemistry study showed that all three NOS isoforms are coexpressed in human skeletal muscles (63) and showed strong NOS expression in fast-oxidative glycolytic fibers, whereas oxidative or glycolytic fibers showed only weak NOS expression suggesting that •NO may be involved in the oxidative metabolism in connection with fast force development. Buchwalow and colleagues (64) also demonstrated that three NOS isoforms coexisted not only in the sarcolemma but also in the intracellular compartments such as sarcoplasmic reticulum, mitochondria, and along contractile fibers. Localization of NOS both in the mitochondria and along contractile fibers emphasizes the role of •NO in the respiratory and contractile functions of the skeletal muscle. A growing body of evidence suggests that •NO modulates mitochondrial respiration via cytochrome c oxidase activity (65–68).
To our knowledge, these are the first data to indicate that aging increases iNOS protein levels and activity in fast (type II) and activity levels in slow (type I) skeletal muscle. Increased levels of iNOS (Figure 5) in aging skeletal muscle support the hypothesis that aging increases the proinflammatory state. Upregulation of iNOS in aging skeletal muscle is also consistent with data from other tissues (e.g., brain, heart, liver, kidney) that indicate proinflammatory pathways become elevated as aging progresses (11,12,69). Indeed, a direct relationship between elevated levels of iNOS, •NO, and protein nitration with impaired function has been observed in a number of tissues (13,70). Previously, iNOS gene expression was increased with age in the kidney (14), vascular smooth muscle (71), and heart (69). In addition, protein nitration in the liver and subcortical brain also increased with aging (13,72). In contrast, caloric restriction protected against age-induced elevation in protein nitration, indicating reduced nitrosative and oxidative stress (70). Yang and colleagues (69) demonstrated that reduction of iNOS activity through both pharmacological (e.g., aminoguanidine) or genetic (iNOS KO) means significantly protected old hearts from age-related impairment of ejection fraction, stroke volume, and pressure generation. When combined with our data, we suggest a systemic age-induced increase in iNOS consistent with the iNOS theory of aging model (13).
Skeletal muscles from old animals are more susceptible to injury and inflammation myopathies related to oxidative stress than those from young animals (3,73). In addition, clinical inflammatory myopathies increase as a function of age (73). Moreover, aging also increases susceptibility of skeletal muscle to myotoxicity from excess •NO (74). The pathological importance of age-induced upregulation of iNOS protein expression and activity in skeletal muscle (Figures 2 and 3) could be linked to a role of iNOS in age-induced increase in apoptosis (70) and impaired contractile function (75).
Although a direct causal link between age-induced elevation of iNOS and impaired skeletal muscle contractile function has not been tested, iNOS upregulation is a commonality among numerous pathologies and models that result in skeletal muscle wasting (20,22,76). Growing evidence links upregulation of iNOS and proinflammatory signaling with skeletal muscle wasting, impaired function, and damage in a number of pathologies including heart failure, chronic obstructive pulmonary disease (COPD), AIDS, cancer, sepsis, and type II diabetes (7,20,76–79). For example, iNOS levels were markedly increased in skeletal muscle of chronic heart failure patients, and iNOS upregulation was linked to exercise intolerance (6,23,76,77). Gielen and colleagues (76) also observed elevated levels of nitrotyrosine in skeletal muscles of chronic heart failure patients. Our laboratory previously demonstrated that a 158% elevation in iNOS protein expression occurs concomitant with 50% muscle atrophy in the SOL with 28 days of unloading (73). It is possible that exercise-induced downregulation of iNOS protein expression and activity in skeletal muscle, particularly in fast-twitch muscle, could protect against weakness, wasting, and apoptosis (27). This is a focus of future investigations.
