Abstract
Fabrication and utilization of mesh materials specifically designed to capture analytes from solution facilitates the direct coupling of affinity capture and ambient ionization mass spectrometry via surface-enhanced transmission mode desorption ionization (TM-DESI). Incorporation of photolabile groups within the linkage between the mesh surface and the covalently modified reactive probe affords facile release of mass tagged analytes directly to mesh surfaces that have been rinsed free of matrix interferences. The approach introduces increased specificity to the already rapid TM-DESI analysis technique, resulting in a powerful tool for high throughput screening of targeted analytes. Specific capture of thiols is discussed herein, but the surface-enhanced TM-DESI technique can be readily extended to other functional groups by alteration of the capture agent.
A new era of high-throughput mass spectrometry emerged with the nearly simultaneous introduction of two ambient ionization techniques: desorption electrospray ionization (DESI)1 and direct analysis in real time (DART).2 Recognition of the enormous potential of these ionization methods has resulted in a growing number of related techniques, including ones that integrate laser desorption, the use of plasmas, and extraction methods.3 The most compelling motivations for ambient ionization mass spectrometry are the ability to analyze surfaces directly, the speed of the analysis and the elimination of difficult or time consuming sample preparation steps. Most ambient ionization methods require only seconds per sample, which is a substantial improvement in throughput compared to the multiple minutes required to separate and analyze components in GC-MS and LC-MS analyses. Moreover most of the cumbersome sample preparation steps such as derivatization reactions and extensive sample cleanup protocols are alleviated. Although one of the major benefits touted for ambient ionization mass spectrometric methods is the direct analysis of complex samples, the elimination of chromatographic separation generally results in reduced specificity and ion suppression for low concentration species. One set of performance metrics (i.e., specificity and/or sensitivity) has been compromised for another (i.e., analytical speed) in desorption-based ambient ionization mass spectrometry. A newer variation of DESI, termed reactive DESI, has been previously reported to address this issue and exploits the addition of specific reagents to the spray solution that undergo ion/molecule reactions with the analytes of interest, thus offering improved selectivity and sensitivity for the detection of targeted molecules.4-6 In the present report, a surface-enhanced DESI-MS strategy is described which allows selective capture, release, and analysis of targeted analytes from complex mixtures, thus uniting the analytical merits of specificity and speed.
DESI is among the most prominently utilized ambient ionization methods.7 In the DESI process, ions are produced by directing charged solvent droplets from an electrospray source toward a sample that is either a bulk material or one that has been deposited onto a sampling surface. A recent adaptation of DESI, transmission mode desorption electrospray ionization (TM-DESI), is a new mode of operation in which the sample is suspended as macro scale droplets in the open space between strands of a mesh.8 Analysis is then performed by scrolling the mesh orthogonally into the path of an electrospray plume positioned coaxial to the inlet capillary of the mass spectrometer, thereby resulting in the transmission of the ionizing plume directly through the mesh. The transmission mode results in desorption and ionization typical of DESI, but with the added benefits of a simpler experimental geometry and the convenient analysis of both dry (i.e., following evaporation of the deposition solvent) and wet (i.e., solvated) samples.
Recent studies utilizing TM-DESI have shown that the chemical characteristics of the mesh strands can influence the ability to desorb species from the surface.9 The present study expands on those observations by demonstrating that the specificity of TM-DESI analyses can be dramatically increased by fabrication and utilization of mesh materials that have been specifically designed to capture selected analyte molecules from solution. Subsequent interaction of the mesh with UV light results in cleavage of a photolabile unit linking the mesh to the captured analyte. The mesh materials are then analyzed immediately by TM-DESI-MS without any additional sample preparation. In addition to providing a method of releasing the covalently bound analyte, cleavage of the photolabile linker also provides a means of tagging the analyte with an easily ionizable mass tag that facilitates electrospray ionization and subsequent tandem mass spectrometry experiments.
