Abstract
This study compared serum antibody titers and granzyme B (GrzB) levels in virus-stimulated peripheral blood mononuclear cells following influenza vaccination. Twelve of 239 older adults who subsequently developed laboratory-diagnosed influenza illness (LDI) had significantly lower GrzB levels compared to subjects without LDI (P=0.004). Eight subjects with LDI in the previous year showed an enhanced GrzB response to vaccination (P=0.02). Serum antibody titers following vaccination did not distinguish those older adults who developed LDI from those who did not. These results suggest that GrzB levels could be combined with antibody titers to more effectively predict vaccine efficacy in older adults.
Keywords: granzyme B, antibody, influenza vaccination
1. Introduction
Influenza is foremost among all infectious diseases in the age-related increase in risk for serious complications and death. Immunosenescence diminishes the ability of older adults to resist influenza (particularly the A/H3N2 strains) and respond to vaccination. Rising hospitalization and death rates have been observed despite widespread influenza vaccination programs [1, 2]. The need to develop more effective influenza vaccines for older adults is widely recognized, but whether or not the aged immune system has the capacity to respond, and what specific components of the immune response would need to be stimulated for improved vaccine efficacy, are poorly understood. Although the limitations of antibody titers as a sole measure of vaccine efficacy in this population are increasingly recognized [3], alternate correlates of protection are not readily available.
The importance of cytotoxic T lymphocytes (CTL) as a major contributor to defense mechanisms against influenza in humans has long been recognized [4]. In peripheral blood mononuclear cells (PBMC) from adults, influenza-specific memory CTL can be stimulated to rapidly develop effector function that is below the sensitivity of traditional 51Cr-release assays [5]. For this reason, we have developed an assay of granzyme B (GrzB), a key cytolytic mediator released from the CTL into the virus-infected target through a process facilitated by perforin. GrzB then stimulates a cascade of events within in the target cell that lead to apoptotic cell death [6], and clearance of virus from the lungs [7]. GrzB activity in influenza-stimulated peripheral blood mononuclear cells (PBMC) correlates with traditional measures of cytolytic activity [8], and has been shown to be the earliest and main contributor to CTL-mediated killing of influenza virus-infected cells in the mouse [9, 10]. We have shown prospectively that prior to influenza illness, GrzB levels are lower in the influenza-stimulated PBMC from older adults who subsequently develop influenza illness compared to those who do not [11, 12]. Further, antibody titers prior to exposure to influenza did not distinguish between those who developed influenza illness from those who did not [12].
To develop an assay of cell-mediated immunity that would have broad application to the older adult population, the study protocol was designed with limited exclusion criteria and recruited a subset of very high-risk older adults with congestive heart failure (CHF). The method is a relatively simple assay of GrzB activity in lysates of PBMC stimulated ex vivo with live influenza virus, as a potential predictor of protection against influenza in vaccinated older adults. The GrzB assay has been further developed and is now standardized against a commercially available GrzB standard so that it can be validated for use across different studies and laboratories.
Herein, we report data showing that GrzB activity not only correlates with protection against influenza but also demonstrates the potential capacity of older adults to mount an enhanced response to influenza. We also show that in the ex vivo response to influenza virus, increased levels of GrzB are largely produced in the T cell (CD3+ or CD3+CD56+) subset with relatively little increase in the activated natural killer (NK) cell (CD3−CD56+) subset. Further, the phenotype of virus-activated GrzB+ T cells is consistent with a virus-specific effector function. Given that the antibody response to influenza vaccination was again shown to have limited ability to predict protection from influenza in older adults [12], measures of both antibody and CTL/GrzB responses are proposed as a more robust evaluation of protection against influenza in this population.
2. Material and Methods
2.1. Study design and participants
This was a prospective study of 239 adults age 60 years and older (median, 75 years old; range, 60–95 years old) recruited in the vicinity of the Greater Hartford Area of Connecticut in each of two consecutive influenza seasons (2003–04 and 2004–05). A second subset of subjects from the study conducted during the 2008–09 season was also studied. All subjects were recruited through written informed consent. The Institutional Review Board of the University of Connecticut Health Center approved the protocol and informed consent document. Older adults without CHF were recruited through mailings to a registry list of older adults in the community who indicated an interest in participating in research studies at the University of Connecticut Health Center (UCHC). Older adults with CHF were recruited through the UCHC Congestive Heart Failure Center. Subjects were excluded for an acute respiratory illness in the two weeks preceding study enrolment, insulin-requiring diabetes in subjects without CHF, any conditions or medications causing immunosuppression such as prednisone > 10 mg/day, or any contraindications to influenza vaccination.
2.2. Procedures
Study participants were characterized according to demographics, presence or absence of CHF, medications including HMG CoA reductase inhibitors (statins), and functional capacity according to performance on the 6-Minute Walk Test (6-MWT). All subjects received the standard dose of the licensed trivalent split-virus influenza vaccine containing A/New Caledonia/20/99 (H1N1), A/Panama/2007/99 (H3N2), and B/Hong Kong/330/2001-like virus in 2003–04, and A/New Caledonia/20/99 (H1N1), A/Fujian/41/2002-like (H3N2), and B/Shanghai/361/2002 in 2004–05, and A/Brisbane/59/2007 (H1N1)-like, A/Brisbane/10/2007 (H3N2)-like, and B/Florida/4/2006-like in 2008–09. Serum and heparinized blood samples were collected prior to vaccination and at 4 and 10 weeks post-vaccination in 2003–04 and 2004–05, or at 4 weeks post-vaccination in 2008–09; a serum sample was collected at the end of the influenza season for all subjects.