Exercise-induced downregulation of iNOS with a concomitant upregulation of nNOS and eNOS is consistent with exercise modulating a shift toward more of a contractile and less of an inflammatory role for NOS (13). The shift in the NOS isoform profile could alter the role of •NO in skeletal muscle due to changes in location, •NO flux, localized changes in production of superoxide and other ROS, peroxynitrite generation, reduced glutathione concentration, and so forth. Potential upstream mechanisms that could contribute to age-induced upregulation of skeletal muscle iNOS levels include cytokines, such as TNF-α and IL-1ß, and NF-ΚB (80,81). iNOS may be upregulated by cytokines through the cytokine response element or through binding of activated NF-ΚB to the promotor region of the iNOS gene (1,20,21). Cytokines are postulated as critical mediators of elevated NF-ΚB and iNOS levels in skeletal muscle in heart failure patients (7,77,82). Conceptually, exercise-induced reduction in proinflammatory cytokines and oxidative stress, upstream to iNOS, in the elderly adult may be an immunologic adaptation that could provide protection against cachexia, muscle damage, and inflammatory diseases (82). Regular exercise and physical activity appear to reduce proinflammatory cytokines in aging individuals (83,84). Reduction of cytokines and inflammation may protect against loss of strength and sarcopenia in aging muscles (85,86). Interestingly, recent data indicate that exercise elevates NF-ΚB in skeletal muscle, which is critical in cell signaling, protein production, and cell protection (27,50), suggesting that exercise may reduce iNOS levels via an alternative pathway or more directly by reducing TNF-α.
Skeletal muscle suffers wasting with advancing age because of both (a) atrophy and (b) loss of muscle fibers, possibly through apoptosis (26,86). Exercise-induced reduction of iNOS may protect against age-induced elevation of oxidative stress as well as apoptosis, which could contribute to muscle wasting via cell death and fiber atrophy (26,87). Recent data confirmed apoptosis in the aged gastrocnemius muscle, where DNA fragmentation was increased by 50% in the old rats compared with the adult animals (27,88). Adams and colleagues (77) reported that apoptosis is frequently found in skeletal muscle obtained from chronic heart failure patients and iNOS and Bcl-2 are possibly involved in the regulation of apoptosis.
Both resistive and endurance training may protect against muscle atrophy, injury, and fiber loss in skeletal muscle, especially in type II fibers (26,89). Exercise training can reduce the risk for injury and inflammation against mechanical and oxidant perturbations, as long as overtraining does not occur (90). These findings are consistent with a protective role of exercise training against heightened inflammatory signaling in skeletal muscle and recent exercise data in heart failure patients. Given that the incidence of heart failure and sepsis increases with age (81), our data indicate a prophylactic and therapeutic role for habitual exercise against dysfunction in aging muscle. Indeed, exercise training significantly reduced iNOS and inflammatory cytokine levels in the muscles of heart failure patients, while increasing insulin-like growth factor (IGF-1) and cytochrome oxidase (76,91). Additional study is warranted to better understand the cellular mechanisms by which changes in the NOS isoform profile (e.g., iNOS/(nNOS + eNOS) ratio) by exercise training improves function and reduces pathology in aging skeletal muscle.
FUNDING
This study was supported by the American College of Sports Medicine, Glenn/American Federation for Aging Research, the American Heart Association—Texas Affiliate (GIA 0555064Y), NIH (AR054084), and the Korea Science and Engineering Foundation (KOSEF-R01-2007-000-20546-0).
Acknowledgments
We thank Dr. Guoyao Wu for his technical support.
