Abstract
Prior work has shown that a high dose (20 mg/kg) of systemic morphine, required to produce significant analgesia in the acute phase of a contusion injury, undermines the long-term health of treated subjects and increases lesion size. Moreover, a single dose of systemic morphine in the early stage of injury (24 h post-injury) led to symptoms of neuropathic pain 3 weeks later, in the chronic phase. The present study examines the locus of the effects using intrathecal morphine administration. Subjects were treated with one of three doses (0, 30, or 90 μg) of intrathecal morphine 24 h after a moderate contusion injury. The 90-μg dose produced significant analgesia when subjects were exposed to noxious stimuli (thermal and incremented shock) below the level of injury. Yet, despite analgesic efficacy, intrathecal morphine significantly attenuated the recovery of locomotor function and increased lesion size rostral to the injury site. A single dose of 30 or 90 μg of intrathecal morphine also decreased weight gain, and more than doubled the incidence of mortality and autophagia when compared to vehicle-treated controls. Morphine is one of the most effective pharmacological agents for the treatment of neuropathic pain and, therefore, is indispensable for the spinally injured. Treatment can, however, adversely affect the recovery process. A morphine-induced attenuation of recovery may result from increases in immune cell activation and, subsequently, pro-inflammatory cytokine concentrations in the contused spinal cord.
Key words: contusion, inflammation, neuropathic pain, opiate
Introduction
Neuropathic pain is cited as one of the most debilitating consequences of spinal cord injury (SCI) (Anderson, 2004). Chronic pain affects approximately two-thirds of the injured population, with one-third of these patients describing the pain as severe (Siddall and Loesser, 2001). Opioids are highly effective at treating pain. Widerström-Noga and Turk (2003) found that approximately 11% of spinally injured patients using opiates reported feeling “pain free,” 22% were “considerably better,” 48% were “slightly better,” and 19% reported “no effect.” Opiates are clearly an indispensable therapy for the spinally injured population.
Despite widespread use of opiate analgesics, little is known about how this treatment interacts with SCI. To address this issue, we have begun to explore how opiate treatment affects recovery using a rodent contusion injury model. A rodent contusion injury not only produces a pathophysiological profile that closely resembles the clinical SCI condition (Bunge et al., 1993; Hulsebosch, 2002), but also results in symptoms of chronic pain in approximately 80% of subjects (Mills et al., 2001). Rats with a contusion injury exhibit thermal, mechanical, and girdle allodynia (Hulsebosch et al., 2000; Lindsey et al., 2000), making them a valid model for the analyses of neuropathic pain after SCI.
Hook et al. (2007) used the contusion model to assess the effects of systemic morphine given in the early phase of recovery (24 h post-injury). We hypothesized that decreased pain transmission after opiate administration could reduce over-excitation at the site of a contusion injury, and thereby reduce secondary damage and perhaps the development of chronic central pain. To test this hypothesis, our initial studies examined whether morphine could block the adverse effects of uncontrollable nociceptive stimulation applied below the level of injury (Hook et al., 2007). Uncontrollable nociceptive stimulation could result from peripheral injuries that accompany spinal trauma. Moreover, previous studies have shown that this form of stimulation undermines the recovery of motor and sensory function (Grau et al., 2004). Unfortunately, preemptive morphine treatment did not block these adverse effects. In fact, our study showed that a single dose of systemic morphine on the day after a spinal contusion injury exacerbated the effects of nociceptive stimulation (Hook et al., 2007). In this study, contused rats were given one of three doses of morphine (0, 10, or 20 mg/kg) prior to exposure to uncontrollable electrical stimulation or restraint alone. Despite decreasing nociceptive reactivity, rats treated with morphine and shock had higher mortality rates, and displayed allodynic responses to innocuous sensory stimuli 3 weeks later. Independent of shock, morphine also undermined the recovery of sensory function, and rats treated with morphine alone had significantly larger lesions than those treated with vehicle. These results suggest that morphine can attenuate recovery despite a blockade of pain-elicited behavior.
The current study aimed to further the understanding of the effects of morphine in a spinal contusion model by determining its site of action. The effects of morphine could result from a direct pharmacological interaction with molecular processes at the level of the spinal cord. Alternatively, morphine may impact spinal function indirectly by engaging brain-dependent or peripheral processes that adversely affect the recovery process. To address this issue, the current experiment examined whether the local application (intrathecal [i.t.]) of morphine, directly onto the spinal cord, affects recovery after a moderate contusion injury. Morphine was applied 2 cm caudal to the injury site to minimize effects mediated at a supraspinal level, and preclude peripheral effects. Pilot studies indicated that 30 and 90 μg, but not 10 μg of i.t. morphine produced analgesia in the acute phase of injury, compared with vehicle controls. This study assessed the long-term effects of a single dose of 0, 30, and 90 μg of i.t. morphine given in the acute phase of injury. Motor and sensory recovery was monitored for 21 days after morphine administration. Unfortunately, as seen in the systemic model, i.t. morphine administration increased lesion size and undermined the general health of the subjects. Morphine (i.t.) also resulted in signficant attenuation of the long-term recovery of locomotor function.
Methods
Subjects
The subjects were male Sprague-Dawley rats obtained from Harlan (Houston, TX). They were approximately 90–110 days old (300–350 g), and were individually housed in Plexiglas bins [45.7 (length) × 23.5 (width) × 20.3 (height) cm] with food and water continuously available. To facilitate access to the food and water, extra bedding was added to the bins after surgery and long mouse sipper tubes were used so that the rats could reach the water without rearing. Subjects were weighed on days that they were assessed for locomotor function, and were checked daily for signs of autophagia and spasticity. A subject was classified as having spasticity if the limb was in an extended, fixed position and was resistant to movement. Bladders were manually expressed in the morning (8–9:30 a.m.) and evening (6–7:30 p.m.) until subjects regained bladder control, which was operationally defined as three consecutive days with an empty bladder at the time of expression. The rats were maintained on a 12-h light/dark cycle and tested during the last 6 h of the light cycle.
All of the experiments were reviewed and approved by the institutional animal care committee at Texas A&M and all NIH guidelines for the care and use of animal subjects were followed.
Surgery
Subjects received a contusion injury using the MASCIS device (Constantini and Young, 1994; Gruner, 1992). Subjects were anesthetized with isoflurane (5%, gas). Once a stable level of anesthesia was achieved the inspired concentration of isoflurane was lowered to 2–3% and an area extending approximately 4.5 cm above and below the injury site was shaved and disinfected with iodine. A 7.0-cm incision was made over the spinal cord. Next, two incisions were made on either side of the vertebral column, extending about 3 cm rostral and caudal to the T12-T13 segment. The dorsal spinous processes at T12-T13 were removed (laminectomy), and the spinal tissue exposed. The dura remained intact. The vertebral column was fixed within the MASCIS device and a moderate injury was produced by allowing the 10-g impactor (outfitted with a 2.5-mm tip) to drop 12.5 mm. After injury, a 15-cm-long polyethylene (PE-10) cannula, fitted with a 0.23-cm (diameter) stainless steel wire (SWGX-090; Small Parts), was threaded 2 cm under the vertebrae immediately caudal to the injury site. The tubing was inserted into the subarachnoid space. To prevent cannula movement, the exposed end of the tubing was secured to the vertebrae rostral to the injury using an adhesive (Superglue). The wire was then pulled from the tubing and the wound was closed using Michel clips. To help prevent infection, subjects were treated with 100,000 units/kg Pfizerpen (penicillin G potassium) immediately after surgery and again 2 days later. For the first 24 h after surgery, rats were placed in a recovery room maintained at 26.6°C. To compensate for fluid loss, subjects were given 2.5 ml of saline after surgery. Michel clips were removed 14 days after surgery.