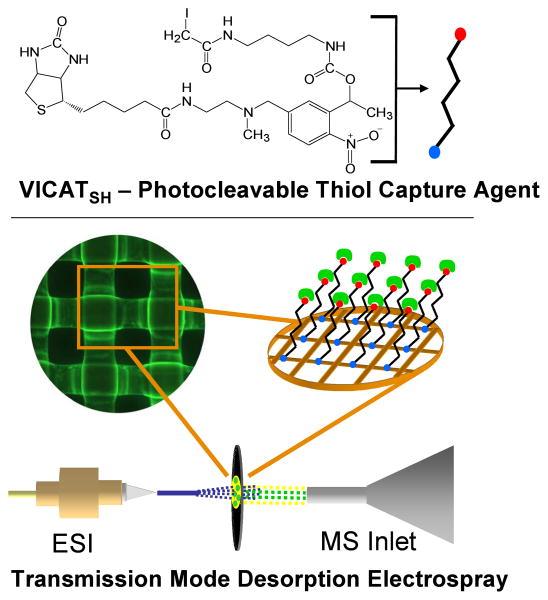
Mesh materials for surface-enhanced TM-DESI analyses have been fabricated using one of several multi-step procedures. An acid-catalyzed hydrolysis of a polyamide mesh (nylon 6,6) provides a pathway to both accessible primary amines and carboxyl groups for further surface derivatization. Many potential derivatization pathways stem from these two reactive groups and promising candidates continue to be pursued in an effort to maximize the efficiency of the surface enhancement. For example, surface carboxyl groups have been coupled both directly to neutravidin (a deglycosylated form of avidin) via carbodiimide chemistry and following the incorporation of spacers such as polyethyleneimine (PEI), polylysine, and glutaraldehyde to reduce steric hindrance and provide additional surface functionality. The derivatization of neutravidin coated mesh materials is completed by the incorporation of biotinylated, photolabile (o-nitrobenzyl) capture reagents such as VICATSH10 or PC-BIOTIN,11 each of which is designed to provide selective enrichment of particular functional groups. This final derivatization step transforms the nylon mesh into a selective capture material that is particularly suited for TM-DESI analyses.
The performance of each derivatization step was quantified using a series of fluorimetric assays that utilize the chemical functionalities present in common dyes such as fluorescein, fluorescein isothiocyanate, fluorescamine and rhodamine 123 to provide fluorescent surrogates for each derivatization reaction. In this way each batch of materials has been assessed quantitatively prior to proceeding to the subsequent derivatization step, and each derivatization step is optimized independently. Figure 1 provides a schematic overview of the surface-enhanced TM-DESI method. The protocol for thiol capture and analysis is straightforward. The pH of the sample is adjusted to the appropriate range for the capture agent (e.g., ∼9.5 for VICATSH), and the mesh is submerged in the solution for 5 minutes to capture any free sulfhydryl-containing analytes via reaction with the iodoacetaminyl unit of the VICATSH reagent. Following capture the mesh is removed from the sample, rinsed thoroughly with water or methanol to remove matrix interferences (e.g., salts) and placed under a UV lamp for 5 minutes to induce photocleavage of the o-nitrobenzyl linkage. Samples are then analyzed directly by TM-DESI-MS using methanol as the electrospray solvent.
Figure 1.
Schematic view of surface-enhanced transmission mode desorption electrospray ionization mass spectrometry employing VICATSH as a thiol capture reagent attached to a Neutravidin-coated mesh. The modified mesh is submerged in a sample to selectively and covalently capture thiols, rinsed free of matrix interferences (e.g., salts, buffers), photocleaved under a UV lamp and analyzed by TM-DESI-MS.
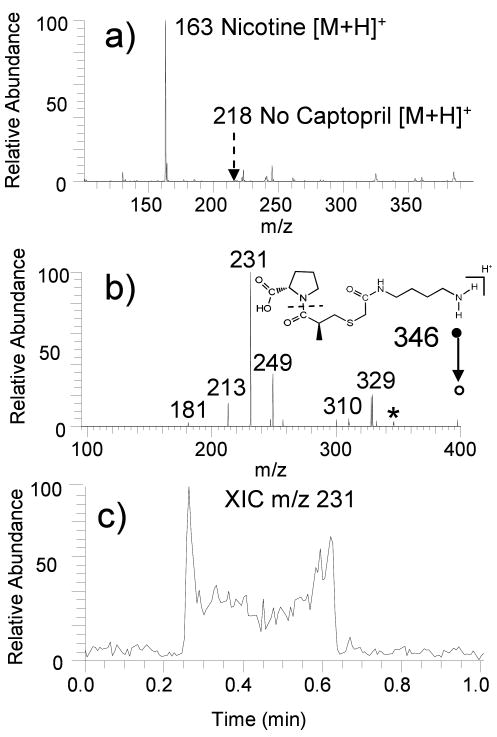
To illustrate the utility of this approach, derivatized mesh materials were used to selectively capture sulfhydryl drugs from aqueous solutions containing a variety of matrix interferences. The results for one of these studies are summarized in Figure 2. The sample in this experiment was an aqueous solution (2 mL) containing nicotine (1 μM), captopril (10 uM), sodium chloride (10 mM) and potassium chloride (10 mM), a composition chosen to mimic a physiological solution containing an easily ionizable but potentially non-targeted interference (nicotine). The sample was analyzed by direct infusion electrospray, TM-DESI without surface enhancement, and TM-DESI using a mesh derivatized with VICATSH. When analyzed directly by ESI, the resulting mass spectrum was dominated by the ion of m/z 163 and m/z 185, which correspond to protonated and sodium-cationized nicotine. Captopril was not observed (protonated captopril has a m/z of 218). Similar results, albeit with reduced salt interference, were obtained by TM-DESI-MS analysis of a sample aliquot spotted and dried on an underivatized nylon mesh. (Figure 2A) In both cases, the presence of the nicotine, with its high basicity and high ESI efficiency, precluded detection of captopril.