2.3. Influenza surveillance
Influenza surveillance for laboratory-diagnosed influenza illness included weekly telephone reminders to participants during the influenza season to report any respiratory (cough, sore throat, shortness of breath, or runny or stuffy nose) and related systemic (fever or feverishness, muscle aches, malaise, or fatigue) symptoms. Any subjects reporting at least two respiratory symptoms or one respiratory and one systemic symptom during the influenza season were identified as influenza-like illness, and laboratory-diagnosed influenza (LDI) confirmed by virus culture from nasopharyngeal swabs or seroconversion (4-fold rise in antibody titer from pre- to post-illness).
2.4. Cell culture, virus stimulation and granzyme B assay
PBMC were isolated from venous blood samples at 0, 4 and 10 weeks post-vaccination by Histopaque gradient purification and stimulated in AIM V medium (GIBCO) containing 1.5 × 106 lymphocytes/ml, 3 × 106 TCID50/ml of influenza A/H3N2 or B strains (cell-derived live virus preparations, generous gift from Solvay Pharmaceuticals, The Netherlands) similar to the vaccinating strains and as previously published for PBMC prepared and stimulated in the first year [12]. PBMC lysates were harvested after 20 hours of culture and frozen at −80° C until completion of the study in each year. GrzB activity was measured in PBMC lysates (20 μl) by cleavage of the substrate, IEPDpna, (BACHEM) as previously described [12]. The standard for the GrzB assay was prepared as a lysate of interleukin-2-stimulated YT cells (human natural killer cell line) in which GrzB activity is calculated against a commercially available GrzB standard (Biomol) supplemented with bovine serum albumin (2 μg/μl). A 1:2 serial dilution of YT lysate was included on each assay plate and a standard curve generated against which GrzB activity was calculated as A405 units (first year) or Biomol GrzB units (second year). GrzB activity is calculated as units per mg protein (BCA assay, Pierce) in the PBMC lysates based on the concentration of GrzB activity (Biomol standard units) and adjusted for the protein concentration (an estimate of the number of cells in the lysate at the time of harvest).
2.5. Antibodies and flow cytometry
To confirm an age-dependent differential expression of cell surface markers, 1.0 × 106 cells/well human PBMC were suspended in 500 μl AIM V medium containing PE-anti-CD107a (H4A3) and 4 × 106 TCID50/ml of influenza A/Aichi/68 (H3N2) virus or without virus stimulation in a 48-well multiplate for 12 hours. Cells were prepared for flow cytometry as previously described [12]. PerCP-anti-CD8, APC-anti-CD56, PE-anti-CD107a, and FITC-anti-GrzB antibodies were purchased from BD Biosciences. Briefly, resuspended 1×106 cells were washed with cold 0.2% FBS/PBS twice before incubation with Abs to CD8 (SK1), CD56 (B159) and CD107a for 20 minutes on ice, and following washing, the cells were fixed with 2% paraformaldyhyde and permeabilized with permeabilization buffer (eBioscience, Inc. San Diego, CA), then incubated with FITC-anti-GrzB (GB11) antibody for 20minutes on ice. All samples were acquired with LSRII (BD Biosciences), and data were analyzed by using CellQuest (BD Biosciences).
2.6. Assays of serum antibodies to influenza
Serum antibody titers measured by hemagglutination inhibition (HAI) assays were performed as previously described [13] using 2-fold dilutions of serum from 1/10 to 1/1024 and a single stock source for each of the hemagglutinin antigens (Centers for Disease Control, Atlanta, GA) and representing the strains of virus contained in the vaccine. Geometric mean titers were calculated using log10 conversion for each dilution. Seroprotection is defined as an antibody titer ≥ 40 and seroconversion as a 4-fold or greater rise in antibody titer.
2.7. Statistical Methods
GrzB levels were log transformed (log10) to approximate a normal distribution of the data and in the completed analysis, reported as the geometric mean (antilog of mean log10 GrzB) for the group or subset. To analyze the combined data from the two study years, A405 units/mg protein (A405) for GrzB activity used in the first year needed to be converted to Biomol units/mg protein (Biomol), as measured in the second year. Given the linear relationship between A405 units and Biomol units, a regression analysis of the mean log GrzB activity for each of the time points and types of responses to vaccination in the two study years generated the following regression equation for the linear relationship: Biomol = 2.094 + 0.447 * A405, with coefficient of determination r2=0.978. GrzB results of the two studies were combined as log10 GrzB (Biomol U/mg protein) for the analysis. Subjects were divided into four subsets for the analysis including: 1) subjects who developed LDI after 4-weeks post-vaccination (Flu subset), 2) subjects who did not develop LDI (NoFlu subset), 3) subjects who had a LDI concurrent with the 4-week post-vaccination time point in the first year (Flu at 4-wks), and 4) subjects who had LDI in the first year and response to vaccination measured in the second year to determine the effect of a prior influenza illness (Prior Flu). The analysis compared ex vivo levels of GrzB in the NoFlu subset to the Flu, Prior Flu and Flu at 4-weeks subsets. Because age, the presence or absence of CHF, performance on the 6-MWT, lipid-lowering drugs [statins], and antibody titers may be important in the immune response, they were assessed as potential confounders in the relationship between GrzB levels and the development or not of LDI.