Morphine administration
Morphine was administered on the day following surgery. After baseline motor and sensory assessments, rats were assigned to one of three drug conditions (0, 30, or 90 μg of morphine sulfate, n = 8 per condition). Subjects then received their assigned dose of morphine sulfate (Sigma-Aldrich, St. Louis, MO) dissolved in 2 μL of distilled water. The drug injection was followed by a 20-μL injection of saline, to flush the catheter.
Assessments of nociceptive reactivity and sensory function
For the assessment of morphine efficacy, nociceptive reactivity was assessed immediately before and 30 min after i.t. morphine administration. A change from baseline score (reactivity after morphine minus reactivity before morphine) was calculated and used as an index of morphine efficacy. Reactivity was assessed with gradually incremented shock and radiant heat, as described in prior studies (King et al., 1996; McLemore et al., 1999; Crown et al., 2000). Briefly, subjects were placed in the restraining tubes with their tail positioned in a 0.5-cm-deep groove that was cut into an aluminum block. Next, subjects were allowed to acclimate to the apparatus for 15 min. Thermal and shock thresholds were then assessed two times using the tail-flick test. These tests occurred at 2-min intervals, and the last tests were used as baseline tail-flick latencies. The order of thermal and shock assessment were counterbalanced across groups. To confirm that subjects did not respond in the absence of the stimuli, blank trials were also performed. A “false alarm” was recorded if subjects made a motor or vocalization response during the blank tests. The blank trials were performed 1 min before or after each sensory test (in counterbalanced fashion). No false alarms were recorded.
Thermal reactivity was tested using a 375-W movie light that was focused onto the rat's tail with a condenser lens positioned 8 cm below the light source. Shock thresholds were assessed using a manual shocker (BRS/LVE, model SG-903) that allowed continuous variation of shock intensity of 0–2 mA (AC, constant current). Test shocks were applied 7 cm from the base of the tail through electrodes constructed from lightweight fuse clips. Test shock intensity was gradually incremented at a rate of 0.05 mA every 3 s. For testing shock and thermal reactivity, a wire hook that was 10 cm long and covered with heat shrink tubing was taped to the last 2.5 cm of the tail. The hook was placed over an elastic band located 11 cm behind the aluminum block. The flexibility of the elastic band allowed for a tail-flick response while maintaining the rat's tail under the heat source. The latency to vocalize was then assessed. After both movement and vocalization responses were detected, the shock or heat was terminated. If a subject failed to respond, the test trial was automatically terminated after 8 s of heat exposure or after shock intensity reached 1.2 mA.
Locomotor recovery
Locomotor behavior was assessed for 21 days post-injury, using the Basso, Beattie and Bresnahan (BBB) scale (Basso et al., 1995), in an open enclosure (a blue children's wading pool, 99 cm in diameter, 23 cm deep). Baseline motor function was assessed on the day following injury and prior to drug treatment. Locomotor behavior was then scored once per day for 1 week (days 2–7). Subjects were scored every other day from day 9 to day 15 and every third day on days 18 and 21. A video recording of each subject's performance in the open field was obtained on days 1, 2, 4, 7, and 21. Because rodents often remain motionless (freeze) when first introduced to a new apparatus, subjects were acclimated to the observation fields for 5 min per day for 3 days prior to surgery. Each subject was placed in the open field and observed for 4 min. Locomotor behavior was scored using the procedure developed by Basso et al. (1995). Care was taken to ensure that the investigators' scoring behavior had high intra- and inter-observer reliability (all r's > 0.89) and that they were blind to the subject's experimental treatment.
Locomotor scores were transformed, as described in Ferguson et al. (2004), to help assure that the data were amendable to parametric analyses. Briefly, this transformation pools BBB scores of 2–4, removing a discontinuity in the scale. The transformation also pools scores from a region of the scale (scores of 14–21) that is very seldom used under the present injury parameters. By pooling these scores, we obtain an ordered scale that is relatively continuous with units that have approximately equivalent interval spacing. Meeting these criteria allows us to apply metric operations (computation of mean performance across legs), improves the justification for parametric statistical analyses, and increases statistical power. Additional statistical power was achieved by obtaining a measure of locomotor performance 24 h after injury, prior to morphine treatment. Pre-treatment locomotor performance, assessed with the BBB scale, accounted for 56% of the variance in recovery across subjects. By using day 1 as a covariate in an analysis of covariance (ANCOVA), therefore, we substantially reduced unexplained variance and thereby increased statistical power.
Additional measures of motor recovery were obtained at the end of the 3-week recovery period using ladder walk (Soblosky et al., 1997) and beam walk (Hicks and D'Amato, 1975; Von Euler et al., 1996, 1997) tasks. Prior to testing, subjects were habituated to the experimental situation for 3 days (8 min per day). During this period of familiarization, they were trained to traverse a wide beam (48.3 cm) to enter a black box positioned at the end of the beam runway. The beginning of the runway is brightly lit, motivating subjects to move toward the dark box. They were left in the box for 2 min after they had traversed the beam. Subjects were then tested on the beam and ladder walk test.
The beam walk test provides a comparative index of the postural stability of the subjects, as well as a gross measure of paw placement abilities. In this test, the subject's ability to traverse a tapered beam (0.375–6.75 in wide) was assessed. We recorded the width at which each foot failed to plantar place on the beam. The average width across the two legs was used as an index of beam walk performance.
The ladder task provides a measure of the extent to which experimental manipulations affect the fine motor abilities of the hindpaws. In the ladder walk test, the subjects were required to cross a horizontal ladder (20 cm wide; 37 rungs at 2.5 cm spacing) in order to reach the black box. Using post hoc frame-by-frame video analyses, we then ascertained how many times the subjects did not successfully place their hindpaws (their paws slipped between the rungs). The total number of footslips were recorded as a measure of ladder walk performance. Subjects that failed to plantar place on the ladder were given a maximum score of 22 footslips.
Recovery of sensory function
Sensory function was assessed after day 21. We assessed sensory reactivity using the nociceptive stimuli (a gradually incremented shock and radiant heat) and the procedures described previously. On an alternate day (test order was counter-balanced across groups) reactivity to von Frey stimulation was assessed. Subjects were placed into Plexiglas tubes [7.0 cm (internal diameter) × 20 cm (length)] that had 6 (length) × 1.7 (width) cm notches removed from the sides of the base. These slots allowed the hindlegs to hang freely below the tube. Progressively stronger tactile stimuli (von Frey stimuli formed from nylon monofilaments; Semmes-Weinstein Anesthesiometer, Stoelting Co., Chicago, IL) were applied sequentially to the plantar surface of the paw at approximately 2-s intervals until subjects exhibited a paw withdrawal (motor response) and vocalization. If one or both responses were not observed, testing was terminated at a force of 300 × g. Each subject was tested twice on each foot in a counterbalanced ABBA order. Test sequences were spaced 2 min apart. Stimulus intensity was reported using the formula provided by Semmes-Weinstein: Intensity = log10 (10,000 * g force).
