Abstract
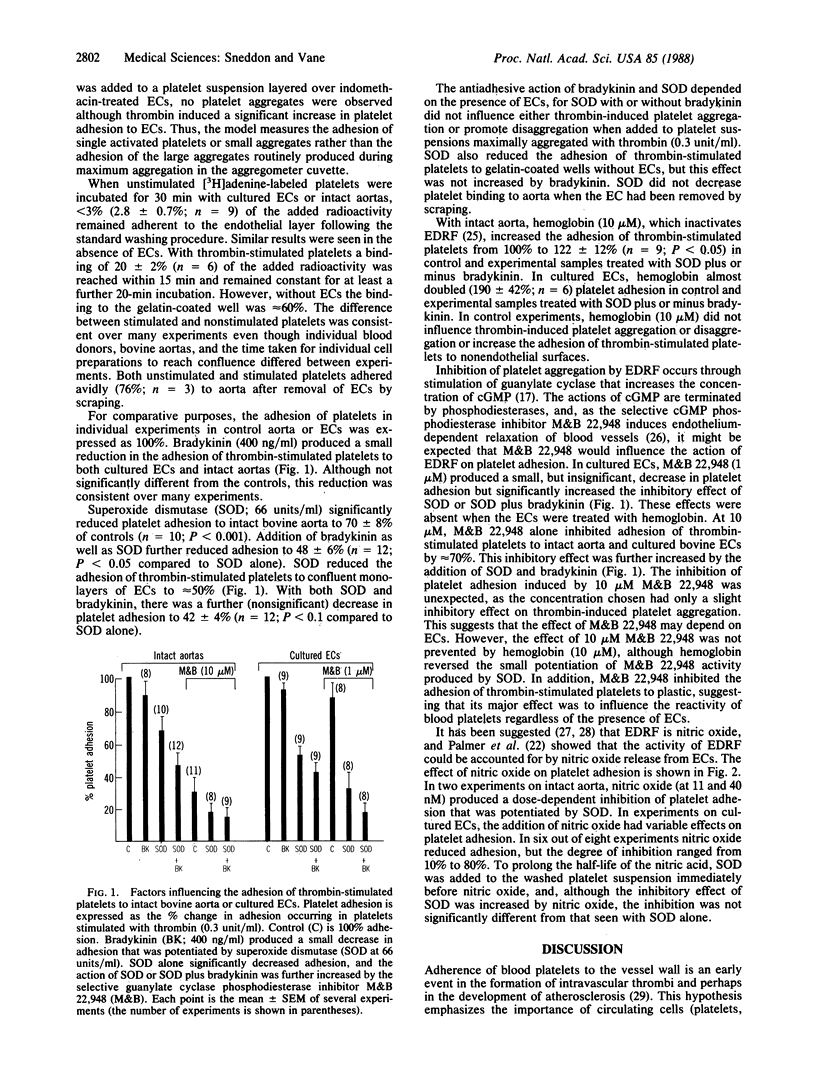
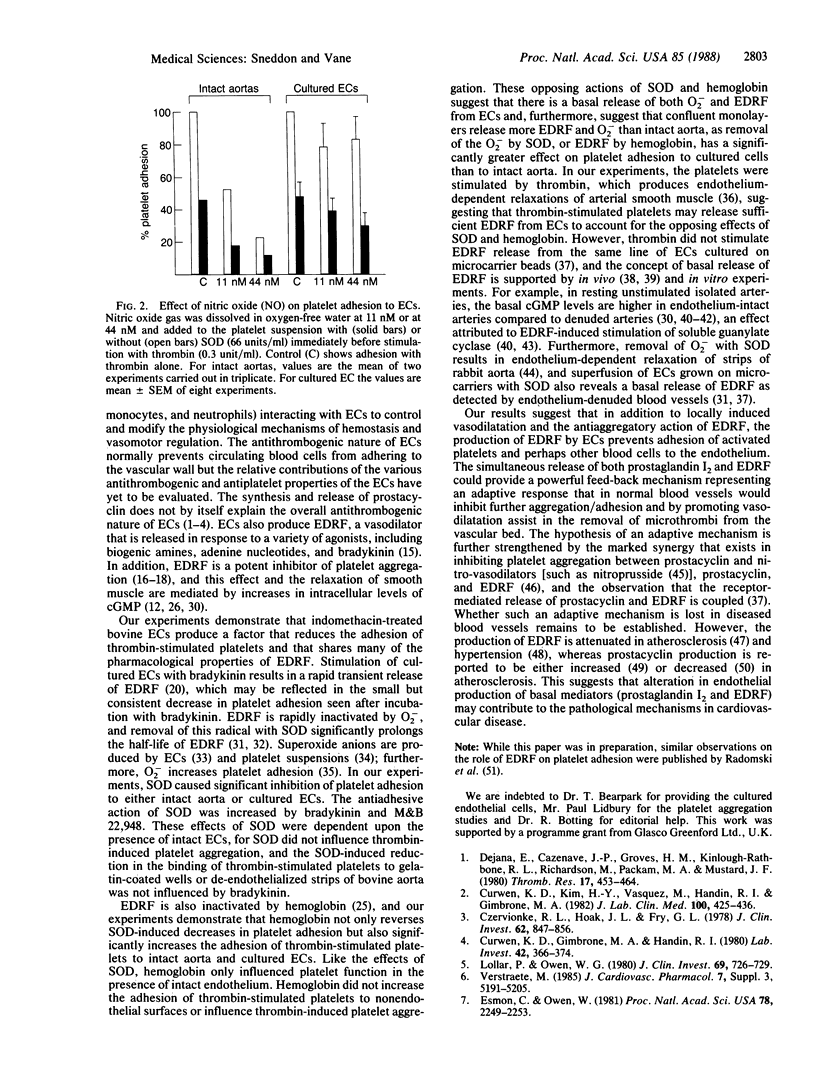
The adhesion of thrombin-stimulated human blood platelets to either the endothelial surface of intact bovine aorta or cultured bovine aortic endothelial cells was studied to determine the role of endothelium-derived relaxing factor in the regulation of platelet adhesion. Endothelial cells and platelets were pretreated with indomethacin to prevent the formation of prostaglandins. The adhesion of thrombin-stimulated platelets to endothelial cells was reduced by superoxide dismutase and bradykinin. The inhibitory effect of both drugs was abolished by hemoglobin and was absent in strips of bovine aorta where the endothelial cells had been removed by scraping. It is suggested that the effects of bradykinin are mediated by the release of endothelium-derived relaxing factor, which is protected from destruction by superoxide dismutase, and that endothelium-derived relaxing factor contributes to the nonadhesive properties of the vascular endothelium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetens J. R., Coene M. C., Verheyen A., Zonnekeyn L., Herman A. G. Biphasic response of intimal prostacyclin production during the development of experimental atherosclerosis. Prostaglandins. 1986 Sep;32(3):319–334. doi: 10.1016/0090-6980(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Buchanan M. R., Butt R. W., Magas Z., van Ryn J., Hirsh J., Nazir D. J. Endothelial cells produce a lipoxygenase derived chemo-repellent which influences platelet/endothelial cell interactions--effect of aspirin and salicylate. Thromb Haemost. 1985 Jun 24;53(3):306–311. [PubMed] [Google Scholar]
- Buchanan M. R., Haas T. A., Lagarde M., Guichardant M. 13-Hydroxyoctadecadienoic acid is the vessel wall chemorepellant factor, LOX. J Biol Chem. 1985 Dec 25;260(30):16056–16059. [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Curwen K. D., Gimbrone M. A., Jr, Handin R. I. In vitro studies of thromboresistance: the role of prostacyclin (PGI2) in platelet adhesion to cultured normal and virally transformed human vascular endothelial cells. Lab Invest. 1980 Mar;42(3):366–374. [PubMed] [Google Scholar]