Serum antibody levels were calculated as the log10-transformed reciprocal titer and reported as the geometric mean titer (GMT; antilog of mean log10 reciprocal titer) for each group or subset. GMT, seroconversion and seroprotection rates were compared between LDI and non-LDI groups.
To evaluate the effect of the 6-MWT, the continuous variable was transformed to a nominal variable based on whether the distance walked was above or below 1100 ft (> or ≤ 1100 ft). Differences in lab values between groups were assessed using the t-test for parametric variables. Significance of the response to vaccination was tested using a paired t-test.
Analyses were performed using SPSS 12.0 (SPSS Inc., Chicago, Ill). For initial exploratory data analysis, a general linear model (GLM) was used to determine the relationships between antibody titers or GrzB levels, and the development or not of LDI and for confounders in the analysis. Analysis of variance (ANOVA) was used to compare antibody titers and Grz B levels within and across different time points in the study. A threshold level of protection was defined as a GrzB level greater than the upper limit of the 95% confidence interval for the Flu subset at 4-weeks post-vaccination.
Flow cytometry experiments analyzed the response to virus stimulation as the change in the proportion of lymphocytes in unstimulated compared to stimulated PBMC, for each group and CD8+ or NK (CD8−CD56+) lymphocyte subset using a repeated measures analysis of variance. The overall response to virus stimulation was compared across the three study groups using the Kruskal-Wallis test (Statview 5.0.1).
3. Results
3.1. Influenza Outcomes
A total of 239 participants were enrolled in the two years of the study; 63 subjects were re-enrolled from the first to the second year. In the first year, nine of 90 subjects developed LDI but five subjects who developed LDI before 4-weeks post-vaccination were excluded; natural infection in these five subjects enhanced the GrzB response masking the effect of vaccination [12]. Thus only the four remaining cases from the first year were included in this analysis. An additional eight of 149 older adults developed LDI in the second year and were also included; one subject was culture positive for and seven subjects seroconverted to the circulating A/Wyoming (A/H3N2) strain. Thus, a total of 12 subjects (Flu subset; four from the first year and eight from the second year) could prospectively be evaluated for the ability of GrzB levels to predict risk for LDI. A second subset was identified as eight of the nine subjects who developed LDI in the first year; these eight subjects (Prior Flu) enrolled in the study in the second year and none of these subjects developed LDI in the second year. This Prior Flu subset was treated as a separate subset in the analysis. Two subjects were withdrawn in the first year due to serious adverse events unrelated to the study, which resulted in admission to a skilled nursing facility. Two subjects deceased, one in the first and one in the second year. None of these events were attributed to influenza illness. These four subjects were excluded from the analysis for a total of 235 subjects who completed the study in the two influenza seasons.
3.2. Clinical variables and interactions with immunologic measures
Over the two studies, influenza attack rates were similar in CHF and non-CHF subjects, six of 86 older CHF subjects and 11 of 139 older subjects. A comparison of the clinical profiles for CHF versus non-CHF subjects showed that the groups were similar in age but in the CHF group, a larger proportion of subjects were male, had ischemic heart disease, diabetes and/or COPD, walked less than 1100 ft. on the 6-MWT, and were taking statins, ACEI or β-blocker medications (Table I). However, none of these factors including age, gender, medications or performance on the 6-MWT had a statistically significant interaction with GrzB levels or antibody titers.
Table 1.
Subject Characteristics
| Variable | CHF (n=96) | Non-CHF (n=143) | P Value* |
|---|---|---|---|
| Mean Age, years (median; range) | 76.2 (75.5; 60–95) | 74.9 (75.7; 61–95) | 0.19 |
| Gender [female] n (%) | 44 (46%) | 108 (75%) | <0.001 |
| 6-MWT [≥1100ft] n(%) | 42 (44%) | 128 (89%) | <0.001 |
| Hypertension | 54 (56%) | 73 (51%) | 0.51 |
| Diabetes | 18 (19%) | 2 (1%) | <0.001 |
| Stroke | 2 (2%) | 4 (3%) | 0.99 |
| Ischemic Heart Disease | 34 (35%) | 21 (15%) | <0.001 |
| COPD | 9 (8%) | 4 (3%) | 0.04 |
| Statins n (%) | 64 (67%) | 56 (39%) | <0.001 |
| ACEI n (%) | 43 (45%) | 30 (21%) | <0.001 |
| b-blockers n (%) | 53 (55%) | 33 (23%) | <0.001 |
| NSAIDs n (%) | 5 (5%) | 17 (12%) | 0.11 |
Chi square Fisher's exact test (2 sided) for p value (except age).
6-MWT = subjects who walked ≥1100ft in the 6-minute walk test
Statins = HMG CoA reductase inhibitor drugs
ACEI = angiotensin converting enzyme inhibitor drugs
β-blockers = β-blocker drugs
NSAIDs = non-steroidal anti-inflammatory drugs including cyclooxygenase II inhibitors but not including aspirin in doses ≤ 325 mg
3.3. Association between granzyme B levels and influenza outcomes
Overall, there was a significant increase in GrzB levels from pre- to 4-weeks post-vaccination as measured ex vivo (20-hour cultures) in lysates of PBMC stimulated with live influenza virus similar to the A/H3N2 vaccine strain (paired t-test, p<0.0001). We postulated that the development of influenza illness following vaccination is due to a poor CTL response to influenza; the GrzB level in ex vivo virus-stimulated PBMC is used as a marker of CTL cytolytic activity. Consistent with this hypothesis, the NoFlu subset produced significantly higher levels of GrzB compared to the Flu subset (ANOVA, p=0.004; Figure 1A). At 4-weeks post-vaccination, this result corresponded with a geometric mean GrzB level of 143 U/mg protein in the Flu subset compared to 212 U/mg protein in the NoFlu subset (GLM, p=0.02). Consistent with the lack of cross-reactivity between influenza A and B strains, there was no significant difference in GrzB levels between Flu, NoFlu and Prior Flu subsets in PBMC cultures stimulated with the influenza B virus at 4 and 10 weeks post-vaccination (Figure 1B).
