Abstract
Recent behavioural findings using dual-task paradigms demonstrate the importance of both spatial and non-spatial working memory processes in inefficient visual search (Anderson et al. in Exp Psychol 55:301–312, 2008). Here, using functional magnetic resonance imaging (fMRI), we sought to determine whether brain areas recruited during visual search are also involved in working memory. Using visually matched spatial and non-spatial working memory tasks, we confirmed previous behavioural findings that show significant dual-task interference effects occur when inefficient visual search is performed concurrently with either working memory task. Furthermore, we find considerable overlap in the cortical network activated by inefficient search and both working memory tasks. Our findings suggest that the interference effects observed behaviourally may have arisen from competition for cortical processes subserved by these overlapping regions. Drawing on previous findings (Anderson et al. in Exp Brain Res 180:289–302, 2007), we propose that the most likely anatomical locus for these interference effects is the inferior and middle frontal cortex of the right hemisphere. These areas are associated with attentional selection from memory as well as manipulation of information in memory, and we propose that the visual search and working memory tasks used here compete for common processing resources underlying these mechanisms.
Keywords: fMRI, Attention, Dual-task interference, Frontal cortex
Introduction
Visual search for items that are difficult to discriminate from surrounding distractor items often take several seconds to perform (Duncan and Humphreys 1989). Such demanding searches are thought to benefit from memory-based mechanisms that guide attention away from previously searched items and towards fresh sources of information. However, there has been much debate over the nature of the memory processes utilised and any possible interaction between attention and memory.
Initial studies suggested a specific role for spatial memory in visual search, such that previously searched locations were held in memory, serving to guide attention towards unsearched areas in a visual scene (Klein 1988; Peterson et al. 2001; McCarley et al. 2003). Supporting this hypothesis, dual-task paradigms in which visual search is performed concurrently with a spatial working memory task demonstrate a decline in search efficiency, compared to search performed in isolation (Experiment 1, Oh and Kim 2004; Woodman and Luck 2004). Such dual-task interference is thought to occur when concurrent tasks compete for the same limited processing resource (Klingberg and Roland 1997; Klingberg 1998; Morey and Cowan 2004). In this case, visual search performance declined due to limited available spatial working memory resources. In contrast, no such decline in efficiency was found for search performed concurrently with an object working memory task (Woodman et al. 2001), or a memory for colour task (Experiment 2, Oh and Kim 2004), suggesting that visual search utilises working memory processes specific to the spatial domain, which are distinct from non-spatial memory processes. Such findings support a ‘domain-specific’ model of working memory, in which functionally separable spatial and non-spatial working memory subsystems are thought to exist within the human brain (Wilson et al. 1993; Smith et al. 1996; Ungerleider et al. 1998; Sala et al. 2003).
However, we have recently shown in a behavioural study that processes associated with non-spatial working memory also play an important role in visual search (Anderson et al. 2008). When inefficient visual search was performed concurrently with either a spatial or a visually matched non-spatial (verbal) working memory task, search efficiency declined by a comparable degree for both. The working memory tasks used were highly demanding, requiring information to be maintained, updated, rehearsed, retrieved and selected for successful performance. Our findings are consistent with a previous study in which a non-spatial working memory task, requiring both the maintenance and manipulation of information in memory, interfered with a concurrent visual search task (Han and Kim 2004). However, in that study, when only simple maintenance of the same information was required no such interference occurred. Together, these findings suggest that working memory resources, in either the spatial or non-spatial domain, associated with monitoring and manipulating information are recruited during difficult visual search tasks. The absence of dual-task interference effects found in previous studies between inefficient visual search and non-spatial working memory tasks, in which only a simple detection task was required, may have been due to the lack of executive processes performed on the information held in memory (Woodman et al. 2001; Oh and Kim 2004).
Here, using behavioural measures and fMRI, we sought to determine whether visual search and working memory interfere because they compete for common, limited capacity processing resources within overlapping areas of the cerebral cortex. We have previously shown in a behavioural study that both a spatial and non-spatial working memory task interferes with concurrent performance of an inefficient search (rotated T amongst Ls), but not with an efficient search for a highly salient target (rotated X amongst Ls) (Anderson et al. 2008). We have also shown, using fMRI, that inefficient search uniquely activates regions of prefrontal cortex, in addition to common regions of occipital and parietal cortex which were also activated by efficient search (Anderson et al. 2007). Here, we sought to determine whether these unique areas of prefrontal cortex—associated with inefficient, but not efficient search—are also associated with our working memory tasks. Such a finding would indicate that the behavioural interference effects observed may result from competition for processing resources within common cortical areas. Furthermore, the behavioural interference effects observed previously were comparable across the spatial and non-spatial domain, indicating that both working memory tasks may engage common regions of frontal cortex (Anderson et al. 2008). Despite the vast literature concerning the frontal cortex and working memory for different types of information (Petrides 1989), the existence of functional subdivisions within the lateral prefrontal cortex for spatial and non-spatial working memory is still a matter of intense debate (Owen et al. 1998; Curtis et al. 2000; Levy and Goldman-Rakic 2000; Nystrom et al. 2000; Walter et al. 2003; Manoach et al. 2004). Indeed, recent meta-analyses of the neuroimaging literature suggest that the lateral prefrontal cortex is organised according to the process performed on stored information, rather than the type of information stored (Owen 1997; D’Esposito et al. 1998).
Here, we employed a dual-task paradigm in which an inefficient visual search task (search for a rotated T amongst rotated Ls) was performed within the retention interval of either a spatial working memory task or a verbal working memory task. The working memory tasks were visually matched and also matched for task difficulty. These stimuli have been used previously in a purely behavioural study and shown to produce significant dual-task interference (Anderson et al. 2008). Using a blocked-design fMRI paradigm, we measured cortical activity associated with the spatial and the verbal working memory tasks and compared this with the cortical network previously found to be associated with inefficient search (Anderson et al. 2007). We were specifically interested in two regions of prefrontal cortex previously identified as being uniquely involved in inefficient search. Specific regions of interest (ROI) in the right inferior and middle frontal gyrus were defined based on the results of our previous study in order to perform detailed analyses. In addition, we also examined the neural correlates of performing the working memory and visual search tasks concurrently compared to performing the working memory tasks alone.
Experiment 1: behavioural study
Methods
Participants
Twelve naïve participants, all right handed, aged 19–32 years with normal, or corrected to normal, visual acuity gave written informed consent to take part in both the behavioural and functional imaging study. Ethical approval was received from the local ethics committee.
Stimuli and behavioural task
There were five experimental conditions (described in detail below): (1) visual search only, (2) spatial working memory only (SWM only), (3) verbal working memory only (VWM only), (4) spatial working memory & search (SWM & search), (5) verbal working memory & search (VWM & search). After an initial practice session (6 trials of each condition), participants performed one block, comprising 40 trials of a single condition, for each of the five conditions.
Stimuli were written in Matlab 6.5.1 (http://www.mathworks.co.uk), using Cogent2000 graphics (http://www.fil.ion.ucl.ac.uk/cogent2000.html) and presented on a 19” PC monitor, 800 × 600 resolution at 60 Hz.
Visual search only
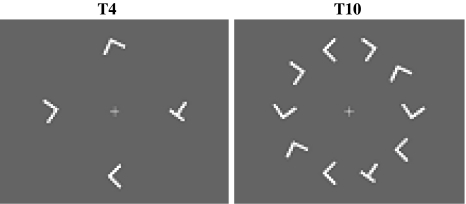
Stimuli comprised a uniform grey background (subtending 18° × 13.6°) with an array of randomly rotated white letter L distractors (subtending 0.7°) equally spaced in a concentric ring (at an eccentricity of 2.4°) around a central white fixation cross. On target present trials one of the distractor was replaced by a randomly rotated letter T (Fig. 1). Participants were required to report the presence or absence of the rotated letter T as fast and as accurately as possible. Two set sizes were used, n = 4 (T4) and n = 10 (T10). These stimuli have previously been shown to elicit an inefficient search profile (Anderson et al. 2007), and hence the term ‘inefficient search’ is used here to describe these search tasks to maintain consistency with this earlier related study.