- Curwen K. D., Kim H. Y., Vazquez M., Handin R. I., Gimbrone M. A., Jr Platelet adhesion to cultured vascular endothelial cells. A quantitative monolayer adhesion assay. J Lab Clin Med. 1982 Sep;100(3):425–436. [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- DeBono D. P., Macintyre D. E., White D. J., Gordon J. L. Endothelial adenine uptake as an assay for cell- or complement-mediated cytotoxicity. Immunology. 1977 Feb;32(2):221–226. [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Cazenave J. P., Groves H. M., Kinlough-Rathbone R. L., Richardson M., Packham M. A., Mustard J. F. The effect of aspirin inhibition of PGI2 production on platelet adherence to normal and damaged rabbit aortae. Thromb Res. 1980 Feb 1;17(3-4):453–464. doi: 10.1016/0049-3848(80)90080-8. [DOI] [PubMed] [Google Scholar]
- Dembinska-Kiec A., Gryglewska T., Zmuda A., Gryglewski R. J. The generation of prostacyclin by arteries and by the coronary vascular bed is reduced in experimental atherosclerosis in rabbits. Prostaglandins. 1977;14(6):1025–1034. doi: 10.1016/0090-6980(77)90282-9. [DOI] [PubMed] [Google Scholar]
- Diamond J., Chu E. B. Possible role for cyclic GMP in endothelium-dependent relaxation of rabbit aorta by acetylcholine. Comparison with nitroglycerin. Res Commun Chem Pathol Pharmacol. 1983 Sep;41(3):369–381. [PubMed] [Google Scholar]
- Dieterle Y., Ody C., Ehrensberger A., Stalder H., Junod A. F. Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ Res. 1978 Jun;42(6):869–876. doi: 10.1161/01.res.42.6.869. [DOI] [PubMed] [Google Scholar]
- Edwards D. H., Griffith T. M., Ryley H. C., Henderson A. H. Haptoglobin-haemoglobin complex in human plasma inhibits endothelium dependent relaxation: evidence that endothelium derived relaxing factor acts as a local autocoid. Cardiovasc Res. 1986 Aug;20(8):549–556. doi: 10.1093/cvr/20.8.549. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furlong B., Henderson A. H., Lewis M. J., Smith J. A. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br J Pharmacol. 