Figure 1.
PBMC stimulated 20 hours with live influenza virus and granzyme B (GrzB) activity measured in PBMC lysates. Geometric mean GrzB levels are shown for each of the study subsets including subjects who did not develop influenza in either influenza season (No flu), subjects who subsequently developed influenza (Flu), and subjects who had had influenza during the previous influenza season (Prior Flu). Results are shown for the pre- (0 wks) and post-vaccination (4- and 10-wks) time points. (A) Results for PBMC stimulated with the A/H3N2 strain of influenza virus. In the Flu subset (n=12), the GrzB response to vaccination was poor and mean GrzB levels were significantly lower post-vaccination compared to the No flu subset (n=210, p=0.004). The effect of a previous influenza A/H3N2 illness on the response to vaccination was demonstrated in the Prior flu subset (n=8), showing an enhanced response with significantly increased post-vaccination levels of GrzB when compared to the No flu subset (p=0.02). (B) Results for PBMC stimulated with the influenza B strain at 4-weeks and 10-weeks post-vaccination (pre-vaccination not tested) in the second year showed no significant difference between the three subsets, consistent with the lack of cross-reactivity between strains of influenza A and B. Error bars represent standard error of the mean.
3.4. Prior influenza illness improves the GrzB response to vaccination
Of the nine subjects who had LDI in the first year, eight subjects participated in the second year (Prior Flu subset). In the second year, these eight subjects showed an enhanced response to vaccination with significantly increased post-vaccination GrzB levels compared to the NoFlu subset (ANOVA, p=0.02; Figure 1A). In terms of absolute differences, the geometric mean GrzB level achieved post-vaccination was approximately 50% higher in the Prior Flu subset compared to the NoFlu subset (308 U/mg protein vs. 212 U/mg protein; p<0.05) at 4-weeks post-vaccination. These results suggest that CTL from aged individuals have the capacity to mount an effective CTL response to influenza and that new vaccines could be designed to effectively restimulate CTL memory and improve vaccine efficacy in older adults.
3.5. GrzB is produced by cytolytic effector T cells
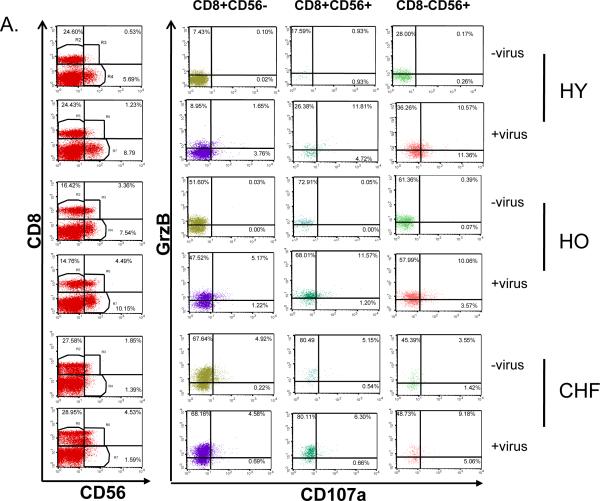
To characterize the CTL (CD8+) that activate GrzB in response to influenza virus, PBMC were stimulated for 12 hours with live influenza virus and analyzed by flow cytometry for the expression of CD107a and intracellular GrzB in CD8+ T cells and activated NK cells (CD8−CD56+). Cell surface expression of CD107a is an indicator of degranulating activity of GrzB+ CTL responding to live influenza virus [14–16], and CD56 was used an activation marker for both CTL (CD8+CD56+) and NK (CD8−CD56+) cells. Representative dot plots for each of the CD8+CD56−, CD8+CD56+ and CD8−CD56+ subsets show higher proportions of GrzB+ lymphocytes within the CD8+CD56+/− and CD8−CD56+ subsets in unstimulated PBMC from both older adult groups (more that one-half) compared to young adults in whom the proportion of GrzB+ lymphocytes was less one-fifth of these two subsets. Because GrzB is activated by a terminal dipeptidylase cleavage [6] at the time of activation of the cytolytic cell, commercially available antibodies to GrzB may not distinguish between active and inactive GrzB in flow cytometric analyses of different CD8+ subsets. This presents a similar challenge to determining the contribution of NK cell to GrzB activity as a large proportion of NK (CD16+) cells contain GrzB. Thus, antibodies to CD56 and CD107a were used to identify cytolytic effector cells within each of the CD8 and NK subsets. Compared to unstimulated controls, influenza-stimulated PBMC showed a substantial increase in the proportion of CD8+ and activated NK (CD8−CD56+) cells that express CD107a (Figure 2A), consistent with the expected proportion of virus-specific CTL responding to virus stimulation.
Figure 2.