Fig. 1.
Search stimuli. Visual search stimuli comprised a uniform grey background with an array of randomly rotated white letter L distractors equally spaced in a concentric ring, around a central white fixation cross. On target present trials one of the distractors was replaced by a randomly rotated letter T. Participants were required to report the presence or absence of the rotated letter T as fast and as accurately as possible. Two set sizes were used, n = 4 (T4) and n = 10 (T10)
Conventionally, search performance is characterized by a ‘search function’ representing the change in RT as a function of set size. The slope of this function is taken as a measure of search efficiency. In this study only two set sizes were used (4 & 10); therefore we have taken the difference in RT for search at set size 10 and search at set size 4, divided by the change in set size (6 items) as our search ‘slope’ and hence a measure of search ‘efficiency’.
Working memory only
A sequence of 5 letters was presented at 5 different locations on a circular arc (eccentricity 2.4°) around fixation. Locations were chosen at random from 10 possible locations (corresponding to the 10 positions used in the search array at n = 10). Letters were chosen at random, without repeats, from 10 possible letters: A D E H N P U V Y Z. The letters T and L were not used to avoid priming the target or distractors in the visual search task during ‘dual-task’ trials (see below).
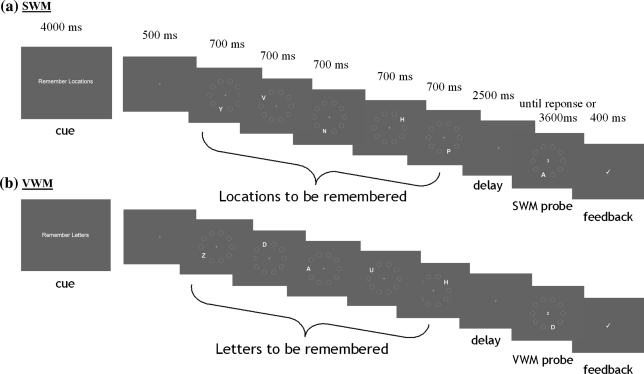
In the SWM only task participants were instructed to remember the locations of the letters and the temporal order in which they were presented, but to ignore the identity of the letters (Fig. 2a). In the VWM only task participants were instructed to remember the identity of the letters and the temporal order in which they were presented, but to ignore the locations in which they appeared (Fig. 2b). The sequence of locations/letters had to be retained across a 2,500 ms interval after which a probe screen appeared. For the SWM probe a new letter was presented at one of the locations used in the initial sequence, along with a number at fixation. Participants were asked whether the central number corresponded to the temporal position in which that location had been presented. For example, in Fig. 2a, the probe is asking whether the location indicated by the letter A was presented third in the sequence, and the answer would be ‘yes’. For the VWM probe one of the letters used in the initial sequence appeared at a new location, along with a number in the centre of the screen. Participants were asked whether the central number corresponded to the temporal position in which that letter had been presented. For example, in Fig. 2b, the probe is asking whether the letter D had been presented 2nd in the sequence, and the answer would be ‘yes’. The location used for the probe was not one of the locations used in the initial sequence.
Fig. 2.
Visually matched spatial working memory and verbal working memory tasks. Example trial sequences for experiment 1 (a) SWM only and (b) VWM only. For the SWM only task participants were instructed to remember the locations of the letters (and the temporal order in which they were presented), but to ignore the identity of the letters. For the VWM only task participants were instructed to remember the identity of the letters, but to ignore the locations in which they appeared. The sequence of locations/letters had to be retained across a 2,500 ms interval after which a probe screen appeared. Participants were instructed to make a button response ‘yes’ or ‘no’ to indicate whether the central number corresponded to the temporal position in which that location (SWM)/letter (VWM) had been presented (see “Methods”). Previous data confirms that these tasks are matched for task difficulty (Anderson et al. 2008)
All locations/letters in the sequence were probed with equal frequency, except for the 1st which was never probed. Participants were instructed to respond ‘yes’ or ‘no’ to the probe as accurately as possible using a manual key press. When a response had been made (or 3,600 ms time had elapsed) feedback was given: a tick for correct, a cross for incorrect or no response. The probe was correct on 50% of trials and emphasis was heavily placed on response accuracy, rather than response speed.
Dual-tasks
For the dual-task conditions, a single visual search task (T4 or T10) was performed within the retention interval of the working memory task (spatial or verbal) and participants performed both tasks concurrently. That is, participants observed the initial sequence to be remembered for the working memory task, then, whilst maintaining and rehearsing this information in memory, simultaneously performed the visual search task and indicated target presence or absence with an immediate key press. A probe was subsequently presented for the working memory task and participants were asked to make a second un-speeded response to indicate whether the probe was correct or incorrect. To avoid priming the location of the target in the visual search array the target location was never used for the working memory task. All other details were the same as for the ‘working memory only’ conditions.
In all conditions participants were required to maintain steady fixation on the central fixation cross throughout, head movement was constrained using a chin rest. Manual responses were collected using the right and down arrow keys on a computer keyboard. One key indicated ‘target present’ for the search task and ‘yes’ to the working memory probe, the other key indicated ‘target absent’ for the search task and ‘no’ to the working memory probe. All participants used their dominant hand and the key mappings were counterbalanced across participants.
Analysis of behavioural data
Mean manual response times (RTs) and response accuracy were calculated separately for the visual search tasks and working memory tasks for every condition. Trials with incorrect responses were discarded and RT values further than 2.5 standard deviations from the mean were considered outliers and removed; the mean was then re-calculated.
Eye movement recording and analysis
An SMI (http://www.smi.de) Eyelink infrared pupil tracker with Eyelink 2.3 software, sampling at 250 Hz, spatial resolution of ~0.5°, was used to record fixation accuracy for all participants throughout all experimental conditions. Eye movement data were analysed off-line using custom programs written in Matlab. A saccade velocity criterion of 30° per second was used. Any trials on which a blink or a saccade greater than 1.2° was made were automatically eliminated.
The number and amplitude of saccades made during the search tasks and the working memory tasks were calculated separately for each condition. A criterion for steady fixation was applied, such that any participants who made saccades of amplitude greater than 1.2° (half the eccentricity of the stimuli) on more than 10% of trials were excluded from the analysis.
Results experiment 1
Fixation analysis
All participants reached criterion for steady fixation (see “Methods”). Analysis of variance, with search set size (4, 10) and working memory domain (spatial, verbal) as within participant factors, confirmed there was no effect of search set size (F(1,11) = 1.278, p = 0.282), or working memory domain (F(1,11) = 0.072, p = 0.794), and no interaction (F(1,11) = 0.281, p = 0.607), on the number of saccades made. Similarly, there was no effect of search set size (F(1,11) = 0.083, p = 0.779), or working memory domain (F(1,11) = 0.820, p = 0.385), and no interaction (F(1,11) = 0.641, p = 0.440), on the amplitude of saccades made.
Search-only data
RTs for the search-only conditions confirmed that search for a T took longer to perform as set size increased from 4 to 10 (i.e. an inefficient search profile), with greater effect for target absent than target present search (target present search ‘slope’ 15 ms per item, target absent search ‘slope’ 38 ms per item). Analysis of variance, with set size and target presence as within participant factors, confirmed these effects were significant (significant main effect of set size (F(1,11) = 44.901, p < 0.001), significant main effect of target presence (F(1,11) = 15.506, p = 0.002), and a significant interaction between set size and target presence (F(1,11) = 23.605, p = 0.001)). See Table 1a for exact values.
Table 1.