Histology
At the end of behavioral testing, subjects were deeply anesthetized (100 mg/kg of pentobarbital, i.p.) and perfused (intracardially) with 4% paraformaldehyde. A 1-cm-long segment of the spinal cord that included the lesion center was taken and prepared for cryostat sectioning. The tissue was sectioned coronally in 20-μm-thick sections, and every 10th slice was preserved for staining. All sections were stained with cresyl violet for Nissl substance and luxol fast blue for myelin (Beattie, 1992; Behrmann et al., 1992).
The total cross-sectional area of the cord and spared tissue was assessed at the lesion center using MicrobrightField software. Sections of ± 600, 1200, 1800, and 2400 μm from the lesion center (rostral and caudal) were also traced and analyzed. Assessments were made by an experimenter who was blind to the subject's treatment condition. Four indices of lesion magnitude were derived: lesion, residual gray matter, residual white matter, and width. To determine the area of lesion, an observer who was blind to the experimental treatments traced around the boundaries of cystic formations and areas of dense gliosis (Basso et al., 1995). Nissl-stained areas that contained neurons and glia of approximately normal densities denoted residual gray matter. White matter was judged spared in myelin-stained areas lacking dense gliosis and swollen fibers. The total area of each cross-section was derived by summing the areas of damage, and gray and white matter. Width was determined from the most lateral points along the transverse plane. These analyses yielded six parameters for each section: white matter area, gray matter area, spared tissue (white + gray), damaged tissue area, net area (white + gray + damage), and section width.
To control for variability in section area across subjects, we applied a correction factor derived from standard undamaged cord sections, taken from age-matched controls. This correction factor is based on section widths and is multiplied by all area measurements to standardize area across analyses (Grau et al., 2004). By standardizing area across sections we were able to estimate the degree to which tissue is “missing” (i.e., tissue loss from atrophy, necrosis, or apoptosis). An accurate assessment of the degree to which a treatment has impacted, or lesioned, the cord includes both the remaining “damaged” tissue as well as resolved lesioned areas. When we sum the amount of “missing” tissue and the measured “damaged” area, we can derive an index of the relative lesion (% relative lesion) in each section that is comparable across sections. We can also compute the relative percent of gray and white matter remaining in each section, relative to intact controls. These measures are highly correlated with various measures of behavioral performance including BBB locomotor scores, recovery of bladder function, and reactivity to shock (Grau et al., 2004).
Statistical analysis
The results were analyzed using analysis of variance (ANOVA). In experiments with a continuous independent variable (e.g., recovery period, rostral-caudal histological sections), mixed-design ANOVAs were used. Trend analyses were also used to identify dose-dependent changes in behavior. In cases where significant between-subject differences were obtained (main effect of a single variable), group means were compared using the Duncan's New Multiple Range Test (p < 0.05).
Group differences on dichotomous variables (e.g., mortality) were evaluated using chi-square or Fisher exact probability tests. These tests allow for comparisons of simple (2 × 2) frequency tables with relatively small samples.
Results
Intrathecal morphine attenuates pain reactivity in the acute stage of injury
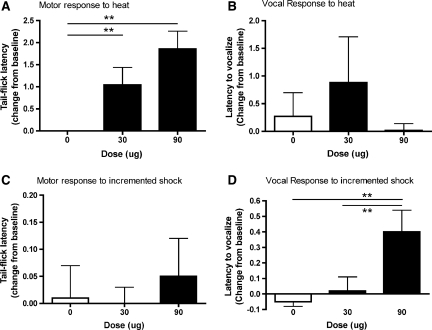
To verify the effectiveness of drug treatment, both a spinal (tail withdrawal from radiant heat) and supraspinal (vocalization to shock) measure of nociceptive reactivity was recorded. An ANCOVA (with pre-treatment baseline scores as the covariate) revealed a significant effect of drug dose on the spinal reactivity tests (F (2, 26) = 3.58, p < 0.05), which assessed the latency to flick the tail away from a radiant heat source. As can be seen in Figure 1A, both the 30- and 90-μg dose significantly increased the latency to tail-flick compared with vehicle controls. There was also a tendency for an increased latency to vocalize to the heat stimulus in the 30-μg treatment group, but this was not significant (F (2, 26) = 3.23, p = 0.06; Fig. 1B). There was no effect of drug dose on the latency to tail-flick to the incremented shock stimulus (F (2, 26) = 2.01, p > 0.05; Fig. 1C). However, there was a main effect of drug dose on the latency to vocalize in response to the shock stimulus (F (2, 26) = 5.41, p < 0.05). As shown in Figure 1D, the 90-μg dose of morphine significantly increased the latency to vocalize when compared with vehicle controls and subjects treated with 30 μg of morphine (p < 0.05).
FIG. 1.
Morphine efficacy was determined by comparing motor and vocal responses to noxious stimulation given before and after intrathecal morphine administration (day 1 post-injury). Change from baseline scores (response after morphine treatment-response before morphine treatment) is depicted. As can be seen in the graphs, the 90-μg dose of intrathecal morphine produced significant analgesia at both a spinal and supraspinal level. Both the 30- and 90-μg dose significantly reduced the latency to flick the tail away from a noxious heat stimulus when compared to vehicle controls (A). There was also a tendency for an increased latency in vocal response to the heat stimulus in the 30-μg treatment group, but it was not significant (B). The 90-μg dose did not affect the motor response to incremented shock (C), but it did increase the latency to make a vocal response to the shock stimulus when compared with subjects treated with 0 or 30 μg of morphine (D). n = 8 for all groups, and in all figures, **p < 0.05.
It should be noted that even the highest (90 μg) dose of i.t. morphine did not eliminate reactivity to noxious stimulation (Fig. 1A,D). There was no evidence of motor paralysis on the tail-flick tasks, or the elimination of a vocal response within a treatment group. These data suggest that despite achieving significant analgesia with i.t. morphine treatment, relative to vehicle controls, the doses were not at the threshold required for complete analgesia.
Intrathecal morphine undermines recovery of locomotor function
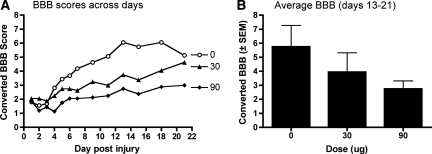
Locomotor scores, collected before treatment on day 1, did not differ across treatment groups (F (2, 21) < 1.0, p > 0.05; Fig. 2). Mean BBB scores on day 1 ranged from 1.81 ± 0.35 (prior to transformation BBB score = 2.11 ± 0.46) for the vehicle-treated group, to 2.06 ± 0.56 (prior to transformation BBB score = 2.55 ± 0.70) for subjects treated with 30 μg of i.t. morphine.
FIG. 2.