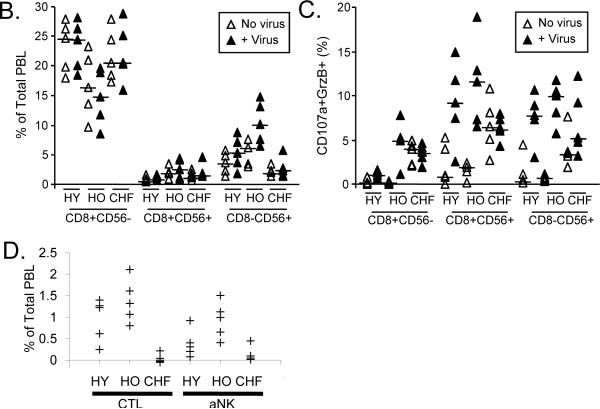
PBMC from healthy young (HY) and older (HO) adults, and older adults with congestive heart failure (CHF) obtained at 4 weeks post-vaccination (2008–09 season) were cultured for 12 hours in the absence (No virus) or presence (+ Virus) of live influenza virus. The proportion of CD8+ (CD56− or CD56+) and CD8−CD56+ within the peripheral blood lymphocyte (PBL) population, and the proportion expressing intracellular granzyme B (GrzB) and the degranulation marker, CD107a, are shown. (A) Representative dot plots are shown for lymphocytes expressing CD8 and/or CD56 (1st column), and the expression of GrzB and CD107a in subsets of CD8+CD56− (2nd column), CD8+CD56+ (3rd column), and CD8−CD56+ (4th column) subsets. (B) The graph shows the individual results for the proportions of lymphocytes that were CD8+CD56−, CD8+CD56+ or CD8−CD56+ in virus-stimulated PBMC and controls; the crossbar indicates the median for each group. The CD8+CD56− subset did not increase with virus stimulation and healthy older adults showed significantly lower proportion of PBL in this subset compared to the other two groups (p=0.04). In contrast, there was a significant increase in the proportion of CD8+CD56+ (activated CTL; p=0.03) and CD8−CD56+ (activated NK cells; p=0.003) in response to virus stimulation. (C) The proportion of lymphocytes expressing CD107a and GrzB is shown for each of the three CD8/CD56 subsets. Overall, there was a significant increase in the proportion of CD107a+GrzB+ in the CD8+CD56− (p=0.0003), CD8+CD56+ (p<0.0001) and CD8−CD56+ (p<0.0001) subsets with virus stimulation. (D) Individual results for the overall response to influenza virus within each of the effector subsets of CTL (CD8+CD56+/−CD107a+GrzB+) and activated NK cells (aNK, CD8−CD56+CD107a+GrzB+) are shown as the % of total PBL. There was a statistically significant increase in the proportion of CD8+ and CD8−CD56+ subsets within the total PBL population (p<0.0001) but the response in both subsets was significantly lower for the CHF group compared to the healthy young and older groups (p<0.005 for CTL, p<0.02 for activated NK cells),
To determine the effect of age and CHF on the response to influenza virus, PBMC from healthy young and older adults and older adults with CHF (n=5/group) were analyzed at 4 weeks post-vaccination (2008–09 season) by flow cytometry for the expression of intracellular granzyme B (GrzB) and the degranulation marker, CD107a, on CD8+ T cells (CD56− or CD56+) and activated NK cells (CD8−CD56+) after 12 hours in culture in the presence or absence of live influenza virus (Representative dot plots, Figure 2A). The proportion of peripheral blood lymphocytes (PBL) that were CD8+CD56− was significantly lower in healthy older adults compared to the other two groups (p=0.04) but did not increase in response to virus stimulation for any of the three study groups. In contrast, there was an overall significant increase in the proportion of cells that were CD8+CD56+ (activated CTL; p=0.03) or CD8−CD56+ (activated NK cells; p=0.003) in response to virus stimulation (Figure 2B). In unstimulated PBMC, both older adult groups compared to the young adult group showed a higher proportion of GrzB+ PBL in the CD8+ subsets (data not shown); in the CHF group, this was associated with an increased proportion of lymphocytes in all three subsets (CD8+CD56+, CD8+CD56−, CD8−CD56+) expressing CD107a (Figure 2C). With virus stimulation, there was an overall significant increase in the proportion of CD107a+GrzB+ in each of the CD8+CD56− (p=0.0003), CD8+CD56+ (p<0.0001) and CD8−CD56+ (p<0.0001) subsets but this response was statistically greater in healthy young and older adults compared to older adults with CHF in all three subsets (Figure 2C). These differences between the three groups resulted in a significantly reduced overall response to influenza virus in the CHF group (Figure 2D); the change with virus stimulation in the proportion of PBL that were CD107a+ and GrzB+ within the CTL (CD8+CD56+/−) and activated NK cell (CD8−CD56+) subsets was significantly lower for the CHF group compared to the healthy young and older groups (p<0.005 for CD8+, p<0.02 for CD8−CD56+). There were too few subjects to determine whether differences between healthy young and older adults were statistically significant.