References
- 1.Adams V, Yu J, Mobius-Winkler S, et al. Increased inducible nitric oxide synthase in skeletal muscle biopsies from patients with chronic heart failure. Biochem Mol Med. 1997;61:152–160. doi: 10.1006/bmme.1997.2598. [DOI] [PubMed] [Google Scholar]
- 2.Adams V, Jiang H, Yu J, et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33:959–965. doi: 10.1016/s0735-1097(98)00626-3. [DOI] [PubMed] [Google Scholar]
- 3.Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279:C611–C618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- 4.McArdle A, Jackson MJ. Exercise, oxidative stress and ageing. J Anat. 2000;197(pt 4):539–541. doi: 10.1046/j.1469-7580.2000.19740539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasilaki A, Jackson MJ, McArdle A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve. 2002;25:902–905. doi: 10.1002/mus.10094. [DOI] [PubMed] [Google Scholar]
- 6.Zarzhevsky N, Menashe O, Carmeli E, Stein H, Reznick AZ. Capacity for recovery and possible mechanisms in immobilization atrophy of young and old animals. Ann N Y Acad Sci. 2001;928:212–225. doi: 10.1111/j.1749-6632.2001.tb05651.x. [DOI] [PubMed] [Google Scholar]
- 7.Agusti A, Morla M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004;59:483–487. doi: 10.1136/thx.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol. 1998;509(pt 2):577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin AC, Stevenson SW, Dudley GA. Nonsteroidal anti-inflammatory therapy after eccentric exercise in healthy older individuals. J Gerontol A Biol Sci Med Sci. 2001;56:M510–M513. doi: 10.1093/gerona/56.8.m510. [DOI] [PubMed] [Google Scholar]
- 10.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 11.Chung HY, Kim HJ, Kim KW, Choi JS, Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech. 2002;59:264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- 12.Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- 13.McCann SM, Licinio J, Wong ML, Yu WH, Karanth S, Rettorri V. The nitric oxide hypothesis of aging. Exp Gerontol. 1998;33:813–826. doi: 10.1016/s0531-5565(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 14.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 15.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 16.Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol. 1999;277:E390–E394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- 17.Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- 18.Rando TA. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 19.Kroncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities. Biol Chem Hoppe Seyler. 1995;376:327–343. doi: 10.1515/bchm3.1995.376.6.327. [DOI] [PubMed] [Google Scholar]
- 20.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 21.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 22.Bia BL, Cassidy PJ, Young ME, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–1862. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 23.Hambrecht R, Adams V, Gielen S, et al. Exercise intolerance in patients with chronic heart failure and increased expression of inducible nitric oxide synthase in the skeletal muscle. J Am Coll Cardiol. 1999;33:174–179. doi: 10.1016/s0735-1097(98)00531-2. [DOI] [PubMed] [Google Scholar]
- 24.Tews DS, Goebel HH. Cell death and oxidative damage in inflammatory myopathies. Clin Immunol Immunopathol. 1998;87:240–247. doi: 10.1006/clin.1998.4527. [DOI] [PubMed] [Google Scholar]
- 25.Pizza FX, Hernandez IJ, Tidball JG. Nitric oxide synthase inhibition reduces muscle inflammation and necrosis in modified muscle use. J Leukoc Biol. 1998;64:427–433. [PubMed] [Google Scholar]
- 26.Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol. 2002;92:2617–2624. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- 27.Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006;8:517–528. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- 28.Koh TJ, Tidball JG. Nitric oxide synthase inhibitors reduce sarcomere addition in rat skeletal muscle. J Physiol. 1999;519(pt 1):189–196. doi: 10.1111/j.1469-7793.1999.0189o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki N, Motohashi N, Uezumi A, et al. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest. 2007;117:2468–2476. doi: 10.1172/JCI30654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon HJ, Kim JJ, Kang MJ, et al. The activation mechanisms of NF-kB and inflammatory enzymes during aging. Ann N Y Acad Sci. 2001;928:381. [Google Scholar]
- 32.Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci U S A. 2001;98:5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capanni C, Squarzoni S, Petrini S, et al. Increase of neuronal nitric oxide synthase in rat skeletal muscle during ageing. Biochem Biophys Res Commun. 1998;245:216–219. doi: 10.1006/bbrc.1998.8404. [DOI] [PubMed] [Google Scholar]
