Abstract
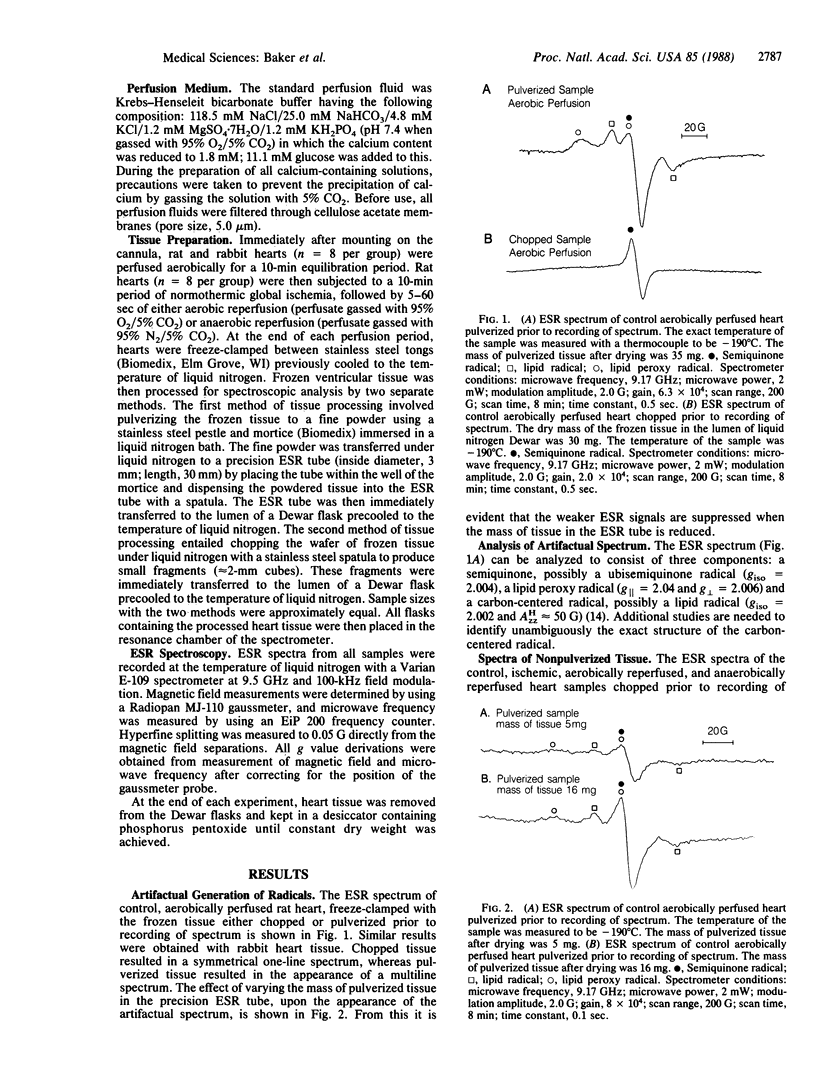
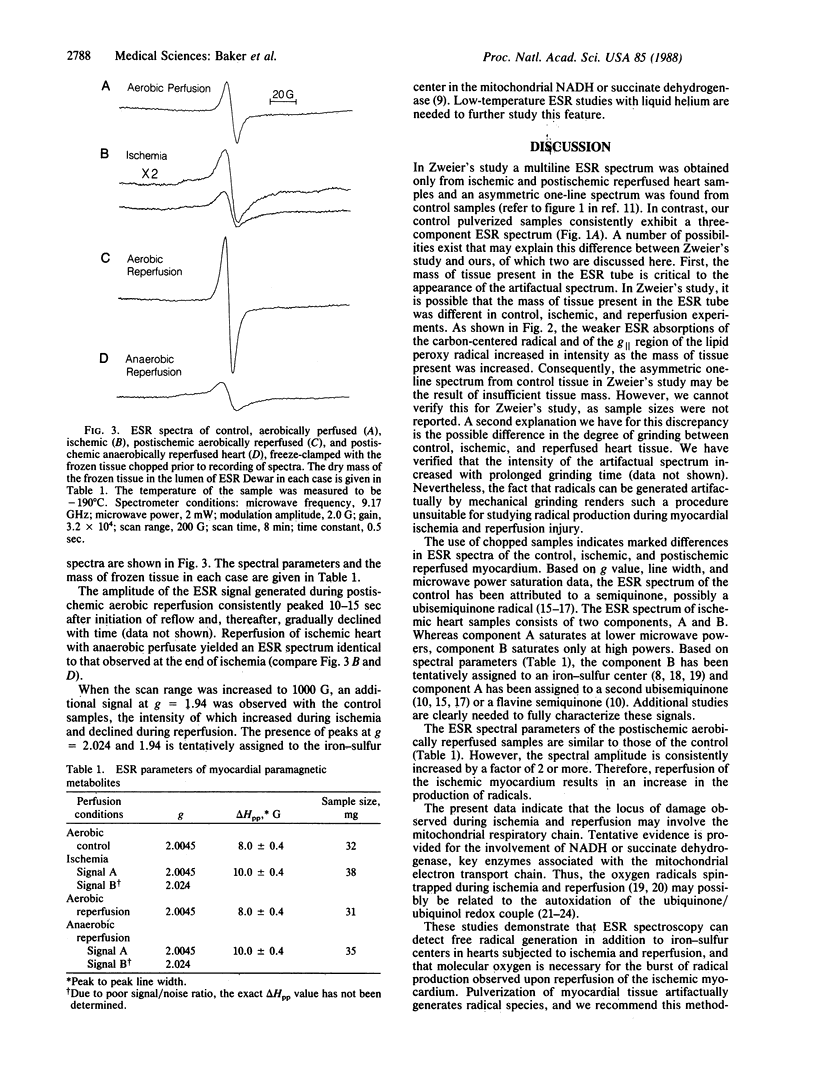
Electron spin resonance spectroscopy has recently been used by others to detect directly radical species in isolated perfused hearts. Sample processing prior to spectroscopy in this study involved pulverization of tissue, which can artifactually generate radical species. We assessed in isolated perfused hearts the influence of tissue pulverization on the identity of radical species detected by spectroscopy and then, using a processing technique less likely to induce artifacts, whether myocardial ischemia and reperfusion generate radical species. Rat and rabbit hearts (n = 8) were perfused aerobically for 10 min and freeze-clamped to -196 degrees C. Frozen tissue was processed at -196 degrees C for spectroscopic analysis by pulverization vs. chopping. Spectra of pulverized tissue consisted of three components: a semiquinone (g = 2.004), a lipid peroxy radical (g [ = 2.04 and g = 2.006), and a carbon-centered radical that is possibly a lipid radical (giso = 2.002 and AHzz approximately equal to 50 G). Chopped tissue consisted of a single component, a semiquinone (g = 2.004). Rat hearts (n = 8 per group) also underwent 10-min global no-flow normothermic ischemia followed by 5-60 sec of either aerobic or anaerobic reperfusion, with frozen tissue chopped prior to spectroscopy. Spectra of ischemic tissue consisted of an iron-sulfur center and a semiquinone. Aerobic reperfusion resulted in a spectrum similar to the control but with increased amplitude that peaked after 10-15 sec of reflow. Anaerobic reperfusion yielded a spectrum identical to that of ischemic tissue. We conclude that pulverization of frozen myocardial tissue arti-factually generates radical species. Using a nonpulverization technique for tissue processing, we found that myocardial ischemia and reperfusion produce radical species but that molecular oxygen is necessary for the burst of radical production during reflow.