3.7. Association between antibody titers and influenza outcomes
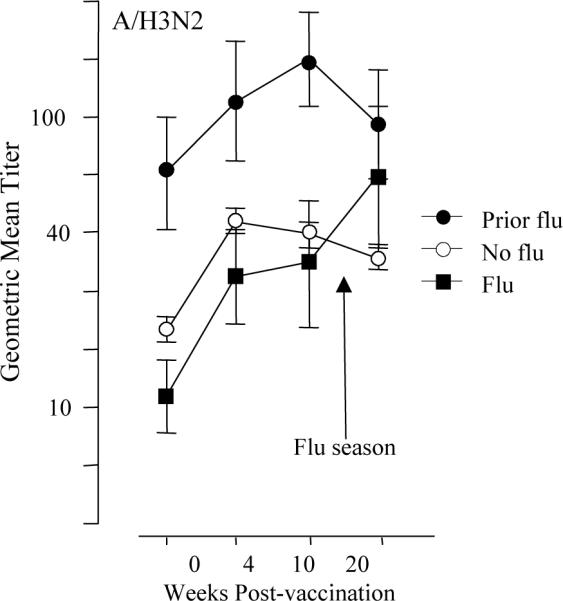
Geometric mean antibody titers for the first year of the study have been previously reported [12]. All subjects in the second year showed a significant antibody response to vaccination for all three strains of virus (p<0.0001 for all three stains; Figure 3, data shown for the A/H3N2 vaccine strain only). The eight subjects with previous LDI expectedly had elevated antibody titers but showed a similar antibody response to vaccination compared to the Flu and NoFlu subsets. Consistent with the first year results, geometric mean antibody titers to the infecting A/H3N2 strain in the second year were not significantly different in the subjects who developed LDI compared to those who did not (Figure 3). Similarly, the mean-fold increase in titer and seroconversion and seroprotection rates to vaccination with the H3N2 strain were not statistically different across the three subsets (Table II). As expected, the prior influenza A/H3N2 infection did not affect antibody titers to the A/H1N1 or B strains (data not shown). However, seroprotection rates were significantly higher for the H1N1 strain in the NoFlu subset compared to the other subsets (Table II), an unexplained observation that requires further exploration.
Figure 3.
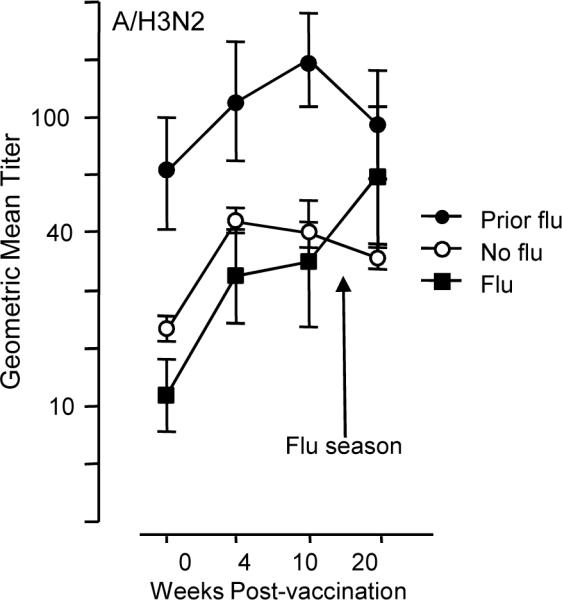
Geometric mean antibody titers measured in serum, pre- (0 wks) and post-vaccination (4- and 10-wks) are shown for the 2004–05 influenza season. In subjects who developed influenza illness (Flu, n=8), antibody titers to the infecting strain, A/Wyoming (A/H3N2), were similar to those who did not develop influenza except at 20-weeks post-vaccination where elevated titers reflect the response to influenza infection in the Flu subset. Prior Flu subjects (n=8) maintained high titers to A/Wyoming due to influenza infection in the previous influenza season but the response to vaccination was similar to the Flu and No Flu subsets. Error bars represent standard error of the mean
Table II.
Seroconversion and Seroprotection Rates to Influenza Vaccination in 2nd Year
| Seroconversion Rates % (n) | Seroprotection Rates % (n) | |||||
|---|---|---|---|---|---|---|
| A/NewCal | A/Wyoming | B/Jillian | A/NewCal | A/Wyoming | B/Jillian | |
| NoFlu | 28 (35) | 37 (46) | 33 (41) | 45 (57)* | 65 (83) | 59 (75) |
| Flu | 13 (1) | 38 (3) | 25 (2) | 13 (1) | 63 (5) | 50 (4) |
| Prior flu | 25 (2) | 13 (1) | 13 (1) | 13 (1) | 88 (7) | 38 (3) |
NoFlu = no influenza in 1st or 2nd year
Flu = influenza in 2nd year
Prior Flu = influenza in 1st year and no influenza in 2nd year
A/NewCal = A/New Caledonia/20/99 (H1N1)
A/Wyoming = A/Wyoming/3/2003 (H3N2)
B/Jillian = B/Jillian/20/2003
p=0.03
4. Discussion
The association between declining cellular immune function and loss of influenza vaccine efficacy with aging is well documented but the underlying mechanism is poorly understood. Clearly, additional correlates of protection to that provided by antibody titers are needed for advances in vaccine technology and the development of more efficacious vaccines for the older adult population. Current influenza vaccines contain split virus particles and thus as killed viruses, are poor stimulators of the CTL response that is largely directed by epitopes derived from the internal proteins of influenza virus in humans. Probably because of CTL memory from prior exposure to natural influenza infection, a CTL response to vaccination can be observed as a change in multiple read-outs of the CTL response to influenza vaccination [17–19] including GrzB activity in PBMC stimulated with live virus[12]. Although both antibody and CTL responses to influenza vaccination may contribute to protection, these responses may not necessarily be correlated [20].