- 34.Richmonds CR, Boonyapisit K, Kusner LL, Kaminski HJ. Nitric oxide synthase in aging rat skeletal muscle. Mech Ageing Dev. 1999;109:177–189. doi: 10.1016/s0047-6374(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 35.Hammeren J, Powers S, Lawler J, et al. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med. 1992;13:412–416. doi: 10.1055/s-2007-1021290. [DOI] [PubMed] [Google Scholar]
- 36.Naito H, Powers SK, Demirel HA, Aoki J. Exercise training increases heat shock protein in skeletal muscles of old rats. Med Sci Sports Exerc. 2001;33:729–734. doi: 10.1097/00005768-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc. 1993;25:1259–1264. [PubMed] [Google Scholar]
- 38.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 39.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 40.Smith LW, Smith JD, Criswell DS. Involvement of nitric oxide synthase in skeletal muscle adaptation to chronic overload. J Appl Physiol. 2002;92:2005–2011. doi: 10.1152/japplphysiol.00950.2001. [DOI] [PubMed] [Google Scholar]
- 41.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol. 2000;88:1192–1198. doi: 10.1152/jappl.2000.88.4.1192. [DOI] [PubMed] [Google Scholar]
- 42.Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- 43.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires α-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res. 2003;92:554–560. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- 45.Wells KE, Torelli S, Lu Q, et al. Recolization of neuronal nitric oxide synthase as a marker for complete restoration of the dystrophin associated protein complex in skeletal muscle. Neuromuscul Disord. 2003;13:21–31. doi: 10.1016/s0960-8966(02)00191-8. [DOI] [PubMed] [Google Scholar]
- 46.Boushel RL, Langberg H, Gemmer C, et al. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284:L452–L457. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Momken I, Lechene P, Ventura-Clapier R, Veksler V. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am J Physiol Heart Circ Physiol. 2004;287:H914–H920. doi: 10.1152/ajpheart.00651.2003. [DOI] [PubMed] [Google Scholar]
- 52.Le Gouill E, Jimenez M, Binnert C, et al. Endothelial nitric oxide synthase knockout mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56:2690–2696. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- 53.Sellman JE, DeRuisseau KC, Betters JL, et al. In vivo inhibition of nitric oxide synthase impairs upregulation of contractile protein mRNA in overloaded plantaris muscle. J Appl Physiol. 2006;100:258–265. doi: 10.1152/japplphysiol.00936.2005. [DOI] [PubMed] [Google Scholar]
- 54.Spier SA, Laughlin MH, Delp MD. Effects of acute and chronic exercise on vasoconstrictor responsiveness of rat abdominal aorta. J Appl Physiol. 1999;87:1752–1757. doi: 10.1152/jappl.1999.87.5.1752. [DOI] [PubMed] [Google Scholar]
- 55.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 57.McAllister RM, Laughlin MH. Vascular nitric oxide: effects of physical activity, importance for health. Essays Biochem. 2006;42:119–131. doi: 10.1042/bse0420119. [DOI] [PubMed] [Google Scholar]
- 58.McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab. 2008;33:173–178. doi: 10.1139/H07-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gill RM, Braz JC, Jin N, Etgen GJ, Shen W. Restoration of impaired endothelium-dependent coronary vasodilation in failing heart: role of eNOS phosphorylation and CGMP/cGK-I signaling. Am J Physiol Heart Circ Physiol. 2007;292:H2782–H2790. doi: 10.1152/ajpheart.00831.2006. [DOI] [PubMed] [Google Scholar]
- 60.Hellsten Y, Nielsen JJ, Lykkesfeldt J, et al. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med. 2007;43:353–361. doi: 10.1016/j.freeradbiomed.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 61.Brown MD. Exercise and coronary vascular remodeling in the healthy heart. Exp Biol Med. 2003;88:645–658. doi: 10.1113/eph8802618. [DOI] [PubMed] [Google Scholar]
- 62.Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodeling. Exerc Sports Sci Rev. 2003;31:26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Punkt K, Fritzsche M, Stockmar C, et al. Nitric oxide synthase in human skeletal muscles related to defined fibre types. Histochem Cell Biol. 2006;125:567–573. doi: 10.1007/s00418-005-0108-7. [DOI] [PubMed] [Google Scholar]
- 64.Buchwalow IB, Minin EA, Samoilova VE, et al. Compartmentalization of NO signaling cascade in skeletal muscles. Biochem Biophys Res Commun. 2005;330:615–621. doi: 10.1016/j.bbrc.2005.02.182. [DOI] [PubMed] [Google Scholar]