Search times and accuracy
| Search RTs (ms) | Search errors (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Target present | Target absent | |||||||
| n = 4 | n = 10 | ‘Slope’ | n = 4 | n = 10 | ‘Slope’ | n = 4 | n = 10 | |
| A. Experiment 1 | ||||||||
| Search alone | 642 (29) | 731 (39) | 15 (3) | 714 (51) | 942 (70) | 38 (6) | 1.0 (0.7) | 4.6 (1.2) |
| Search + SWM | 792 (30) | 945 (38) | 26 (4) | 853 (30) | 1,235 (73) | 64 (10) | 1.7 (0.9) | 6.6 (1.5) |
| Search + VWM | 771 (26) | 923 (42) | 25 (6) | 847 (32) | 1,245 (96) | 66 (15) | 2.1 (0.7) | 6.9 (1.5) |
| B. Experiment 2 | ||||||||
| Search alone | 756 (37) | 1,012 (90) | 7.6 (1.8) | |||||
| Search + SWM | 814 (48) | 1,059 (81) | 4.5 (1.4) | |||||
| Search + VWM | 835 (38) | 1,035 (69) | 5.3 (1.4) | |||||
Manual responses for target present and target absent search at a set size of 4 and 10 for all single and dual task conditions. Values in brackets indicate the standard error of the mean (SEM)
Effect of memory load on search efficiency
Initial analysis of variance, performed on the spatial and verbal data separately, confirmed that both the spatial and the verbal working memory tasks significantly interfered with visual search performance. With spatial working memory load (no load, load), search set size (4, 10), and target presence (present, absent) as within participant factors, analysis of variance confirmed there was a main effect of spatial memory load (F(1,11) = 88.497, p < 0.001), a main effect of set size (F(1,11) = 55.623, p < 0.001) and a main effect of target presence (F(1,11) = 22.461, p = 0.001). There was also significant interaction between spatial memory load and set size (F(1,11) = 18.824, p = 0.001) and between set size and target presence (F(1,11) = 36.340, p < 0.001). Similarly, for the verbal working memory load, analysis of variance confirmed there was a main effect of verbal memory load (F(1,11) = 41.454, p < 0.001), a main effect of set size (F(1,11) = 33.033, p < 0.001) and a main effect of target presence (F(1,11) = 29.897, p < 0.001). There was also significant interaction between spatial memory load and set size (F(1,11) = 4.820, p = 0.050) and set size and target presence (F(1,11) = 27.370, p < 0.001). All other effects did not reach significance.
The above analyses confirm that adding a spatial or verbal memory load does indeed interfere with search performance. However, it is possible that these effects are driven by different cognitive strategies being adopted when performing two tasks concurrently (i.e. under dual-task conditions), compared to when performing each task alone (Herath et al. 2001). Therefore, to remove this potential confound, we prefer to focus our analysis on comparing search performance across different dual-task conditions, rather than comparing dual-task with single-task conditions. To achieve this, RTs for the ‘search-only’ condition were simply used as a baseline and subtracted from search RTs during dual-task conditions.
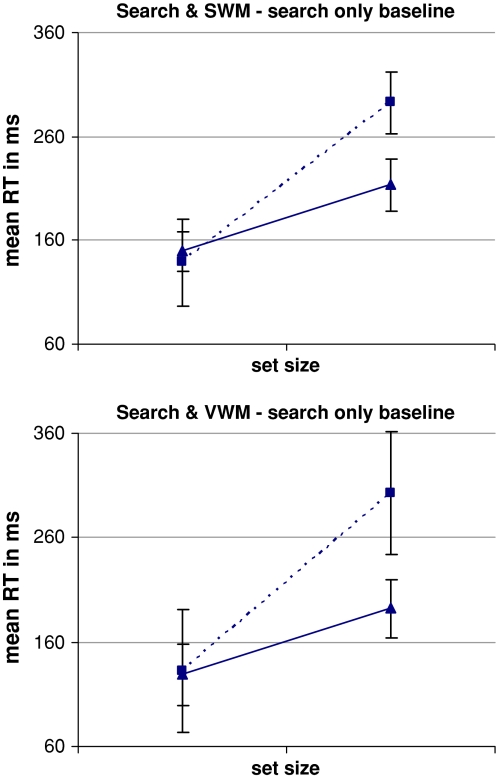
Once the baseline search RT values have been subtracted from the search RTs for the spatial dual-task conditions, there is still a residual search ‘slope’ of 11 ms per item for target present search and 26 ms per item for target absent search. Similarly, once the baseline search RT values have been subtracted from the search RTs for the verbal dual-task conditions, there is still a residual search ‘slope’ of 11/28 ms per item for target present/absent search. Thus both the spatial and verbal memory load significantly interfered with search performance. These data are illustrated in Fig. 3 (see Table 1 for exact RT values). Importantly, analysis of variance, with working memory domain (spatial, verbal), search set size (4, 10), and target presence (present, absent) as within participant factors, confirmed there was no main effect of working memory domain (F(1,11) = 0.639, p = 0.441), no interaction between working memory domain and set size (F(1,11) = 0.044, p = 0.837) and no three-way interaction between memory domain, set size and target presence/absence (F(1,11) = 0.418, p = 0.531). This confirms that the spatial and verbal working memory tasks induced comparable interference effects with the visual search task. There was the expected main effect of set size (F(1,11) = 9.117, p = 0.012) and a trend for a significant interaction between set size and target presence (F(1,11) = 4.006, p = 0.071). All other effects did not reach significance.
Fig. 3.
Results Experiment 1—the effects of dual-task performance on search times. RTs for inefficient search performed in isolation have been subtracted from search RTs during the dual-task conditions, for target present (filled triangle) and target absent search (filled square). On average, an additional 11 ms per item was required to perform target present search concurrently with the SWM task and 26 ms per item for target absent search, compared to search performed in isolation. Similarly an extra 11 ms per item was required for target present search performed concurrently with the VWM task and 28 ms per item for target absent search. Error bars indicate standard error of the mean
Effect of memory load on search accuracy
Search accuracy declined under dual-task conditions compared to search performed in isolation (Table 1a). However, when the ‘search only’ error data was used as a baseline and subtracted from the dual-task error data, analysis of variance, with working memory domain (spatial, verbal) and search set size (4,10) as within participant factors, revealed no effect of working memory domain (F(1,11) = 0.525, p = 0.484), no effect of search set size (F(1,11) = 1.767, p = 0.211), and no interaction between memory domain and set size (F(1,11) = 0.013, p = 0.912).
Effects of search on working memory performance
For the SWM only and VWM only conditions there was no significant difference in error rate (paired t test: t(11) = 1.041, p = 0.320) (Table 2a), and all participants achieved greater than 70% accuracy in both tasks. However, under dual-task conditions errors on the SWM task were higher than those for the VWM task: analysis of variance, with working memory domain (spatial, verbal) and search set size (4, 10) as within participant factors, confirmed a main effect of working memory domain (F(1,11) = 8.006, p = 0.016) but no effect of increasing search set size (F(1,11) = 990, p = 0.341), and no interaction (F(1,11) = 0.728, p = 0.412) .
Table 2.
Working memory response times and accuracy
| Working memory RTs (ms) | Working memory errors (%) | |||
|---|---|---|---|---|
| SWM | VWM | SWM | VWM | |
| A. Experiment 1 | ||||
| + no search | 1,274 (66) | 1,342 (60) | 12.1 (1.4) | 9.2 (2.5) |
| + inefficient search n = 4 | 1,275 (63) | 1,377 (58) | 22.3 (2.1) | 11.3 (3.2) |
| + inefficient search n = 10 | 1,327 (76) | 1,389 (60) | 19.4 (2.7) | 11.0 (2.7) |
| B. Experiment 2 | ||||
| + no search | 1,364 (80) | 1,269 (56) | 14.2 (2.3) | 9.0 (1.7) |
| + inefficient search n = 10 | 1,331 (86) | 1,354 (74) | 19.1 (1.6) | 9.7 (1.7) |
Manual RTs and response accuracy for the working memory tasks for dual and single-task conditions. Values in brackets indicate the standard error of the mean (SEM)
No apriori predictions were made about the effect search would have on working memory response times and analysis of variance revealed no effect of memory domain (F(1,11) = 3.796, p = 0.077), no effect of search set size (F(1,11) = 0.894, p = 0.365) and no interaction (F(1,11) = 0.306, p = 0.591). There was also no significant difference in the RTs for either working memory task under single or dual-task conditions (Table 2a).