The data presented shows that the ex vivo CTL response to vaccination measured in an assay of GrzB activity predicts susceptibility to influenza illness. This GrzB activity correlates with cytolytic activity in 51Cr-release assays in influenza-stimulated PBMC [8, 21]. GrzB is co-expressed with perforin and is contained in most CD8+ T cells expressing the degranulation marker, CD107a, consistent with a cytolytic effector function. Our results also suggest that activated NK cells contribute to the GrzB activity measured; however, it has been previously shown that expression of IFN-γ and perforin by these activated NK cells is a T-cell dependent process [22]. Thus, the total GrzB response to influenza that is derived from or depends on T cells may be important for protection against influenza. Consistent with the substantial increased risk of complicated influenza illness in older adults with CHF, there was a marked reduction in the proportion of CTL and NK cells responding to influenza virus stimulation.
Preliminary results suggest that the high proportion of GrzB+ CTL in unstimulated PBMC and the poor response to virus stimulation in the CHF group is due to an increase in terminally differentiated effector memory CTL. Further experiments are planned to determine the amount of GrzB activity in purified CTL and NK cell subsets of resting and virus-stimulated PBMC and whether these terminally differentiated CTL can produce active GrzB In response to virus stimulation.
Previous studies in humans included only non-influenza-infected lung tissue. Thus, the question of function and differentiation of T cells can be answered at the site of but not at the time of influenza challenge. These studies in human lung tissue have shown much higher frequencies of influenza virus-specific memory CD8 T cells compared to peripheral blood. However, these lung resident CTL had a relatively late differentiation phenotype and low levels of GrzB compared to the higher levels expressed in virus-specific T cells in peripheral blood [23]. The authors concluded that these lung resident CTL were non-cytotoxic memory cells. Our results are consistent with the findings of this study showing that memory CTL are in the peripheral blood rather than the lungs. In contrast to the CTL response to viral peptides alone [24], we have shown that T cells responding to live influenza virus express high levels of GrzB and significantly increase the expression of CD107a on the cell surface, consistent with the development of a cytolytic effector function in the early stage of infection.
Unlike antibody responses to split-virus vaccines that are largely strain-specific, CTL responses are cross-reactive within most strains of influenza A (or within the strains of influenza B) and CTL memory can be restimulated by exposure to the virus. The data presented shows that older adult CTL have the capacity to mount an enhanced response to influenza vaccination, in this case due to restimulation of CTL memory from natural influenza A/H3N2 infection in the previous year. This is a very important observation suggesting that a reformulated influenza vaccine designed to stimulate the CTL response could improve protection in this population.
An important new development in the GrzB assay is the standardization of activity against a commercially available GrzB standard. The assay can now be validated across multiple laboratories and used to establish a threshold level of GrzB activity in ex vivo PBMC lysates as a correlate of protection. As an example of how this might be applied, it is important to understand that a correlate of protection can only be defined in those subjects with known infection, i.e., those who developed influenza illness. From this study, a protective level could be defined as GrzB activity greater than the upper limit of the 95% confidence interval at 4-weeks post-vaccination in the subset of subjects who subsequently developed influenza illness (200 U/mg protein). Future studies are designed to validate the assay in multiple laboratories and prospectively test this threshold of GrzB activity as a correlate of protection and consistency with current estimates of influenza vaccine efficacy and effectiveness in older people [25, 26].
In two separate influenza seasons, it has been shown that antibody titers do not effectively distinguish those older adults who will go on to develop influenza from those who will not. These results are consistent with an earlier study reporting that of 72 vaccinated elderly who were later confirmed to have influenza infection, 60% should have been protected based on classical hemagglutination inhibition (HI) assays (HI titer ≥ 1:40), with 31% of infected vaccinees having very high titers (HI titres ≥ 1:640) [27]. Together, these data indicate antibody titers are a correlate, not a guarantee of protection; there are apparent successes and apparent failures of inactivated vaccine in older adults that cannot be explained by serum hemagglutination inhibition antibody titers [28].
Poor vaccine-mediated protection in older adults has been attributed to age-related defects in cellular immune function based on polyclonal stimulation, which correlate with protective versus non-protective levels of influenza-specific antibody titers [29, 30]. Further studies combining measures of both humoral and cellular immune responses could shed light on how each of these responding lymphocyte subsets may contribute to cytolytic mechanisms of protection against influenza, and to cytokine-mediated cellular immune responses to influenza vaccination with aging [3, 12, 31].
A potential limitation of this study relates to differences in the timing of the influenza seasons, starting in late November in the first year and in late January in the second year. However, the level of immune markers following vaccination appears to have been the same for both the early and later influenza season, and is consistent with previous results showing that the CTL response to influenza vaccination is maintained for 14–16 weeks following vaccination in this population [17]. A second limitation is that influenza attack rates were too low to detect in subjects who developed influenza, a difference in GrzB levels between older adults with and without CHF. Although subjects with significant CHF (walking less than 1100 ft on the 6-MWT) have a slightly increased GrzB levels in influenza-stimulated PBMC, our flow cytometric studies suggest that this activity may be present at baseline and is associated with a poor response to influenza challenge.