- 65.Edmunds NJ, Moncada S, Marshall JM. Does nitric oxide allow endothelial cells to sense hypoxia and mediate hypoxic vasodilation? In vivo and in vitro studies. J Physiol. 2003;546:521–527. doi: 10.1113/jphysiol.2002.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Cir Res. 2000;87:1108–1117. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- 68.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993–C2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- 69.Yang B, Larson DF, Watson RR. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004;75:655–667. doi: 10.1016/j.lfs.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 70.Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann NY Acad Sci. 2002;959:66–81. doi: 10.1111/j.1749-6632.2002.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 71.Chan GH, Fiscus RR. Exaggerated production of nitric oxide (NO) and increases in inducible NO-synthase mRNA levels induced by the pro-inflammatory cytokine interleukin-1beta in vascular smooth muscle cells of elderly rats. Exp Gerontol. 2004;39:387–394. doi: 10.1016/j.exger.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Siles E, Martinez-Lara E, Canuelo A, et al. Age-related changes of the nitric oxide system in the rat brain. Brain Res. 2002;956:385–392. doi: 10.1016/s0006-8993(02)03575-8. [DOI] [PubMed] [Google Scholar]
- 73.Song W, Lawler J, Bloomfield S. Hindlimb unloading increases iNOS protein expression and NF-kappaB DNA binding activity in rat skeletal muscle. [abstract] FASEB J. 2003;17:A945. [Google Scholar]
- 74.Yazici Y, Kagen LJ. Clinical presentation of the idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 2002;28:823–832. doi: 10.1016/s0889-857x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 75.Radak Z, Pucsok J, Mecseki S, Csont T, Ferdinandy P. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic Biol Med. 1999;26:1059–1063. doi: 10.1016/s0891-5849(98)00309-8. [DOI] [PubMed] [Google Scholar]
- 76.Gielen S, Adams V, Mobius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 77.Adams V, Nehrhoff B, Spate U, et al. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 78.Torres SH, De Sanctis JB, de Briceno LBM, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol. 2004;181:419–427. doi: 10.1677/joe.0.1810419. [DOI] [PubMed] [Google Scholar]
- 79.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R50–R56. doi: 10.1152/ajpregu.00564.2002. [DOI] [PubMed] [Google Scholar]
- 80.Chang CK, LoCicero J., 3rd Overexpressed nuclear factor kappaB correlates with enhanced expression of interleukin-1beta and inducible nitric oxide synthase in aged murine lungs to endotoxic stress. Ann Thorac Surg. 2004;77:1222–1227. doi: 10.1016/j.athoracsur.2003.09.128. ; discussion 1227. [DOI] [PubMed] [Google Scholar]
- 81.Adams V, Spate U, Krankel N, et al. Nuclear factor-kappa B activation in skeletal muscle of patients with chronic heart failure: correlation with the expression of inducible nitric oxide synthase. Eur J Cardiovasc Prev Rehabil. 2003;10:273–277. doi: 10.1097/00149831-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. 2003;13:56–62. doi: 10.1034/j.1600-0838.2003.20218.x. [DOI] [PubMed] [Google Scholar]
- 83.Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–964. doi: 10.1249/01.mss.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- 84.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 85.Bruunsgaard H, Bjerregaard E, Schroll M, Pedersen BK. Muscle strength after resistance training is inversely correlated with baseline levels of soluble tumor necrosis factor receptors in the oldest old. J Am Geriatr Soc. 2004;52:237–241. doi: 10.1111/j.1532-5415.2004.52061.x. [DOI] [PubMed] [Google Scholar]
- 86.Pahor M, Kritchevsky S. Research hypotheses on muscle wasting, aging, loss of function and disability. J Nutr Health Aging. 1998;2:97–100. [PubMed] [Google Scholar]
- 87.Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58:999–1001. doi: 10.1093/gerona/58.11.m999. [DOI] [PubMed] [Google Scholar]
- 88.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 89.Tarpenning KM, Hamilton-Wessler M, Wiswell RA, Hawkins SA. Endurance training delays age of decline in leg strength and muscle morphology. Med Sci Sports Exerc. 2004;36:74–78. doi: 10.1249/01.MSS.0000106179.73735.A6. [DOI] [PubMed] [Google Scholar]
- 90.Fielding RA, Meydani M. Exercise, free radical generation, and aging. Aging (Milano) 1997;9:12–18. doi: 10.1007/BF03340124. [DOI] [PubMed] [Google Scholar]
- 91.Schulze PC, Gielen S, Schuler G, Hambrecht R. Chronic heart failure and skeletal muscle catabolism: effects of exercise training. Int J Cardiol. 2002;85:141–149. doi: 10.1016/s0167-5273(02)00243-7. [DOI] [PubMed] [Google Scholar]