Figure 2.
(a) TM-DESI-MS of a mock sample containing nicotine (1 μM), captopril (10 μM), sodium chloride (10 mM), and potassium chloride (10 mM) from an underivatized nylon mesh. (b) CID spectrum for captured and tagged captopril (precursor m/z 346 Da) (c) Extracted ion chronogram for ion m/z 231 indicating the distribution of the captured analyte across the mesh surface.
In contrast, successful detection and confirmation via collision induced dissociation (CID) of captopril from the same solution was easily obtained using the VICATSH-based surface-enhanced technique with TM-DESI-MS analysis. In this case, protonated nicotine (m/z 163) was not detected in the TM-DESI mass spectrum, indicating that the mesh material was selective against the non-thiol containing alkaloid. Furthermore, CID of the ion of m/z 346 produced the spectrum displayed in Figure 2B. This spectrum is indicative of the mass tagged version of captopril (m/z 346), confirming the successful capture and release of this thiol-containing analyte. The extracted ion chronogram of m/z 231, an ion that corresponds to loss of the pyrrolidine carboxylic acid group, in Figure 2C illustrates the distribution of the captured analyte across the mesh (Analysis time = 0.25 min to 0.65 min).
This example clearly illustrates the ability of the surface-enhanced TM-DESI technique to overcome the presence of highly basic interferences that typically result in ion suppression and false negative detection for lower abundance, lower basicity analytes in standard direct infusion ESI and DESI analyses. The specific type of surface-modified mesh described in Figure 1 could be applied for the capture and analysis of other types of biologically relevant thiols, such as endogenous metabolites like cysteine, homocysteine, glutathione, as well as sulfhydryl-containing xenobiotics.
Acknowledgments
Support from the Welch Foundation (F1155, JSB) and NIH (1RC1AG035713, JSB, and DK67869, MHG) is acknowledged.
References
- 1.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 2.Cody RB, Laramee JA, Durst HD. Anal Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 3.Venter A, Nefliu M, Cooks RG. Trends Anal Chem. 2008;27:284–290. [Google Scholar]
- 4.Huang G, Chen H, Zhang X, Cooks RG, Ouyang Z. Anal Chem. 2007;79:8327–8332. doi: 10.1021/ac0711079. [DOI] [PubMed] [Google Scholar]
- 5.Nyadong L, Green MD, De Jesus VR, Newton PN, Fernandez FM. Anal Chem. 2007;79:2150–2157. doi: 10.1021/ac062205h. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Ifa DR, Manicke NE, Cooks RG. Anal Chem. 2009;81:7618–7624. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Science. 2006;311:1566–1569. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 8.Chipuk JE, Brodbelt JS. J Am Soc Mass Spectrom. 2008;19:1612–1620. doi: 10.1016/j.jasms.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Chipuk JE, Brodbelt JS. J Am Soc Mass Spectrom. 2009;20:584–592. doi: 10.1016/j.jasms.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Bottari P, Turecek F, Aebersold R, Gelb MH. Anal Chem. 2004;76:4104–4111. doi: 10.1021/ac049905b. [DOI] [PubMed] [Google Scholar]
- 11.Olejnik J, Sonar S, Krzymañska-Olejnik, Rothschild KJ. Proc Natl Acad Sci. 1995;92:7590–7594. doi: 10.1073/pnas.92.16.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]