There was a dose-dependent attenuation of locomotor function throughout the 21-day recovery period (A). Indeed, subjects treated with 90 μg of intrathecal morphine displayed significantly lower levels of recovery from days 13–21, when locomotor performances had stabilized, than vehicle controls (B). In fact, the 90-μg dose appeared to completely undermine recovery: these subjects began with a converted Basso, Beattie and Bresnahan (BBB) score of 2 prior to treatment and did not improve on the converted (or untransformed) BBB scale.
Morphine treatment resulted in a dose-dependent attenuation of locomotor recovery, assessed with the BBB scale, after injury. Using day 1 scores as a covariate, an ANCOVA verified that there was a significant main effect of drug dose on recovery (F (2, 20) = 5.33, p < 0.05) and a significant interaction between drug dose and days of recovery (F (22, 220) = 1.77, p < 0. 05). As can be seen in Figure 2, treatment with the 90-μg dose significantly attenuated locomotor performance compared with vehicle controls. Trend analyses revealed a significant linear relation between average locomotor scores of the groups over days 13–21, when performances had stabilized (F = 6.43, p < 0.05). Post hoc analyses comparing the group means confirmed that subjects treated with the 90-μg dose of morphine recovered significantly less motor function than vehicle treated controls (p < 0.05; Fig. 2B).
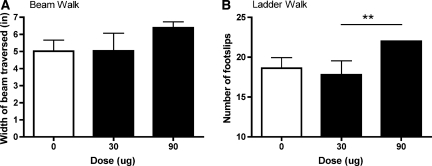
Motor recovery was further assessed at the end of the 21-day recovery period using the tapered beam and ladder walk tests. There was no effect of drug dose on the beam walk task (Fig. 3A). There was, however, a significant main effect of drug dose on the ladder test (F (2, 18) = 3.66, p < 0.05). Subjects treated with 90 μg of morphine made significantly more footfalls than subjects treated with 30 μg of morphine on this task (Fig. 3B). The performance of subjects treated with vehicle and 90 μg of morphine did not differ significantly.
FIG. 3.
Despite achieving analgesic efficacy, subjects treated with 90 μg of morphine displayed lower levels of motor recovery on the ladder task. There was no significant effect of drug dose on the beam walk task (A). However on the ladder task, subjects treated with 90 μg of morphine made significantly more footfalls than the subjects treated with 30 μg of morphine (B). **p < 0.05.
Effects of intrathecal morphine on sensory function
There was no effect of morphine on either motor or vocal reactivity to mechanical stimuli, assessed 21 days after injury (F (2, 15) < 1.33 for both, p > 0.05). There were also no effects of drug dose on motor or vocal responses to either the heat or shock stimuli in the chronic phase of recovery.
Effects of morphine on weight gain after injury
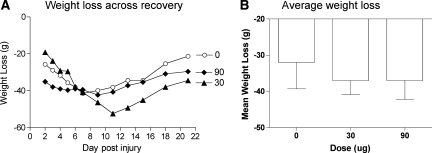
A day after injury, prior to drug treatment, mean weights ranged from 320.33 ± 5.88 to 333.30 ± 5.86 and were not statistically different (F (2, 21) < 1.0, p > 0.05). To control for the variability observed in starting weight within each group, a difference score was calculated by subtracting the starting weight (prior to drug treatment) from the weights observed across recovery. As shown in Figure 4, vehicle-treated rats exhibited weight loss over the first week, and then slowly regained weight over the subsequent two weeks. The impact of morphine treatment depended on both recovery day and dose. Immediately after treatment, subjects given 90 μg exhibited greater weight loss, whereas the 30-μg group showed greater weight loss later in the recovery period. These trends yielded a significant Dose × Days interaction (F (22, 231) = 2.46, p < 0.005).
FIG. 4.
Both the 30- and 90-μg dose of morphine increased weight loss over the recovery period relative to vehicle controls. Changes from baseline weights (weights after day 1−weights on day 1) are depicted across the recovery period (A). Despite beginning at comparable weights, subjects treated with morphine lost weight after morphine treatment and did not regain this weight during the 21-day recovery period. The mean weight loss (±SEM) for each treatment condition is shown (B).
Effects of morphine on autophagia, mortality, spasticity, and bladder function
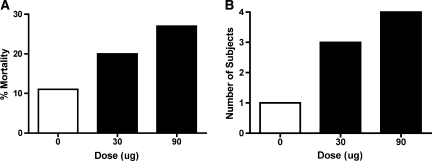
The impact of morphine treatment on mortality and autophagia is illustrated in Figure 5A,B. While morphine treatment was associated with greater mortality, this effect did not approach statistical significance. (To achieve a balanced design, additional subjects were folded into the experimental groups when a subject died. To assess whether this affected the results obtained for locomotor recovery, we examined whether the pattern of results changed when we excluded the added subjects. An ANCOVA verified that there was no change in the overall pattern of results.) Morphine treatment did appear to enhance autophagia, but this effect did not reach statistical significance (χ2 (2) = 5.33, p = 0.07). Morphine treatment had no effect on spasticity (χ2 (2) < 1.0, p > 0.05) or the recovery of bladder function (F (2, 21) < 1.0, p > 0.05).
FIG. 5.
The incidence of mortality (A) and autophagia (B) are presented for each of the groups. While mortality did not differ significantly across groups, treatment with both the 30 and 90 μg of intrathecal morphine more than doubled the incidence of autophagia compared with vehicle controls.
