Abstract
Abnormal misfoldings of the microtubule-associated protein tau, leading to the aggregation of tau into paired helical filaments that are ultimately deposited as neurofibrillary tangles, is a key neuropathologic feature of a number of neurodegenerative disorders collectively referred to as tauopathies. We recently observed that a particular grape seed polyphenolic extract (GSPE), namely, Meganatural-Az® may attenuate the generation and stability of misfolded proteins. We hypothesized that Meganatural-Az® GSPE might also attenuate tau protein misfolding that leads to the generation of tau filamentary aggregates that are critical for the initiation and progression of neurodegeneration and/or cognitive dysfunctions in tauopathies. In this study, we used in vitro aggregations of synthetic Ac-306VQIVYK311 tau peptide as a model system to explore whether Meganatural-Az® GSPE might modulate aggregations of tau protein. We demonstrate that this GSPE is capable of inhibiting tau peptide aggregations, as well as dissociating preformed tau peptide aggregates. Results from this study suggest that this GSPE might provide beneficial disease-modifying bioactivities in tau-associated neurodegenerative disorders by modulating tau-mediated neuropathologic mechanisms. Our observation, in conjunction with the demonstrated bioavailability, as well as safety and tolerability, of this GSPE, supports the development of Meganatural-Az® GSPE for the prevention and/or treatment of tau-associated neurodegenerative disorders.
Keywords: Neurodegeneration, neurofibrillary tangles, polyphenols, tauopathies
INTRODUCTION
Microtubules, major constituents of the intracellular cytoskeleton, play key roles in regulating many essential cellular processes, such as intracellular transport and axonal elongation, as well as generation of cell polarity and shape [1,2]. Tau is a family of closely related intracellular microtubule-associated proteins. In the brain, tau is primarily expressed in neurons, where it is highly concentrated in the axon [3]. Interactions between tau and microtubules promote microtubule functions by promoting their stability and polymerization that are necessary for cytoskeletal functions [2].
Pathological changes in tau are linked to a number of neurodegenerative disorders collectively referred to as tauopathies, including Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), argyrophilic grain disease (AgD), Pick’s disease (PiD), as well as a number of familial frontotemporal dementias with Parkinsonism linked to chromosome 17 (FTDP-17). Common features among tauopathies are abnormal hyperphosphorylation of tau and accumulations of tau into detergent-resistant intracellular inclusions, known as neurofibrillary tangles (NFTs), among neurons and/or glial cells in the brain. Abnormally hyperphosphorylated tau proteins are readily dissociated from microtubules and aggregated into oligomeric tau-paired helical filaments that are ultimately deposited as intracellular NFTs [4].
Aggregation of tau into fibrillary deposits is a seeding-nucleation process. As illustrated in Fig. 1A, formations of hyperphosphorylatedtau oligomers serve as nucleation sites that sequester additional hyperphosphorylated tau as well as normal non-phosphorylated tau into fibrillary aggregates [1]. Thus, a predominant theory of tau-mediated neurodegeneration is based on a “toxic gain of function” model in which abnormally phosphorylated tau promotes sequestration of both hyperphosphorylated and normal tau from microtubules, leading to microtubule instability and alterations of microtubule-mediated processes, including abnormalities in axonal transport, among others (for review, see [1]).
Fig. 1.
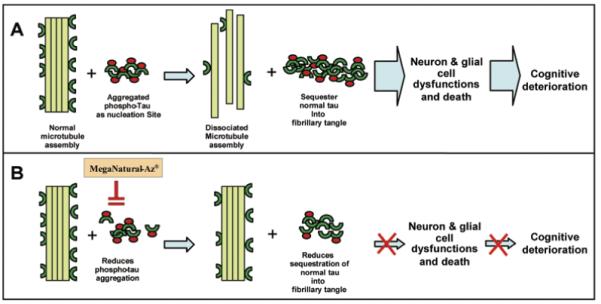
Hypothetical mechanism by which Meganatural-Az® GSPE may attenuate tau-related neurodegeneration and cognitive deterioration in AD and other tauopathies. (A) Aggregation of abnormally hyperphosphorylated tau in neurons and glial cells provides nucleation sites that recruit aggregation of normal, non-phosphorylated tau into intracellular deposits of neurofibrillary tangles. Microtubules are disabled by the loss of tau, leading to neuronal dysfunction, which ultimately culminates in neuronal cell death and cognitive impairment. (B) Meganatural-Az® may attenuate tau-mediated neurodegeneration and cognitive deterioration in AD and other tauopathies by inhibiting aggregation of hyperphosphorylated tau and/or aggregation of normal tau into neurofibrillary tangles.