1987 Apr;90(4):687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Houston D. S., Shepherd J. T., Vanhoutte P. M. Aggregating human platelets cause direct contraction and endothelium-dependent relaxation of isolated canine coronary arteries. Role of serotonin, thromboxane A2, and adenine nucleotides. J Clin Invest. 1986 Aug;78(2):539–544. doi: 10.1172/JCI112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Burke T. M., Wood K. S., Wolin M. S., Kadowitz P. J. Association between cyclic GMP accumulation and acetylcholine-elicited relaxation of bovine intrapulmonary artery. J Pharmacol Exp Ther. 1984 Mar;228(3):682–690. [PubMed] [Google Scholar]
- Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can J Physiol Pharmacol. 1985 Sep;63(9):1206–1209. doi: 10.1139/y85-199. [DOI] [PubMed] [Google Scholar]
- Levin R. I., Weksler B. B., Jaffe E. A. The interaction of sodium nitroprusside with human endothelial cells and platelets: nitroprusside and prostacyclin synergistically inhibit platelet function. Circulation. 1982 Dec;66(6):1299–1307. doi: 10.1161/01.cir.66.6.1299. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Silk S. T., Safier L. B., Ullman H. L. Superoxide production and reducing activity in human platelets. J Clin Invest. 1977 Jan;59(1):149–158. doi: 10.1172/JCI108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Smith J. A., White D. G. The mechanisms by which haemoglobin inhibits the relaxation of rabbit aorta induced by nitrovasodilators, nitric oxide, or bovine retractor penis inhibitory factor. Br J Pharmacol. 1986 Nov;89(3):563–571. doi: 10.1111/j.1476-5381.1986.tb11157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Superoxide anion release by human endothelial cells: synergism between a phorbol ester and a calcium ionophore. J Cell Physiol. 1986 May;127(2):207–210. doi: 10.1002/jcp.1041270203. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M., Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res. 1983 May 15;30(4):383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Lorenz R. R., Vanhoutte P. M. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am J Physiol. 1985 Jul;249(1 Pt 2):H95–101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]