Intrathecal morphine increases tissue loss rostral to the site of injury
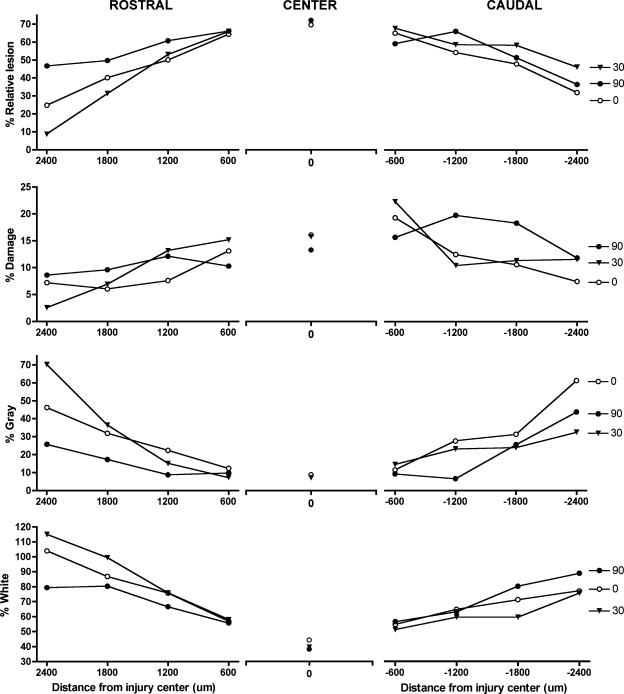
There was no effect of morphine at the lesion center, for any histological indice. The relative lesion (damage + missing), and areas of remaining gray and white matter were comparable across groups. Similarly, no significant effects of drug dose were found caudal to the injury site. In sections rostral to the injury site, however, significant group differences emerged. Morphine significantly affected the relative lesion area and the area of gray matter remaining rostral to the injury site. Although there was no main effect of dose on the relative lesion (F (2, 14) = 2.32, p > 0.05), there was a significant Dose × Section Level interaction (F (6, 42) = 2.74, p < 0.05). As shown in Figure 6, subjects treated with 90 μg of morphine displayed a higher percentage relative lesion across the 1800 μm rostral to the injury site. Individual ANOVAs confirmed that subjects treated with 90 μg of morphine had a larger percentage relative lesion rostral to the injury compared with the 30-μg group (F (1, 8) = 12.87, p < 0.01). No other significant differences emerged between groups for this measure. The increased damage in morphine-treated subjects appeared to be due to a loss of gray matter. Although there was no main effect of dose (F (2, 14) = 1.61, p > 0.05), there was a significant Dose × Section Level interaction for the percentage gray remaining (F (6, 42) = 2.65, p < 0.05). Individual comparisons between percentage gray in subjects treated with 90 μg of morphine and the 30-μg group revealed a main effect of dose (F (1, 8) = 5.52, p < 0.05), and a Dose × Section Level interaction (F (3, 24) = 4.58, p < 0.05). Subjects treated with 90 μg of morphine lost significantly more gray matter rostral to the injury compared to the 30-μg group, but no differences emerged in comparisons to the vehicle group. There were also no significant effects of dose on the percentage white matter remaining rostral to the lesion. Interestingly, there was also a significant Dose × Section Level interaction for the percentage damage rostral to the injury (F (6, 42) = 3.04, p < 0.05), an effect due to higher levels of damage in subjects treated with 30 μg of morphine relative to the 90-μg group and vehicle controls (Fig. 6). Individual ANOVAs indicate that, rather than “missing” tissue rostral to the lesion, subjects treated with 30 μg of morphine displayed larger cystic formations and areas of dense gliosis than subjects in the other groups. There were significant Dose × Section Level interactions when percentage damage was compared for the vehicle and 30-μg treatment groups (F (3, 27) = 5.15, p < 0.05), and the 30-μg and 90-μg conditions (F (3, 24) = 5.44, p < 0.01). There was no significant difference in the percentage damage for the 90-μg treatment group compared with vehicle controls.
FIG. 6.
There were significant effects of morphine on lesion size and the amount of gray tissue remaining rostral (left column) to the center of the injury. In particular, the 90-μg treatment group had significantly more relative lesion (% damage + % missing) rostral to the injury site with less gray and white matter sparing. Data for the center of the lesion (middle column) and caudal to the lesion center (right column) are also depicted.
Discussion
Morphine (i.t.) applied in the acute phase of injury significantly attenuated long-term motor recovery and general health. Subjects treated with a single dose of 30 or 90 μg of morphine displayed significantly lower levels of motor recovery than vehicle controls. In fact, subjects treated with the 90-μg dose recovered very little motor function over the 21-day assessment period. In addition to locomotor function, treatment with the 90-μg dose of morphine increased lesion size rostral to the injury site, decreased weight gain, and increased autophagia. These data extend the results of the systemic study (Hook et al., 2007), demonstrating that changes in the central molecular environment may leave the injured spinal cord vulnerable to the adverse effects linked with morphine administration. Morphine (i.t.) is sufficient to undermine recovery.
Effects of morphine on motor recovery
The data presented here indicate that even relatively low doses of morphine in the acute phase of a contusion injury significantly attenuate recovery of motor function. In fact, the 90-μg dose, which was required to achieve significant analgesia (for both types of nociceptive stimuli and for spinal and supraspinal measures; Fig. 1) when compared with vehicle controls, not only undermined general locomotor recovery it also attenuated fine motor control on the ladder task in subjects tested 3 weeks after morphine administration. These data show that i.t. administration increases the adverse effects of morphine on motor recovery, when compared with systemic administration (Hook et al., 2007). In the clinical setting, SCI patients will generally receive systemic morphine for the initial treatment of acute pain. In cases of epidural administration, however, these data caution against morphine use in the early phase of treatment. These data also underscore the importance of further investigation of the window of vulnerability to i.t. morphine in the contusion model.
To our knowledge, this is the first study to report long-term adverse effects of morphine on recovery of motor function. Others have found, however, that relatively low doses of i.t. morphine can transiently impair motor function (Kakinohana et al., 2003; Yaksh et al., 2003; Fuchigami et al., 2006). Using a clinically low dose of i.t. morphine (30 μg), Kakinohana et al. (2003) found that treatment potentiated transient motor dysfunction following a noninjurious interval of spinal ischemia. The paraparesis observed in this study lasted only 4.5 h, but histological analyses of the spinal cords 48 h after morphine treatment revealed an occasional presence of dark-staining α-motoneurons. Kakinohana et al. (2003) suggest that these dark motoneurons may be markers of pre-degenerative neuronal injury that was aggravated by the administration of opioids.
Yaksh et al. (2003) also reported that chronic (7+ days) i.t. infusion of morphine resulted in the development of spasticity and motor dysfunction in intact dogs. Morphine produced a time and dose-dependent attenuation of motor function due to the development of local inflammatory masses forming at the catheter tip and compressing the spinal cord (causing upper motor neuron lesions). These inflammatory masses consisted of accumulations of neutrophils, monocytes, macrophages, and plasma cells, and displayed significant IL-1β, TNF-α, iNOS, and eNOS immunoreactivity (Yaksh et al., 2003). Importantly, the development of inflammatory masses was not simply related to catheter placement or infusion per se as it was not observed in saline or clonidine controls. Instead it appears that the infusion of morphine led to the development of aseptic i.t. masses. These findings have been replicated in sheep (Gradert et al., 2003) and observed in human patients (Coffey and Burchiel, 2002; Follett, 2003). Alarmingly, in human patients these inflammatory masses have been reported to result in paraplegia and significant spinal cord lesions (Coffey and Burchiel, 2002). Morphine appears to induce a local inflammatory response in the spinal cord, thereby producing neurological changes that can significantly undermine motor and sensory function.
The interactions between morphine and the immune system are particularly important in the contusion model. Inflammation is a hallmark of an acute spinal contusion injury (Popovich and Jones, 2003). Within minutes of injury, there are elevated levels of pro-inflammatory cytokines in the damaged spinal tissue. Recently, Kigerl et al. (2007) demonstrated that the toll-like receptors (TLRs), TLR2 and TLR4, play a significant role in regulating inflammation and gliosis after SCI. TLR4 mutant (C3H/HeJ) and TLR2 knockout mice had impaired locomotor recovery after SCI, and reduced expression of pro-inflammatory cytokines (IL-1β and TNF-α). These data suggest that TLRs are important for coordinating pathophysiological changes after SCI, providing a form of neuroprotection under normal activation conditions. Interestingly, emerging evidence suggests that the TLR4 receptor also plays a significant role in morphine tolerance and the development of “paradoxical pain” with chronic morphine exposure (Tanga et al., 2005; Hutchinson et al., 2007; Ledeboer et al., 2007). Morphine appears to activate spinal glia through non-classic opiate receptors, such as the TLR4 receptor, potentiating the release of neuroexcitatory substances including pro-inflammatory cytokines, nitric oxide, reactive oxygen species, and pro-inflammatory chemokines (Watkins et al., 2007). As even acute morphine exposure appears to increase the expression of TLR4 at both the transcriptional and translational levels (Hutchinson et al., 2007), these data have significant implications for the contusion model. Acute morphine administration may disturb a critical balance in immune cell activation in the injured spinal cord, disrupting the normal physiological sequelae underlying recovery of function after injury and resulting in the adverse effects of morphine seen in the present study. Indeed, emerging evidence suggests that a maladaptive response of glial cells to injury (“gliopathy”) may underlie the development of neuropathic pain in a contusion model (Crown et al., 2008; Detloff et al., 2008; Hulsebosch, 2008).