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio G., Weisfeldt M. L., Jacobus W. E., Flaherty J. T. Evidence for a reversible oxygen radical-mediated component of reperfusion injury: reduction by recombinant human superoxide dismutase administered at the time of reflow. Circulation. 1987 Jan;75(1):282–291. doi: 10.1161/01.cir.75.1.282. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Dickens B. F., Weglicki W. B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987 Aug 31;221(1):101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- Benedetto C., Bocci A., Dianzani M. U., Ghiringhello B., Slater T. F., Tomasi A., Vannini V. Electron spin resonance studies on normal human uterus and cervix and on benign and malignant uterine tumors. Cancer Res. 1981 Jul;41(7):2936–2942. [PubMed] [Google Scholar]
- Burton K. P., McCord J. M., Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984 Jun;246(6 Pt 2):H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Hochstein P. Ubisemiquinone radicals in liver: implications for a mitochondrial Q cycle in vivo. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1292–1299. doi: 10.1016/s0006-291x(82)80138-1. [DOI] [PubMed] [Google Scholar]
- Hearse D. J. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977 Aug;9(8):605–616. doi: 10.1016/s0022-2828(77)80357-x. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Kowal A. T., Morningstar J. E., Johnson M. K., Ramsay R. R., Singer T. P. Spectroscopic characterization of the number and type of iron-sulfur clusters in NADH:ubiquinone oxidoreductase. J Biol Chem. 1986 Jul 15;261(20):9239–9245. [PubMed] [Google Scholar]
- Kramer J. H., Arroyo C. M., Dickens B. F., Weglicki W. B. Spin-trapping evidence that graded myocardial ischemia alters post-ischemic superoxide production. Free Radic Biol Med. 1987;3(2):153–159. doi: 10.1016/s0891-5849(87)80011-4. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Myers M. L., Bolli R., Lekich R. F., Hartley C. J., Roberts R. Enhancement of recovery of myocardial function by oxygen free-radical scavengers after reversible regional ischemia. Circulation. 1985 Oct;72(4):915–921. doi: 10.1161/01.cir.72.4.915. [DOI] [PubMed] [Google Scholar]