Summary experiment 1
The behavioural findings of experiment 1 confirm that dual-task interference effects occur when inefficient visual search is performed within the retention interval of either a spatial or a verbal working memory task—replicating our previous findings. Importantly, for the search RTs, analysis of variance confirmed there was no 3-way interaction between memory domain, set size and target presence/absence (F(1,11) = 0.418, p = 0.531), confirming that the decrease in search efficiency was comparable across the spatial and non-spatial domain.
Experiment 2: functional MRI study
In experiment 2 we seek to determine whether these behavioural interference effects may have arisen as a result of competition for common limited capacity processing resources within overlapping areas of the cerebral cortex.
Methods
Stimuli
All stimuli were projected onto a rear mounted projector screen which subtended 18° × 13.6 , with all stimuli at an eccentricity of 2.4°, viewed via a mirror system mounted on the head coil. ‘Presentation’ software (http://www.neurobs.com) was used to present the visual images and to collect manual response data.
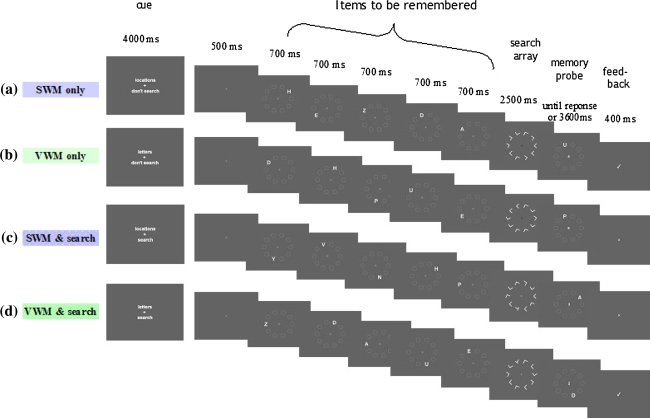
The stimuli from experiment 1 were adapted to ensure trial length, as well as visual and motor responses, were matched across all conditions. There were 4 experimental conditions (Fig. 4a–d) and 1 control condition (described below): (1) SWM only, (2) VWM only, (3) SWM & search, (4) VWM & search, (5) Visual search only—control condition.
Fig. 4.
Four blocked experimental conditions used in the fMRI paradigm. Example trials from the four experimental conditions: (a) SWM only, (b) VWM only, (c) SWM & search, (d) VWM & search. A cue screen appeared at the beginning of each block, indicating which task to perform for the following four trials. All conditions were matched for visual and motor responses. For the working memory only trials (a, b) a target absent visual search array was presented during the retention interval (instead of just a fixation cross used in experiment 1). The colour of the fixation cross changed from white to black to remind participants NOT to search, and subjects were asked to always report target absence. For the dual-task trials (c, d), subjects were required to perform the visual search task, whilst simultaneously rehearsing information required for the working memory task
Working memory only
Participants performed the same SWM only and VWM only tasks used in experiment 1. However, in order to control for visual stimulation across conditions a target absent visual search array was presented during the retention interval, instead of the fixation cross used previously (Fig. 4a, b). The colour of the fixation cross changed from white to black during this interval to remind participants NOT to search. Participants were instructed to press the ‘target absent’ button during the search array, to ensure motor responses were comparable to the dual-task conditions. Subjects also made a second response to the working memory probe to indicate whether it was correct or incorrect. Although the response to the search array was meaningless it kept motor responses constant across all conditions. All other details were the same as for experiment 1.
Dual-tasks
Trials were identical to those for the dual-task conditions used in experiment 1, except that only search at the larger set size of n = 10 was used (Fig. 4c, d).
Visual search only
This condition was included to control for processes associated with visual search, as well as visual and motor responses, when used as a baseline for comparison with other conditions (see below). To ensure trial length and visual stimulation were comparable to conditions a–d, the search task was preceded by a sequence of letter Ks (a letter not used in any other condition) and followed by a dummy ‘probe’ screen. Participants were advised in advance that the sequence of letter Ks was purely a visual control, and that they were not required to remember the sequence and that either button could be pressed in response to the probe at the end of the trial (positive feedback was always given). Although the response to the memory probe was meaningless it ensured that motor responses were controlled for across all conditions. Participants were required to perform the search task as normal and to make an immediate response to indicate target presence or absence. The target was present on 50% of trials.
Experimental block design
There were 4 trials of one condition per block, and 5 block conditions, as detailed above (1) SWM only, (2) VWM only, (3) SWM & search, (4) VWM & search, (5) Visual search only—control condition). Participants performed 2 blocks of each of the 5 conditions per scan run, and there were 3 scan runs per participant (i.e. each participant performed 24 trials of each condition in total (4 trials × 2 blocks × 3 runs)). Block order was randomised for the first 5 blocks of each run and the order of the remaining 5 blocks completed a palindrome. Each active block was interleaved with a 14 s period, to allow brain activity to return to near baseline, this comprised of a 10 s fixation screen and a 4 s cue screen indicating the next block condition. There was an additional 10 s period at the beginning of each scan run to allow the brain to reach steady state magnetisation. Scans during this initial fixation period were discarded from the analysis.
Recording behavioural responses
An MRI compatible button box (Cedrus Corporation) was linked to the data collection PC for recording manual responses. All participants responded with the index and middle finger of their right hand. Half the participants used their index finger for positive responses (‘target present’ for the search task, and ‘yes’ to the working memory probe) and their middle finger for negative responses (‘target absent’ for the search task, and ‘no’ to the working memory probe), the remaining participants used the opposite button mapping.
Eye movement tracking and analysis
Eye movements were recorded from all participants during MRI scanning using an MR compatible ASL504 LRO infrared video-based eye tracker (Applied Science Laboratory, Bedford, MA) sampling at 240 Hz, with a spatial resolution of ~0.5°. Fixation was analysed off-line using the same custom routines used for Experiment 1.
Scanning details
All images were acquired using a Siemens 1.5T Vision MRI scanner (Numaris version 33B) with the standard circularly polarised head coil. T2* weighted images were acquired using Gradient Echo EPI with a 128 × 128 matrix, field of view 240 mm, TE 54 ms and 90° flip-angle. A 64 × 64 axial mosaic sequence was used to acquire 34 slices, parallel to the AC-PC line, in interleaved slice order. Acquired voxel size was 3.5 mm3. Using a TR of 3.6 s, 158 volume acquisitions were acquired per experimental run.
T1 weighted axial anatomical scans with 1 × 1 × 2 mm resolution were also acquired for every participant using an MP-RAGE sequence (TR = 9.7 ms, TE = 4 ms, T1 = 300 ms, flip-angle 8°, 128 partitions, FOV 250 × 250 × 256 mm).
Analysis of imaging data
Image processing and statistical analyses were carried out using SPM99 (Wellcome Trust Centre for Neuroimaging, UCL, http://www.fil.ion.ucl.ac.uk/spm). Each participant’s functional images were realigned to the first image to compensate for head movement, spatially normalised to the SPM99 EPI template, and spatially smoothed with an isotropic smoothing kernel of 6 mm, full width at half maximum.
For each participant, a linear combination of regressors representing the time series for each condition of interest were convolved with a synthetic haemodynamic response function and its temporal derivative, creating a classic box car function. The general linear model, as employed by SPM99, was used to generate parameter estimates of activity at each voxel, for each condition, for each participant. Linear contrasts between regressors, representing the different experimental conditions, were used to determine activated brain areas by generating statistical parametric maps of the t statistic (SPM{t}).
For the group, a random-effects analysis was employed (Friston et al. 1999), so that any inferences drawn from the data can be applied to the general population. For every participant, a single mean image was generated for each contrast between experimental conditions. These images were then used as the basis for inter-participant comparisons and used to generate a statistical parametric map of the t statistic at every voxel. Behavioural measures of RT and accuracy, for both the WM and search tasks, were added as covariates of no interest to the group analysis where appropriate, to ensure that these measures did not influence the observed pattern of activation. A threshold of p < 0.001, uncorrected for multiple comparisons, was used to determine significance, unless otherwise stated.