Loss of tissue rostral to the injury in the rats treated with 90 μg of morphine may be one consequence of the increased glial activation. While increased levels of pro-inflammatory cytokines have not been documented after a single lumbrosacral i.t. infusion of morphine in intact rats (10 μg) (Johnston et al., 2004), the vulnerable state of the contused cord is likely to potentiate the effects of morphine. Indeed, Raghavendra et al. (2002) found that the development of tolerance and increased pro-inflammatory cytokine levels are exaggerated in nerve-injured rats exposed to chronic morphine administration. Increased levels of pro-inflammatory cytokines, and other neuroexcitatory substances, have significant implications for recovery at multiple levels. First, pro-inflammatory cytokines and chemokines appear to reduce the acute analgesic efficacy of morphine (Johnston et al., 2004). Second, these substances have also been implicated in the development of morphine tolerance (Raghavendra et al., 2002; Watkins et al., 2007), an effect that may be mediated, in part, by the downregulation of spinal cord dorsal horn GLAST and GLT-1 glutamate transporters (Tai et al., 2006) and in increased levels of extracellular excitatory amino acids (Mao et al., 2002; Tai et al., 2006). Unfortunately, any increase in glutamate levels due to morphine would further exacerbate the elevated concentrations observed in the acute phase of spinal injury (McAdoo et al., 1997). Increased levels of excitatory amino acids, in turn, may contribute to the development of paradoxical pain with sensitization of pain transmission neurons (Watkins et al., 2007), due to the phosphorylation and upregulation of AMPA (De Vry et al., 2004) and NMDA (Chen and Huang, 1991; Viviani et al., 2003) receptors with a concurrent decrease in GABA receptor levels (Stellwagon et al., 2005). A rise in extracellular glutamate levels could then saturate neuronal NMDA and AMPA receptors promoting increased neuronal excitability as well as excitotoxicity, and the death of spinal neurons. Overall, morphine combined with an intrinsic inflammatory response in the acute contusion injury model is likely to enhance neuronal excitability ultimately potentiating the development of acute morphine tolerance, “paradoxical pain,” and the exacerbation of lesion size.
It should also be noted that there appeared to be a dose-dependent effect of morphine on the loss of tissue rostral to the injury site. There was increased relative lesion and decreased gray matter sparing in subjects treated with 90 μg of morphine relative to those treated with 30 μg. These data further suggest that the detrimental effects of morphine may be due to the disruption of a critical balance between beneficial and toxic effects dependent on cytokine concentrations (Yang et al., 2005). In the acute phase of injury, intrinsic immune activation appears to be necessary for the resolution of injury, with the removal of damaged and dying cells (Kigerl et al., 2007), and it is essential for initiation of the early phases of tissue repair (Li et al., 2001). Based on these beneficial components of immune activation some have advocated amplifying the acute immune response (Rapalino et al., 1998; Schwartz et al., 1999; Schwartz and Kipnis, 2001). Others, however, caution against this approach (Popovich and Jones, 2003). Our data suggest that the arguments of both sides of this controversy may be equally valid, but that the margins for manipulating immune function may be very narrow. While 30 μg of morphine may have increased the beneficial effects of immune function in the injured spinal cord, a single injection of 90 μg of morphine tipped the balance toward detrimental effects. Further studies of the dose-dependent effects of i.t. morphine are warranted.
The loss of neural tissue rostral to the injury site may explain the lack of motor recovery seen in the subjects treated with 90 μg of i.t. morphine. Alternatively, attenuation of recovery may result from a loss in functional plasticity because of the saturation of neural circuits mediating motor behaviors. Other studies in our laboratory focus on spinal cord learning, using a transection model in which communication between the brain and the spinal cord is eliminated. These studies have repeatedly demonstrated that the isolated spinal cord is able to learn response-outcome (instrumental) relationships (Grau et al., 1998, 2006; Crown et al., 2002). This capacity for learning, however, is blocked when subjects are exposed to peripheral inflammatory agents, such as intradermal capsaicin and formalin (Ferguson et al., 2006; Hook et al., 2008). Capsaicin and formalin generate a state of “central sensitization” in the spinal cord. Central sensitization results in the potentiation of responses of nociceptive neurons in the spinal dorsal horn (Simone et al., 1991; Willis, 2002), and is thought to be one mechanism underlying the development of neuropathic pain. Central sensitization may also induce a diffuse state of overexcitation in the spinal cord that saturates plasticity and precludes the subsequent learning of selective response-outcome relations (Ferguson et al., 2006; Hook et al., 2008). In a spinal contusion model accompanying peripheral injuries and/or acute morphine exposure, as discussed above, may also saturate spinal neural circuits that are necessary for “re-learning” or recovering basic locomotor functions (Edgerton et al., 2001). Interestingly, the spinal circuits mediating instrumental learning are localized to the L4-S2 region of the cord (Liu et al., 2005), which is essentially the same location as the central pattern generator supporting locomotor behavior (Langlet et al., 2005; Barthelemy et al., 2006; Lavrov et al., 2006).
Effects of morphine on weight, mortality, and autophagia
In addition to the adverse effects on locomotor recovery, treatment with 30 and 90 μg of i.t. morphine severely compromised the general health of the subjects. These subjects lost significantly more weight than the vehicle-treated subjects throughout the recovery period, and displayed more than twice the incidence of autophagia. In fact, we have replicated the effects of 90 μg of morphine on autophagia in a subsequent study. Combining data across the two studies, we have found that morphine significantly increases the incidence of autophagia (n = 18 for each group, χ2 (1) = 5.79, p < 0.02). These data suggest that like systemic morphine, i.t. morphine produces symptoms of neuropathic pain. (Autophagia is thought to result from neuropathic pain in animals and humans with central nervous system lesions [Mailis, 1996; Frost et al., 2008].)
For weight loss, all groups initially lost some weight following surgery, but whereas subjects in the vehicle treatment group began to regain weight, those in the 30- and 90-μg groups did not. The cause of the demise in general health is difficult to explain given that morphine was applied only once at the spinal level. It is possible, however, that sensitization of pain transmission neurons in the spinal cord, and an enhanced release of Substance P from presynaptic terminals affected the function of the hypothalamic-pituatary-adrenal (HPA) axis and the stress response of subjects treated with morphine. Choi et al. (2004) found that a single i.t. injection of Substance P significantly increased c-fos mRNA levels in the HPA axis, an effect that was consistently demonstrated with repeated i.t. injections. Long-term changes in pain processing at the level of the spinal cord may dramatically alter the stress response of subjects treated with i.t. morphine. Both the weight loss and autophagia could be indicative of increased stress responses in these treatment groups. This alarming consequence of i.t. morphine administration must be further investigated.