- Nohl H., Jordan W., Hegner D. Mitochondrial formation of OH Radicals by an ubisemiquinone-dependent reaction an alternative pathway to the iron-catalysed Haber-Weiss cycle. Hoppe Seylers Z Physiol Chem. 1982 Jun;363(6):599–607. doi: 10.1515/bchm2.1982.363.1.599. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Ubihydroquinone-cytochrome c reductase segment of the electron transfer system and complex mitochondrial fragments. J Biol Chem. 1974 Mar 25;249(6):1928–1939. [PubMed] [Google Scholar]
- Otani H., Tanaka H., Inoue T., Umemoto M., Omoto K., Tanaka K., Sato T., Osako T., Masuda A., Nonoyama A. In vitro study on contribution of oxidative metabolism of isolated rabbit heart mitochondria to myocardial reperfusion injury. Circ Res. 1984 Aug;55(2):168–175. doi: 10.1161/01.res.55.2.168. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H., Schepler K. L., Dunham W. R., Sands R. H. Interaction of ubisemiquinone with a paramagnetic component in heart tissue. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2886–2890. doi: 10.1073/pnas.72.8.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C., Ohnishi T. Studies on the stabilized ubisemiquinone species in the succinate-cytochrome c reductase segment of the intact mitochondrial membrane system. Biochem J. 1980 Dec 15;192(3):769–781. doi: 10.1042/bj1920769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Kirsh M. M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982 Jun;83(6):830–839. [PubMed] [Google Scholar]
- Simpson P. J., Mickelson J. K., Lucchesi B. R. Free radical scavengers in myocardial ischemia. Fed Proc. 1987 May 15;46(7):2413–2421. [PubMed] [Google Scholar]
- Suzuki H., King T. E. Evidence of an ubisemiquinone radical(s) from the NADH-ubiquinone reductase of the mitochondrial respiratory chain. J Biol Chem. 1983 Jan 10;258(1):352–358. [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]