Regions of interest
To determine whether the cortical networks associated with our spatial and/or verbal working memory tasks overlapped with that for inefficient visual search, a ‘mask’ ROI image (thresholded at p < 0.01, uncorrected) was applied to the data to restrict our analysis to regions known to be involved in inefficient search. This mask was generated using independent data from a previous study that specifically investigated the cortical network for inefficient search using a comparable experimental design (Anderson et al. 2007), and hence only includes voxels known to be associated with inefficient search. Data from both studies were normalised to the same standard template. For the ‘search only’ condition included in the present study, the visual search task was sandwiched in the middle of a ‘dummy’ working memory task to ensure comparable visual stimulation and manual responses across all conditions. Therefore, only a small portion of each trial actually involved performing the search task, and brain activity averaged across the block will have been significantly contaminated by neural responses associated with observing and responding to the dummy working memory task. Hence, a mask ROI image was generated from the results of our previous study, which more accurately represents the brain areas involved in inefficient search, and used to determine regions of overlap with the working memory tasks used here.
Further, region of interest analyses were carried out on two specific regions of prefrontal cortex previously associated with inefficient search: the inferior and middle frontal cortex of the right hemisphere. These regions were defined apriori using the same mask ROI image generated from our previous fMRI study described above, and used for the purpose of probing the neural correlates of dual-task processing within these regions (see below). The inferior frontal region was defined by a cluster of activity in the ventro-lateral prefrontal cortex (VLPFC), Brodmann’s areas 44/47, centered around the MNI coordinates [44/20/-6]. The right middle frontal region, in the dorso-lateral prefrontal cortex (DLPFC), Brodmann’s area 9/46, was defined by a sphere with a 15 mm radius centered on the MNI coordinates [48/12/26].
Results experiment 2
Behavioural responses during scanning
Fixation analysis
Fixation for each condition was analysed separately (Table 3). Analysis of variance, with working memory load (single-task, dual-task) and working memory domain (spatial, verbal) as within participant factors, confirmed there was no effect of memory load (F(1,11) = 0.647, p = 0.438), or working memory domain (F(1,11) = 0.024, p = 0.878), and no interaction (F(1,11) = 0.014, p = 0.908), on the number of saccades made. Similarly, there was no effect of memory load (F(1,11) = 0.332, p = 0.576), or working memory domain (F(1,11) = 2.160, p = 0.170), and no interaction (F(1,11) = 0.014, p = 0.908), on the amplitude of saccades made.
Table 3.
Fixation accuracy during scanning
| SWM only | VWM only | SWM & search | VWM & search | Visual search only | |
|---|---|---|---|---|---|
| Number of saccades | 1.2 (0.4) | 1.3 (0.6) | 1.7 (0.7) | 1.7 (0.8) | 1.7 (0.6) |
| Amplitude of saccades | 1.3 (0.1) | 1.2 (0.1) | 1.3 (0.1) | 1.2 (0.1) | 1.2 (0.1) |
The average number and amplitude of saccades for each condition recorded inside the scanner
The numbers in brackets represent the standard error of the mean
Search performance
Similar to experiment 1, search RTs and search accuracy did not significantly differ for search performed within the retention interval of either the spatial or the verbal working memory task (Table 1b). Analysis of variance on search times, with working memory domain (spatial, verbal) and target presence (present, absent) as within participant factors, confirmed a main effect of target presence (F(1,11) = 27.328, p < 0.001) but no main effect of working memory domain (F(1,11) = 0.004, p = 0.950) and no interaction (F(1,11) = 1.223, p = 0.292).
No significant difference in search accuracy was found across all conditions (F(2,22) = 1.564, p = 0.232).
Working memory performance
Error rates for the SWM only condition were slightly higher than those for the VWM only condition, but the difference did not reach significance (see Table 2b, paired t test: t(11) = 1.915, p = 0.082), all participants achieved greater than 71% accuracy in both tasks. However, under dual-task conditions errors on the SWM task were significantly higher than those for the VWM task (paired t test: t(11) = 3.647, p = 0.004). Analysis of variance, with working memory domain, and search (no search, search) as within participant factors, confirmed a main effect of working memory domain (F(1,11) = 15.699, p = 0.002) and a main effect of search (F(1,11) = 18.529, p = 0.001), but no interaction (p = 0.297).
As for experiment 1, no apriori predictions were made about the effect search would have on the working memory response times. Analysis of variance revealed no effect of memory domain (F(1,11) = 0.376, p = 0.552), no effect of search (F(1,11) = 0.363, p = 0.559) and no interaction (F(1,11) = 2.027, p = 0.182) (Table 2b).
fMRI data
The primary aim of this study was to determine whether brain areas known to be recruited during inefficient visual search could be associated with working memory processes, and if so, whether these processes were specific to the spatial domain.
Common pathways for spatial working memory and visual search
First, to determine whether brain activity associated with spatial working memory overlapped with that for inefficient search, the results for the SWM only condition (versus fixation baseline) were masked with an image representing the cortical network for inefficient search previously identified (Anderson et al. 2007) (see “Methods”). This mask acts to restrict the data to voxels known to be involved in inefficient search, so that areas of overlap can be determined.
This revealed overlapping regions of activity in the inferior, middle and superior frontal cortex bilaterally, bilateral parietal cortex extending from posterior to anterior aspects of the intra-parietal sulcus as well as inferiorly to the parieto-occipital junction in the right hemisphere, and bilateral occipital activation (Table 4).
Table 4.
Regions associated with both spatial working memory and inefficient search
| Region | x/y/z coordinates | Z | cl | BA | ||
|---|---|---|---|---|---|---|
| Parietal | aIPS | R | 46/−34/44 | 4.32 | 281 | 40 |
| L | −36/−34/36 | 4.50 | 113 | 40 | ||
| −32/−46/44 | 3.57 | 113 | 40 | |||
| mIPS | R | 32/−56/58 | 4.40 | 281 | 7/40 | |
| 36/−52/52 | 3.70 | 281 | 7 | |||
| L | −26/−56/52 | 4.09 | 539 | 7 | ||
| pIPS | R | 16/−76/52 | 3.82 | 492 | 7 | |
| L | −12/−72/54 | 4.18 | 539 | 7 | ||
| SPC | R | 16/−64/64 | 4.39 | 492 | 7 | |
| L | −16/−72/62 | 4.12 | 539 | 7 | ||
| Parieto-occipital | POJ | R | 28/−72/36 | 3.80 | 405 | 7/19 |
| Occipital | SOG | R | 30/−76/24 | 4.12 | 405 | 19 |
| L | −28/−76/26 | 3.94 | 115 | 19 | ||
| MOG | R | 32/−78/12 | 4.02 | 405 | 19 | |
| IOG | L | −40/−76/−10 | 3.77 | 24 | 19 | |
| Frontal | SFG | R | 28/−2/54 | 3.80 | 152 | 6 |
| L | −22/−6/62 | 4.99 | 194 | 6 | ||
| −20/−10/52 | 3.69 | 194 | 6 | |||
| MFG | R | 50/8/26 | 4.59 | 75 | 9/46 | |
| IFG | R | 32/22/0 | 4.79 | 375 | 45/47 | |
| 32/24/−14 | 4.57 | 375 | 45/47 | |||
| L | −32/26/−8 | 4.76 | 144 | 47 | ||
| −32/24/0 | 4.13 | 144 | 47 | |||
| Cingulate | R | 6/22/48 | 4.03 | 89 | 32 | |
Coordinates of regions of overlap, Z scores, cluster size (cl) and approximate Brodmann Areas (BA)
In addition, we compared activity evoked by the dual-task condition ‘SWM & search’ with the single-task control condition ‘Visual search only’, to identify areas specifically associated with the additional spatial working memory task. Although this comparison has the advantage of controlling for visual and motor responses, it suffers from the assumptions of pure insertion (Friston et al. 1996). Thus, if activity is sub-additive in cortical areas recruited by both tasks, then activity in these areas may be lost in the subtraction; or if the two tasks interact there is potential for additional areas related to dual-task performance to be included in the network of cortical activity. Therefore, this comparison is included purely as supportive data to the above findings. When this comparison was masked with the cortical network for inefficient search found previously, the results were very similar to those documented above confirming their reliability.