In summary, our results suggest that the assay of GrzB activity in ex vivo virus-stimulated PBMC provides a responsive and complementary measure to antibody titers in the evaluation of influenza vaccine effectiveness in older adults. Further studies are needed to define a threshold level of GrzB activity as a correlate of protection in the development of both seasonal and pandemic influenza vaccines. Finally, we have shown that in spite of age-related changes that compromise immune function, older adults may still have to capacity to respond to reformulated influenza vaccines that are targeted to effectively re-stimulate CTL memory.
Acknowledgements
This work was funded by the National Institutes of Health (NIH), National Institute on Aging, R01 AG20634, and National Institute of Allergy and Infectious Diseases, R01 AI68265 (J. E. M., Principal Investigator). The study was conducted through the Lowell P. Weicker, Jr. General Clinical Research Center funded by the NIH, National Center for Research Resources (Grant Number MO1 RR06192) at the University of Connecticut Health Center (UCHC), and in collaboration with the UConn Center on Aging. The work was also supported by grants from the Canadian Institutes of Health Research (CIHR), and the Canadian Network of Vaccine Centers of Excellence (CANVAC). R.C.B is a CIHR Distinguished Scientist, a Medical Scientist of the Alberta Heritage Foundation for Medical Research (AHFMR), a Canada Research Chair, and a Howard Hughes International Scholar. K. P. K. is an AHFMR Scientist.
We thank Martha Hein, Gloria Borders and the staff of the Lowell P. Weicker, Jr. General Clinical Research Center for co-ordination of the study, Lisa Kenyon-Pesce in the UConn Center on Aging for subject recruitment, Ms. Laura Kearney in the Heart Failure Clinic for her clinical support to this study, and Yen Lemire and Norine Kuhn for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. Jama. 2004;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- [2].Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- [3].Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25(4):599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- [4].McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309(1):13–7. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- [5].Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186(6):859–65. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lord SJ, Rajotte RV, Korbutt GS, Bleackley RC. Granzyme B: a natural born killer. Immunol Rev. 2003;193:31–8. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- [7].Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–17. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- [8].Ewen C, Kane KP, Shostak I, Griebel PJ, Bertram EM, Watts TH, et al. A novel cytotoxicity assay to evaluate antigen-specific CTL responses using a colorimetric substrate for Granzyme B. J Immunol Methods. 2003;276(1–2):89–101. doi: 10.1016/s0022-1759(03)00073-5. [DOI] [PubMed] [Google Scholar]
- [9].Johnson BJ, Costelloe EO, Fitzpatrick DR, Haanen JB, Schumacher TN, Brown LE, et al. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc Natl Acad Sci U S A. 2003;100(5):2657–62. doi: 10.1073/pnas.0538056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174(9):5332–40. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- [11].McElhaney JE, Gravenstein S, Upshaw CM, Hooton JW, Krause P, Drinka P, et al. Granzyme B: a marker of risk for influenza in institutionalized older adults. Vaccine. 2001;19(27):3744–51. doi: 10.1016/s0264-410x(01)00087-1. [DOI] [PubMed] [Google Scholar]
- [12].McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–9. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- [13].WHO Collaborating Center for Influenza BPD . The hemagglutination inhibition test for influenza viruses. DHEW, PHS, CDC, Center for Infectious Disease; Atlanta, GA: 1981. pp. 1–21. revised. [Google Scholar]
- [14].Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- [15].Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2(6):401–9. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- [16].Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004 May 15;172(10):6407–17. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- [17].Powers DC, Belshe RB. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993;167(3):584–92. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- [18].He XS, Holmes TH, Mahmood K, Kemble GW, Dekker CL, Arvin AM, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197(6):803–11. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- [19].Terajima M, Cruz J, Leporati AM, Orphin L, Babon JA, Co MD, et al. Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients. J Virol. 2008;82(18):9283–7. doi: 10.1128/JVI.01047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Co MD, Orphin L, Cruz J, Pazoles P, Rothman AL, Ennis FA, et al. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine. 2008;26(16):1990–8. doi: 10.1016/j.vaccine.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McElhaney JE, Pinkoski MJ, Upshaw CM, Bleackley RC. The cell-mediated cytotoxic response to influenza vaccination using an assay for granzyme B activity. J Immunol Methods. 1996;190(1):11–20. doi: 10.1016/0022-1759(95)00235-9. [DOI] [PubMed] [Google Scholar]
- [22].He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114(12):1812–9. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, et al. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191(10):1710–8. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- [24].Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006 Oct 1;177(7):4330–40. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- [25].Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. Jama. 1994;272(21):1661–5. [PubMed] [Google Scholar]
- [26].Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21(16):1769–75. doi: 10.1016/s0264-410x(03)00070-7. [DOI] [PubMed] [Google Scholar]
- [27].Gravenstein S, Drinka P, Duthie EH, Miller BA, Brown CS, Hensley M, et al. Efficacy of an influenza hemagglutinin-diphtheria toxoid conjugate vaccine in elderly nursing home subjects during an influenza outbreak. J Am Geriatr Soc. 1994;42(3):245–51. doi: 10.1111/j.1532-5415.1994.tb01746.x. [DOI] [PubMed] [Google Scholar]
- [28].Govaert TM, Sprenger MJ, Dinant GJ, Aretz K, Masurel N, Knottnerus JA. Immune response to influenza vaccination of elderly people. A randomized double-blind placebo-controlled trial. Vaccine. 1994;12(13):1185–9. doi: 10.1016/0264-410x(94)90241-0. [DOI] [PubMed] [Google Scholar]
- [29].Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75(24):12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–9. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- [31].Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(2–3):427–39. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]