In contrast to the systemic study, i.t. morphine did not increase mortality. It is tempting, therefore, to suggest that the effects of mortality were due to morphine's actions at a non-spinal site. It should be noted, however, that in the systemic model mortality was increased only when morphine administration was combined with a nociceptive shock stimulus. Mortality did not occur with systemic morphine administration alone. In the present study, the mortality that did occur in the morphine- treated groups occurred long after administration (average mortality = 7.8 days) and appeared to parallel a decrease in general health, including the loss of weight and the development of more severe signs of autophagia. We hypothesize that, like autophagia and weight loss, the mortality seen in the systemic model reflects potentiation of a stress response and a demise in general health, rather than any direct effects of morphine.
Clinical implications
The data presented here suggest that morphine given in the acute phase of a contusion injury, and particularly i.t. morphine, can severely compromise recovery of function at multiple levels. Because of the dramatic effects of this treatment, we suggest that morphine should not be used as an analgesic in the acute phase of injury. However, as previously noted, morphine is one of the most effective analgesics for the treatment of neuropathic pain after a SCI and is, therefore, indispensable for this population. Neuropathic pain symptoms tend to develop 6 months to 5 years after injury, presumably as sensory functioning increases. At this stage of injury, morphine's adverse effects may not be as dramatic, but this is unknown. Indeed, glial priming in the acute phase of injury may leave the spinal cord vulnerable to the effects of morphine long after the immediate inflammatory response has subsided. Given the prevalence of neuropathic pain in the spinally injured, and its significant effect on quality of life, it is essential that we further investigate the molecular mechanisms underlying the efficacy and secondary consequences of prescribed analgesics in these populations. In particular, the data presented here must challenge us to identify the mechanisms underlying the development of neuropathic pain and the interactions between morphine and molecular changes inherent to a SCI.
Acknowledgments
We would like to thank Kyle Baumbauer, Russell Huie, Thomas Prentice, Daniel Woodie, and Amanda Brown for their comments on an earlier draft of this manuscript. A portion of the data from this study has been previously presented in abstract form. This study was supported by DA020596, HD058412, and NS041548.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Barthélemy D. Leblond H. Provencher J. Rossignol S. Nonlocomotor and locomotor hindlimb responses evoked by electrical microstimulation of the lumbar cord in spinalized cats. J. Neurophysiol. 2006;96:3273–3292. doi: 10.1152/jn.00203.2006. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beattie M.S. Anatomic and behavioral outcome after spinal cord injury produced by a displacement controlled impact device. J. Neurotrauma. 1992;9:157–160. doi: 10.1089/neu.1992.9.157. [DOI] [PubMed] [Google Scholar]
- Behrmann D.L. Bresnahan J.C. Beattie M.S. Shah B.R. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- Bunge R.P. Puckett W.R. Becerra J.L. Marcillo A. Quencer R.M. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. In: F.J. Seil., editor. Advances in Neurology. Vol. 59. Raven Press; New York: 1993. pp. 328–339. [PubMed] [Google Scholar]
- Chen L. Huang L.M. Sustained potentiation of NMDA receptors mediated glutamate responses through activation of protein kinase C by a μ opioid. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Choi S.S. Lee H.K. Shim E.J. Kwon M.S. Seo Y.J. Lee J.Y. Suh H.W. Alterations of c-Fos mRNA expression in hypothalamic-pituitary-adrenal axis and various brain regions induced by intrathecal single and repeated substance P administrations in mice. Arch. Pharm. Res. 2004;27:863–866. doi: 10.1007/BF02980180. [DOI] [PubMed] [Google Scholar]
- Coffey R.J. Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: report and observations on 41 patients. Neurosurgery. 2002;50:78–86. doi: 10.1097/00006123-200201000-00014. [DOI] [PubMed] [Google Scholar]
- Constantini S. Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Crown E.D. King T.E. Meagher M.W. Grau J.W. Shock-induced hyperalgesia: III. Role of the bed nucleus of the stria terminalis and amygdaloid nuclei. Behav. Neurosci. 2000;114:561–573. [PubMed] [Google Scholar]
- Crown E.D. Ferguson A.R. Joynes R.L. Grau J.W. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav. Neurosci. 2002;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Crown E.D. Gwak Y.S. Ye Z. Johnson K.M. Hulsebosch C.E. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff M.R. Fisher L.C. McGaughy V. Longbrake E.E. Popovich P.G. Basso D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVry J. Kuhl E. Fanken-Kunkel P. Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur. J. Pharmacol. 2004;491:137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Edgerton V.R. Leon R.D. Harkema S.J. Hodgson J.A. London N. Reinkensmeyer D.J. Roy R.R. Talmadge R.J. Tillakaratne N.J. Timoszyk W. Tobin A. Retraining the injured spinal cord. J. Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.R. Hook M.A. Garcia G. Bresnahan J.C. Beattie M.S. Grau J.W. A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma. 2004;21:1601–1613. doi: 10.1089/neu.2004.21.1601. [DOI] [PubMed] [Google Scholar]
- Ferguson A.R. Crown E.D. Grau J.W. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Follett K.A. Intrathecal morphine and inflammatory masses. Anesthesiology. 2003;99:5–6. doi: 10.1097/00000542-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Frost F.S. Mukkamala S. Covington E. Self-inflicted finger injury in individuals with spinal cord injury: an analysis of 5 cases. J. Spinal Cord Med. 2008;31:109–116. doi: 10.1080/10790268.2008.11753991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchigami T. Kakinohana M. Nakamura S. Murata K. Sugahara K. Intrathecal nicorandil and small-dose morphine can induce spastic paraparesis after a noninjurious interval of spinal cord ischemia in the rat. Anesth. Analg. 2006;102:1217–1222. doi: 10.1213/01.ane.0000198634.25504.83. [DOI] [PubMed] [Google Scholar]
- Grau J.W. Barstow D.G. Joynes R.L. Instrumental learning within the spinal cord: I. Behavioral properties. Behav. Neurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau J.W. Washburn S.N. Hook M.A. Ferguson A.R. Crown E.D. Garcia G. Bolding K.A. Miranda R.C. Uncontrollable stimulation undermines recovery after spinal cord injury. J. Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Grau J.W. Crown E.D. Ferguson A.R. Washburn S.N. Hook M.A. Miranda R.C. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav. Cogn. Neurosci. Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Gradert T.L. Baze W.B. Satterfield W.C. Hildebrand K.R. Johansen M.J. Hassenbusch S.J. Safety of chronic intrathecal morphine infusion in a sheep model. Anesthesiology. 2003;99:188–198. doi: 10.1097/00000542-200307000-00029. [DOI] [PubMed] [Google Scholar]
- Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Hicks S.P. D'Amato C.J. Motor-sensory cortex-corticospinal system and developing locomotion and placing in rats. Am. J. Anat. 1975;143:1–42. doi: 10.1002/aja.1001430102. [DOI] [PubMed] [Google Scholar]