Common pathways for verbal working memory and visual search
To determine whether a non-spatial working memory task also activated cortical regions associated with inefficient search, the results for the VWM only condition (versus fixation baseline) were masked with the results for inefficient search.
Cortical areas associated with both VWM and inefficient search included the inferior frontal cortex bilaterally, the right middle frontal cortex, bilateral superior frontal cortex, bilateral parietal cortex extending from posterior to anterior aspects of the intra-parietal sulcus as well as bilateral superior occipital cortex (Table 5).
Table 5.
Regions associated with both verbal working memory and inefficient search
| Region | x/y/z coordinates | Z | cl | BA | ||
|---|---|---|---|---|---|---|
| Parietal | aIPS | R | 48/−36/42 | 4.03 | 27 | 40 |
| L | −44/−32/40 | 3.13 | 21 | 40 | ||
| mIPS | R | 32/−50/48 | 3.69 | 24 | 7 | |
| L | −38/−48/50 | 3.52 | 16 | 7 | ||
| pIPS | R | 18/−68/50 | 3.84 | 119 | 7 | |
| L | −12/−72/54 | 3.47 | 52 | 7 | ||
| SPC | R | 16/−68/62 | 3.58 | 119 | 7 | |
| Occipital | SOG | R | 32/−74/26 | 3.58 | 19 | 19 |
| 32/−76/12 | 3.51 | 16 | 19 | |||
| L | −28/−76/26 | 4.02 | 36 | 19 | ||
| MOG | R | 32/−78/12 | 3.38 | 9 | 19 | |
| Frontal | SFG | L | −24/−6/56 | 3.86 | 32 | 6 |
| −30/−8/48 | 3.84 | 44 | 6 | |||
| −22/−4/68 | 3.57 | 19 | 6 | |||
| MFG | R | 48/4/24 | 3.13 | 3 | 9/46 | |
| IFG | R | 34/18/10 | 3.77 | 19 | 13 | |
| 44/22/2 | 3.35 | 16 | 45/47 | |||
| L | −36/14/−2 | 3.38 | 2 | 45/47 | ||
| Cingulate | R | 10/12/56 | 3.37 | 4 | 6 | |
Coordinates of regions of overlap, Z scores, cluster size (cl) and approximate Brodmann’s Areas (BA)
In addition, we compared activation produced by the dual-task ‘VWM & search’ with the single-task control condition ‘Visual search only’, in order to identify brain areas associated with the verbal working memory task, whilst controlling for visual and motor responses. When this contrast was masked with the results for inefficient search very similar results were obtained to those documented above, adding further support and reliability to the above findings.
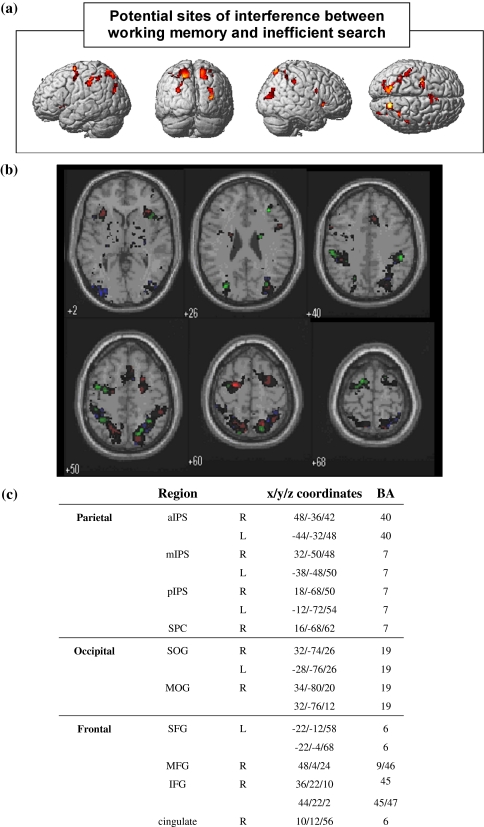
Potential sites of interference between working memory and visual search
The results of the previous sections show considerable overlap in the regions activated by both working memory tasks and inefficient search. Figure 5 shows the activation patterns for all three tasks overlaid on the same axial slices of the brain. Regions of overlap highlight possible sites for the behavioural interference effects observed, these include the right inferior and middle frontal cortex, the left superior frontal gyrus, posterior, middle and anterior regions of the intra-parietal sulcus and the superior occipital gyrus, predominantly in the right hemisphere.
Fig. 5.
Common areas activated by inefficient search and both working memory tasks. a 3D rendering of a standardised T1 brain template with superimposed loci of brain activity showing areas in the brain which are commonly activated by the spatial working memory, verbal working memory and inefficient visual search. b Axial slices of a standardised brain template showing overlapping regions of activity for inefficient visual search (blue), spatial working memory (red) and verbal working memory (green). A threshold of p < 0.005 has been used for illustrative purposes, but all clusters were significant to p < 0.001 (except L IFG). (c) Coordinates of activation maxima for regions of overlap and approximate Brodmann’s Areas (BA)
Although the behavioural interference effects observed in experiment 1 could potentially have arisen from competition for cortical processing within any of these regions, the most likely sites of interference are the right inferior and middle frontal gyrus. Evidence for believing this comes from two related studies that employed the same working memory and search tasks used here. Regions of the right inferior and middle frontal gyrus have been uniquely associated with inefficient search, but not efficient search (such as an X amongst Ls) (Anderson et al. 2007). However, both efficient and inefficient search were found to engage regions of occipital and parietal cortex, which overlap with areas also activated by our spatial and non-spatial working memory tasks. Because dual-task interference has not been found to occur between efficient search and the working memory tasks used here (Anderson et al. 2008) (suggesting that these tasks do not compete for cortical processing), it is unlikely that areas of occipital and parietal cortex are the critical site of interference. Such interference is more likely to occur within regions of prefrontal cortex that are uniquely activated by inefficient search and both the working memory tasks, i.e. the right inferior and middle frontal gyrus. ROI analysis was thus carried out within these two specific regions, to further probe the neural correlates of performing the inefficient visual search tasks simultaneously with the working memory tasks (see below).
Of further interest, in addition to the common cortical areas activated by both the spatial and verbal working memory tasks, we also found evidence for regions of domain-specific activity—there was a relative lateralisation for greater activation of left hemisphere areas during verbal working memory and greater right hemisphere activation during spatial working memory, consistent with previous findings (Nystrom et al. 2000; Postle et al. 2000; Hautzel et al. 2002).
Cortical activations during dual-task conditions
To investigate the neural correlates of dual-task performance, we compared activity during dual-task and single-task conditions within two specific regions of frontal cortex which we predicted to be the most likely site of the dual-task interference found between the inefficient search and working memory tasks used here (Fig. 6).
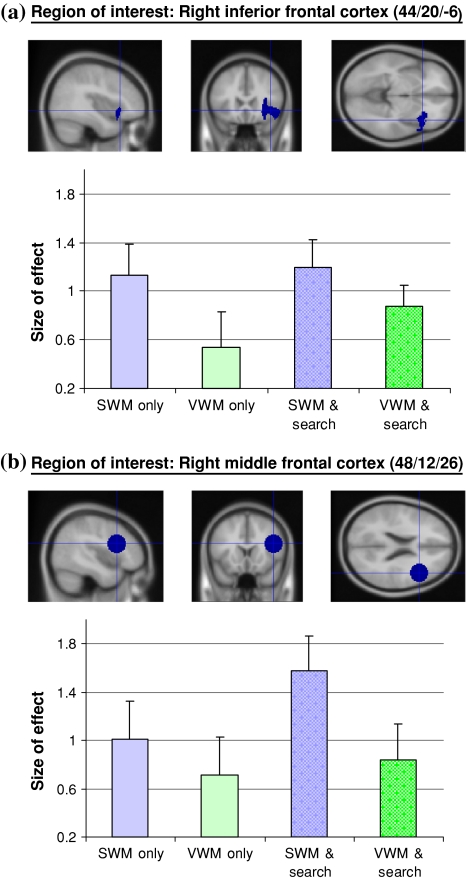
Fig. 6.