- Hook M.A. Liu G.T. Washburn S.N. Ferguson A.R. Bopp A.C. Huie J.R. Grau J.W. The impact of morphine after a spinal cord injury. Behav. Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook M.A. Huie J.R. Grau J.W. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav. Neurosci. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C.E. Xu G.Y. Perez-Polo J.R. Westlund K.N. Taylor C.P. McAdoo D.J. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Hulsebosch C.E. Pharmacology of chronic pain after spinal cord injury: novel acute and chronic intervention strategies. In: R.P. Yezierski., editor; K.J. Burchiel., editor. Spinal Cord Injury Pain: Assessment, Mechanisms, Management. Progress in Pain Research and Management. IASP Press; Seattle: 2002. pp. 189–204. [Google Scholar]
- Hulsebosch C.E. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp. Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M.R. Bland S.T. Johnson K.W. Rice K.C. Maier S.F. Watkins L.R. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci. World J. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I.N. Milligan E.D. Wieseler-Frank J. Frank M.G. Zapata V. Campisi J. Langer S. Martin D. Green P. Fleshner M. Leinwand L. Maier S.F. Watkins L.R. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinohana M. Marsala M. Carter C. Davison J.K. Yaksh T.L. Neuraxial morphine may trigger transient motor dysfunction after a noninjurious interval of spinal cord ischemia: a clinical and experimental study. Anesthesiology. 2003;98:862–870. doi: 10.1097/00000542-200304000-00012. [DOI] [PubMed] [Google Scholar]
- King T.E. Joynes R.L. Meagher M.W. Grau J.W. Impact of shock on pain reactivity: II. Evidence for enhanced pain. J. Exp. Psychol. Anim. Behav. Process. 1996;22:265–278. doi: 10.1037//0097-7403.22.3.265. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A. Lai W. Rivest S. Hart R.P. Satoskar A.R. Popovich P.G. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Langlet C. Leblond H. Rossignol S. Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J. Neurophysiol. 2005;93:2474–2488. doi: 10.1152/jn.00909.2004. [DOI] [PubMed] [Google Scholar]
- Lavrov I. Gerasimenko Y.P. Ichiyama R.M. Courtine G. Zhong H. Roy R.R. Edgerton V.R. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J. Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- Ledeboer A. Liu T. Shumilla J.A. Mahoney J.H. Vijay S. Gross M.I. Vargas J.A. Sultzbaugh L. Claypool M.D. Sanftner L.M. Watkins L.R. Johnson K.W. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2007;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. Carpio D.F. Zheng Y. Bruzzo P. Singh V. Ouaaz F. Medzhitov R.M. Beg A.A. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- Lindsey A.E. LoVerso R.L. Tovar C.A. Hill C.E. Beattie M.S. Bresnahan J.C. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil. Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- Liu G.T. Ferguson A.R. Crown E.D. Bopp A.C. Miranda R.C. Grau J.W. Instrumental learning within the rat spinal cord: localization of the essential neural circuit. Behav. Neurosci. 2005;119:538–547. doi: 10.1037/0735-7044.119.2.538. [DOI] [PubMed] [Google Scholar]
- Mailis A. Compulsive targeted self-injurious behaviour in humans with neuropathic pain: a counterpart of animal autotomy? Four case reports and literature review. Pain. 1996;64:569–578. doi: 10.1016/0304-3959(95)00173-5. [DOI] [PubMed] [Google Scholar]
- Mao J. Sung B. Ji R.R. Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J. Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdoo D.J. Hughes M.G. Xu G. Robak G. de Castro R. Microdialysis studies of the role of chemical agents in secondary damage upon spinal cord injury. Neurotrauma. 1997;14:507–515. doi: 10.1089/neu.1997.14.507. [DOI] [PubMed] [Google Scholar]
- McLemore S. Crown E.D. Meagher M.W. Grau J.W. Shock-induced hyperalgesia: II. Role of the dorsolateral periaqueductal gray. Behav. Neurosci. 1999;113:539–549. doi: 10.1037//0735-7044.113.3.539. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Hains B.C. Johnson K.M. Hulsebosch C.E. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J. Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Jones T.B. Manipulating neuroinflammatory reactions in the injured spinal cord:back to basics. Trends Pharm. Sci. 2003;24:13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Raghavendra V. Rutkowski M.D. DeLeo J.A. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapalino O. Lazarov-Spiegler O. Agranov E. Velan G.J. Yoles E. Fraidakis M. Solomon A. Gepstein R. Katz A. Belkin M. Hadani M. Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat. Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Cohen I. Lazarov-Spiegler O. Moalem G. Yoles E. The remedy may lie in ourselves: prospects for immune cell therapy in central nervous system protection and repair. J. Mol. Med. 1999;77:713–717. doi: 10.1007/s001099900047. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol. Med. 2001;7:252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. Loesser J.D. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Simone D.A. Sorkin L.S. Oh U. Chung J.M. Owens C. LaMotte R.H. Willis W.D. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J. Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Soblosky J.S. Colgin L.L. Chorney-Lane D. Davidson J.F. Carey M.E. Some functional recovery and behavioral sparing occurs independent of task-specific practice after injury to the rat's sensorimotor cortex. Behav. Brain Res. 1997;89:51–59. doi: 10.1016/s0166-4328(97)00049-1. [DOI] [PubMed] [Google Scholar]
- Tai Y.H. Wang Y.H. Wang J.J. Tao P.L. Tung C.S. Wong C.S. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124:77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Tanga F.Y. Nutile-McMenemy N. DeLeo J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B. Bartesaghi S. Gardoni F. Vezzani A. Behrens M.M. Bartfai T. Binaglia M. Corsini E. Di Luca M. Galli C.L. Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Euler M. Akesson E. Samuelsson E.B. Seiger A. Sundstrom E. Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp. Neurol. 1996;137:242–254. doi: 10.1006/exnr.1996.0023. [DOI] [PubMed] [Google Scholar]
- Von Euler M. Seiger A. Sundstrom E. Clip compression injury in the spinal cord: a correlative study of neurological and morphological alterations. Exp. Neurol. 1997;145:502–510. doi: 10.1006/exnr.1997.6481. [DOI] [PubMed] [Google Scholar]
- Watkins L.R. Hutchinson M.R. Ledeboer A. Wieseler-Frank J. Milligan E.D. Maier S.F. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav. Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widerstrom-Noga E.G. Turk D.C. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord. 2003;41:600–609. doi: 10.1038/sj.sc.3101511. [DOI] [PubMed] [Google Scholar]
- Willis W.D. Long-term potentiation in spinothalamic neurons. Brain Res. Brain Res. Rev. 2002;40:202–214. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- Yaksh T.L. Horais K.A. Tozier N.A. Allen J.W. Rathbun M. Rossi S.S. Sommer C. Meschter C. Richter P.J. Hildebrand K.R. Chronically infused intrathecal morphine in dogs. Anesthesiology. 2003;99:174–187. doi: 10.1097/00000542-200307000-00028. [DOI] [PubMed] [Google Scholar]
- Yang L. Jones N.R. Blumbergs P.C. Van Den Heuvel C. Moore E.J. Manavis J. Sarvestani G.T. Ghabriel M.N. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J. Clin. Neurosci. 2005;12:276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]