Region of interest analyses in R MFG and R IFG. Sagittal, coronal and axial views of the right IFG ROI (a) and right MFG ROI (b). These regions were defined apriori using the results of our previous fMRI study on inefficient search (Anderson et al. 2007). The right IFG region was defined by a cluster of activity centered around the MNI coordinates [44/20/-6] (see “Methods”). The right MFG region was defined by a sphere with a 15 mm radius centered on the MNI coordinates [48/12/26]. The bar graphs represent parameter estimates for spatial (blue) and verbal (green) working memory, for single and dual-task conditions, within these two regions. Error bars indicate the SE of the mean for the group
For the VLPFC region there was greater activity for the spatial working memory task than the verbal working memory task (t(11) = 2.513, p = 0.029), and dual-task performance did not significantly increase activity in the right VLPFC region compared to the working memory tasks performed alone (spatial: t(11) = 0.406, p = 0.693; verbal: t(11) = 1.081, p = 0.303).
Activity within the right DLPFC region did not significantly differ for the SWM only and VWM only tasks (t(11) = 1.046, p = 0.318), however, when inefficient visual search was performed within the retention interval of the spatial working memory task there was a significant increase in activity (t(11) = 2.967, p = 0.013), which was not observed for the verbal working memory task (t(11) = 0.349, p = 0.734).
Importantly, behavioural measures of RT and accuracy, for both the WM and search task, were included where appropriate as covariates of no interest in the group analysis (see “Methods”). Hence, the pattern of activity observed in the DLPFC and VLPFC regions of interest did not reflect any correlation with these measures (parameter estimates for all covariates of no interest were less than 0.05 for all conditions, in either region). Thus it is unlikely that the increase in DLPFC activity observed for the SWM & search condition was driven by non-specific processes related to task difficulty.
Of additional interest, we found no evidence for any cortical area specifically associated with ‘dual-task’ performance, regardless of domain. When the dual-task conditions (SWM & search + VWM & search) were compared with the working memory only conditions (SWM only + VWM only), there was no activity above threshold, even when threshold was reduced to p < 0.01.
General discussion
This study aimed to clarify the role of working memory resources in inefficient visual search. Experiment 1 confirmed that dual-task interference effects occur when inefficient visual search is performed within the retention interval of either a spatial or a verbal working memory task, replicating previous findings. The magnitude of the interference effects, which manifest as a decrease in search efficiency and search accuracy, were comparable across the spatial and non-spatial domain, suggesting that visual search competes for processes which are common to both working memory tasks.
In experiment 2, we demonstrated considerable overlap in the cortical activation patterns associated with inefficient visual search and both the spatial and verbal working memory tasks. The behavioural interference effects observed between these tasks are likely to have arisen from competition for cortical processing within these overlapping regions (Klingberg and Roland 1997; Klingberg 1998).
Overlapping pathways for working memory and inefficient visual search
The spatial and verbal working memory tasks used here activated overlapping regions of occipital, parietal and frontal cortex, consistent with many previous imaging studies that show common cortical activation across a variety of working memory domains (Owen et al. 1998; Duncan and Owen 2000; Nystrom et al. 2000; Postle et al. 2000; Hautzel et al. 2002). Importantly, activity in these regions also overlapped with areas known to be associated with inefficient visual search. As discussed previously, although the behavioural interference effects observed in experiment 1 could potentially have arisen from competition for cortical processing within any of the overlapping regions, the results of previous work lead us to believe that the most likely sites of interference are the right inferior and middle frontal gyrus.
What processes might these areas subserve that all three tasks compete for? For the working memory tasks used here, information had to be held on-line and rehearsed throughout the retention interval. Similarly, during inefficient search, information about the target item, as well as information about previously searched items is held ‘on-line’ to facilitate attentional selection and ensure correct target selection (Duncan et al. 1997; Desimone 1998; Shore and Klein 2000). The VLPFC, Brodmann areas 45/47, is thought to play a key role in such functions—maintaining spatial and non-spatial information in memory (Jonides et al. 1993; Courtney et al. 1997; Pollmann and von Cramon 2000) as well as the active retrieval of information from memory (Cadoret et al. 2001).
The middle frontal region of overlap, which fell within the DLPFC (Brodmann’s area 9/46), has repeatedly been implicated in spatial and non-spatial working memory tasks, particularly when the manipulation of information in memory is required (McCarthy et al. 1996; Owen 1997; D’Esposito et al. 1998; Owen et al. 1998; Nystrom et al. 2000; Hautzel et al. 2002; Wager and Smith 2003). Activity in this region has been associated with increased working memory demands (Braver et al. 1997), reconfiguring and continuous updating of information in memory (Owen et al. 1999; Wager and Smith 2003), as well as continuous monitoring of information and actions (Rowe et al. 2000; Rowe and Passingham 2001; Petrides et al. 2002; Lau et al. 2004). Furthermore, neuroimaging in humans and electrophysiological studies in monkeys have found the DLPFC to play an important role in attentional selection (de Fockert et al. 2001; Lebedev et al. 2004), including selecting targets from distractor items during visual search (Iba and Sawaguchi 2002; Iba and Sawaguchi 2003), or from an internal representation (Carlson et al. 1998), including selecting an item from memory according to its temporal position (Rowe and Passingham 2001; Wager and Smith 2003). It is plausible that a common underlying mechanism is responsible for attentional selection as well as manipulation of information in memory, and it is this common mechanism for which the working memory and search task compete.
Monitoring of incoming information and continual updating of information in memory are essential to ‘keep track’ during visual search. The load on these processes increases with set size, as the number of items to monitor increases. Further, if inefficient search proceeds in a serial manner, with the aid of memory for previously searched locations/items, then a process that monitors the order in which items enter memory will also facilitate the search process by preventing re-searching of previously visited items (McCarley et al. 2003). Thus, both the VLPFC and DLPFC subserve cognitive functions associated with monitoring, manipulating and retrieving information from memory considered important for successful performance in both the inefficient search and the working memory tasks. Indeed, single-unit recordings in non-human primates have found a population of DLPFC neurons with properties consistent with a role in both working memory and visual search (Rainer et al. 1998; Iba and Sawaguchi 2002; Iba and Sawaguchi 2003).
Many researchers agree that the function of the prefrontal cortex is broadly one of ‘executive control’ (Miller and Cohen 2001) and a specific frontal network appears to be consistently recruited for the solution of diverse cognitive problems (Duncan and Owen 2000). Indeed, neurons in the prefrontal cortex appear to have a special role in integrating different types of information and can be flexibly ‘tuned’ according to current behavioural demands (Rao et al. 1997; Rainer et al. 1998; Prabhakaran et al. 2000).
Region of interest analysis and dual-task performance
Dual-task performance increased activity within the same cortical network found for the working memory tasks performed alone. However, the region of interest analyses demonstrated that dual-task performance had a differential effect on VLPFC and DLPFC activity (Fig. 6).
Consistent with previous findings that show the VLPFC to be insensitive to the increased task demands required for dual-task performance (Owen et al. 1999; Manoach et al. 2004), activity within this region did not increase for our dual-task conditions compared to the single-task (working memory only) conditions. There was however, greater activity for the spatial tasks compared to the verbal tasks, consistent with the relative tendency for greater involvement of the right hemisphere for spatial compared to non-spatial material (D’Esposito et al. 1998; Wager and Smith 2003).
In contrast, although activity within the right DLPFC region did not significantly differ for the spatial and verbal single-task conditions, when inefficient visual search was performed within the retention interval of the spatial working memory task there was a significant increase in activity. This effect was not observed for the verbal domain. Although the overall working memory load associated with executive functioning should be the same for both dual-task conditions, the fact that memory was split across the verbal and spatial domain in the case of the dual-task verbal working memory and inefficient search condition, may have reduced the processing burden on this region. That is, when two spatial tasks were performed concurrently (spatial working memory and inefficient search) there was greater engagement of DLPFC, than when a verbal working memory task and inefficient search (predominantly spatial task) were performed concurrently. This finding is consistent with previous studies that have shown the DLPFC to be sensitive to increases in spatial memory load (Braver et al. 1997; Cohen et al. 1997; Rypma et al. 1999).
It has been suggested that DLPFC activity during dual-task paradigms may be related to the allocation and coordination of attentional resources, a process that has been proposed to be unique to dual-task performance (D’Esposito et al. 1995). However, we found no specific cortical areas recruited for ‘dual-task’ performance that were not also recruited during our single-task conditions. It has also been argued that increased task difficulty, or effort, can lead to increased DLPFC activity. Although task difficulty was not equated in the two dual-task conditions (indexed by the greater number of errors in the spatial dual-task condition than the verbal dual-task condition), we found no significant correlation of response errors on the working memory tasks with brain activity within this region. The increase in errors for the spatial working memory dual-task conditions is consistent with our previous study, and also with the findings of others (Experiment 1, Oh and Kim 2004; Woodman and Luck 2004). Interestingly, the error rate on the spatial working memory task did not increase with search set size, which might have suggested mutual interference between the search task and the working memory task. Interestingly, a similar increase in error rate also occurs when the spatial working memory task is performed concurrently with an efficient search task (Anderson et al. 2008). Thus, it seems that the mere presence of the search array (regardless of difficulty) acts as a non-specific mask that disrupts the representation of spatial information in working memory, independent of any specific visual search process (Woodman et al. 2001).
Our findings are consistent with previous studies that have shown activity in DLPFC to increase specifically with increasing working memory demands, and not when task difficulty was increased independently of working memory requirements (Barch et al. 1997). Moreover, several studies have even found decreased activation in the right middle frontal gyrus with increasing cognitive load (Barch et al. 1997; Manoach et al. 2004). Therefore, it is unlikely that the increased activity in DLPFC is related to some non-specific increase in task difficulty un-related to working memory demands.
Conclusions
Using a dual-task paradigm, we found significant dual-task interference effects between an inefficient visual search task and both a spatial and non-spatial (verbal) working memory task. These behavioural interference effects, which manifested as a decline in search efficiency, were comparable across the spatial and non-spatial domain—suggesting a critical role for non-spatial as well as spatial working memory in visual search. Consistent with this, using fMRI, we found considerable overlap in the cortical networks recruited during inefficient visual search and both the spatial and the verbal working memory tasks, suggesting that the behavioural interference effects observed may result from competition for processing resources within common cortical areas. Drawing on previous findings, we propose that the most likely site for such competition is within the inferior and middle frontal cortex of the right hemisphere. These areas are associated with attentional selection from memory as well as manipulation of information in memory, and we propose that the search task and working memory tasks used here compete for common processing resources underlying these mechanisms.
Acknowledgments
We thank Dr. Andrew Parton for reading and commenting on a previous version of this manuscript and all the participants for their time. This research was supported by grants from Charing Cross and Hammersmith Special Trustees and the Wellcome Trust (037222/Z/98/A).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ, McRobbie D, Kennard C. Involvement of prefrontal cortex in visual search. Exp Brain Res. 2007;180:289–302. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Mannan SK, Rees G, Sumner P, Kennard C. A role for spatial and non-spatial working memory processes in visual search. Exp Psychol. 2008;55:301–312. doi: 10.1027/1618-3169.55.5.301. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/S0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Cadoret G, Pike GB, Petrides M. Selective activation of the ventrolateral prefrontal cortex in the human brain during active retrieval processing. Eur J Neurosci. 2001;14:1164–1170. doi: 10.1046/j.0953-816x.2001.01737.x. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Zald DH, Pardo JV. Organization of working memory within the human prefrontal cortex: a PET study of self-ordered object working memory. Neuropsychologia. 2000;38:1503–1510. doi: 10.1016/S0028-3932(00)00062-2. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/S0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B Biol Sci. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychol Rev. 1989;96:433–458. doi: 10.1037/0033-295X.96.3.433. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7:255–261. doi: 10.1016/S0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ. The trouble with cognitive subtraction. Neuroimage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Han SH, Kim MS. Visual search does not remain efficient when executive working memory is working. Psychol Sci. 2004;15:623–628. doi: 10.1111/j.0956-7976.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- Hautzel H, Mottaghy FM, Schmidt D, Zemb M, Shah NJ, Muller-Gartner HW, Krause BJ. Topographic segregation and convergence of verbal, object, shape and spatial working memory in humans. Neurosci Lett. 2002;323:156–160. doi: 10.1016/S0304-3940(02)00125-8. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- Iba M, Sawaguchi T. Neuronal activity representing visuospatial mnemonic processes associated with target selection in the monkey dorsolateral prefrontal cortex. Neurosci Res. 2002;43:9–22. doi: 10.1016/s0168-0102(02)00013-5. [DOI] [PubMed] [Google Scholar]
- Iba M, Sawaguchi T. Involvement of the dorsolateral prefrontal cortex of monkeys in visuospatial target selection. J Neurophysiol. 2003;89:587–599. doi: 10.1152/jn.00148.2002. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Klein R. Inhibitory tagging system facilitates visual search. Nature. 1988;334:430–431. doi: 10.1038/334430a0. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Concurrent performance of two working memory tasks: potential mechanisms of interference. Cereb Cortex. 1998;8:593–601. doi: 10.1093/cercor/8.7.593. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Roland PE. Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Brain Res Cogn Brain Res. 1997;6:1–8. doi: 10.1016/S0926-6410(97)00010-4. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biol. 2004;2:e365. doi: 10.1371/journal.pbio.0020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Manoach DS, White NS, Lindgren KA, Heckers S, Coleman MJ, Dubal S, Holzman PS. Hemispheric specialization of the lateral prefrontal cortex for strategic processing during spatial and shape working memory. Neuroimage. 2004;21:894–903. doi: 10.1016/j.neuroimage.2003.10.025. [DOI] [PubMed] [Google Scholar]
- McCarley JS, Wang RF, Kramer AF, Irwin DE, Peterson MS. How much memory does oculomotor search have? Psychol Sci. 2003;14:422–426. doi: 10.1111/1467-9280.01457. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morey CC, Cowan N. When visual and verbal memories compete: evidence of cross-domain limits in working memory. Psychon Bull Rev. 2004;11:296–301. doi: 10.3758/bf03196573. [DOI] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11:424–446. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim MS. The role of spatial working memory in visual search efficiency. Psychon Bull Rev. 2004;11:275–281. doi: 10.3758/bf03196570. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci USA. 1998;95:7721–7726. doi: 10.1073/pnas.95.13.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, Sahakian BJ, Petrides M, Pickard JD. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Peterson MS, Kramer AF, Wang RF, Irwin DE, McCarley JS. Visual search has memory. Psychol Sci. 2001;12:287–292. doi: 10.1111/1467-9280.00353. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and memory. New York: Elsevier; 1989. [Google Scholar]
- Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci USA. 2002;99:5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S, von Cramon DY. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Exp Brain Res. 2000;133:12–22. doi: 10.1007/s002210000396. [DOI] [PubMed] [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corkin S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. Neuroimage. 2000;11:409–423. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Integration of diverse information in working memory within the frontal lobe. Nat Neurosci. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Memory fields of neurons in the primate prefrontal cortex. Proc Natl Acad Sci USA. 1998;95:15008–15013. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Sala JB, Rama P, Courtney SM. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia. 2003;41:341–356. doi: 10.1016/S0028-3932(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Shore DI, Klein RM. On the manifestations of memory in visual search. Spat Vis. 2000;14:59–75. doi: 10.1163/156856801741369. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci USA. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/CABN.3.4.255. [DOI] [PubMed] [Google Scholar]
- Walter H, Bretschneider V, Gron G, Zurowski B, Wunderlich AP, Tomczak R, Spitzer M. Evidence for quantitative domain dominance for verbal and spatial working memory in frontal and parietal cortex. Cortex. 2003;39:897–911. doi: 10.1016/S0010-9452(08)70869-4. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Visual search is slowed when visuospatial working memory is occupied. Psychon Bull Rev. 2004;11:269–274. doi: 10.3758/bf03196569. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK, Luck SJ. Visual search remains efficient when visual working memory is full. Psychol Sci. 2001;12:219–224. doi: 10.1111/1467-9280.00339. [DOI] [PubMed] [Google Scholar]