We recently demonstrated that a specific grape seed polyphenol extract (GSPE), i.e., Meganatural-Az®, can inhibit aggregations of AD-type amyloid-β (Aβ) peptides into high molecular oligomeric Aβ species that play a major role in the development of cognitive dysfunction in AD [5]. More importantly, we found that this GSPE exerts anti-Aβ oligomerization activity at the organism level and that dietary treatments of a transgenic mouse model of AD with Meganatural-Az® significantly attenuated the onset and progression of AD-type Aβ neuropathology and cognitive deterioration [6]. Based on this consideration, we hypothesized that Meganatural-Az® GSPE might also exert anti-oligomerization bioactivities to tau and attenuate abnormal aggregations of tau protein in the brain (see schematics in Fig. 1B). Results from our study support the development of Meganatural-Az® GSPE for the prevention or treatment of tau-mediated neurodegenerative disorders.
MATERIALS AND METHODS
Meganatural-Az® GSPE
Meganatural-Az® GSPE is a highly purified, 100% water-soluble polyphenolicextract from Vitis vinifera grape seeds (Polyphenolics, Inc., Madera, CA). The-geographic source of the grape seeds used to generate Meganatural-Az® GSPE is California, where grapes are usually harvested between August and October. The GSPE extraction procedure is based on standard procedures at Polyphenolics, Inc. Specifically, the solvent used to extract the raw material in the form of driedseeds is water (for two hours at approximately 200 to 210°F), and the ratio of fresh grape/seeds to finished extract ranges from 30:1 to 50:1. No excipients are used in the extraction procedure, and the native extract is 100% from seeds. The finished product is spray dried into a powder and contains more than 90% total polyphenols as gallic acid equivalents and has a moisture content of less than 10%. Meganatural-Az® GSPE is stable in the factory-sealed container at room temperature for at least three years.
HPLC analysis shows that Meganatural-Az® GSPE comprises catechin and epicatechin in monomeric, dimeric, oligomeric, and polymeric forms [6]. Typically, Meganatural-Az® GSPE contains approximately 8% monomers, 75% oligomers, and about 17% polymers. We arbitrarily used the molecular weight catechin and epicatechin dimers (the most abundant forms of oligomer in GSPE) to calculate the molarity of the GSPE in this study. This extract has the unique feature that it is readily absorbed through the intestinal mucosa because of the modification of the constituent polyphenols [7]. Moreover, this GSPE is well tolerated in both humans [8] and rodents [9].
Assessments of Meganatural-Az® GSPE anti-tau aggregation bioactivity, in vitro
A 6-amino acid N-acetylated (Ac-306VQIVYK311) tau peptide, corresponding to residues 306 to 311 of tau, was synthesized commercially. This short peptide segment is derived from the third repeat motif of the tau microtubule-binding region. Oligomerization of the Ac-306VQIVYK311 peptide is essentially as described in Goux et al. [10]. In brief, the synthetic tau peptide was dissolved in 20 mM MOPS, pH 7.2. Polymerization of the tau peptide was conducted in a final 75 μl volume of a solution containing 20 mM MOPS (pH 7.2), 2.2 μM peptide, and 10 μM thioflavin-S (ThS). The reaction was initiated by adding salt to a final 0.15 M concentration. The kinetics of tau peptide aggregation in the absence or presence of the varying concentrations of the GSPE were assessed over one hour by following the increase in ThS fluorescence upon binding of ThS to aggregated peptide species; fluorescent excitation was induced at 436 nm, and fluorescent emission was detected at 470 nm.
Assessments of Meganatural-Az® GSPE dissociating preformed tau aggregates, in vitro
Synthetic Ac-306VQIVYK311 tau peptide was aggregated in the absence of Meganatural-Az® GSPE. After formations of tau aggregates, varying concentrations of the GSPE were added to the reactions, and changes in the contents of tau aggregates, in response to additions of the GSPE, were monitored by following ThS fluorescence.
RESULTS
Meganatural-Az® GSPE interferes with aggregations of the Ac-306 VQIVYK311 tau peptide
The synthetic Ac-306VQIVYK311 tau peptide is a short peptide segment found in the microtubule-binding region of the tau protein. Evidence suggests that this short peptide segment is essential for tau polymerization [10]. This is supported by the in vitro biophysical observation that the short Ac-306VQIVYK311 peptide spontaneously aggregates into filament structures in the presence of salt [10].
Building on this observation, we chose aggregations of the synthetic Ac-306VQIVYK311 tau peptide as a model system to explore whether Meganatural-Az® GSPE might play a role in modulating aggregations of tau protein.
Consistent with previous observations [10], we found that the synthetic Ac-306VQIVYK311 tau peptide readily forms into aggregates over time in the presence of salt, as reflected by increasing ThS fluorescence as a function of reaction time (Fig. 2A).
Fig. 2.
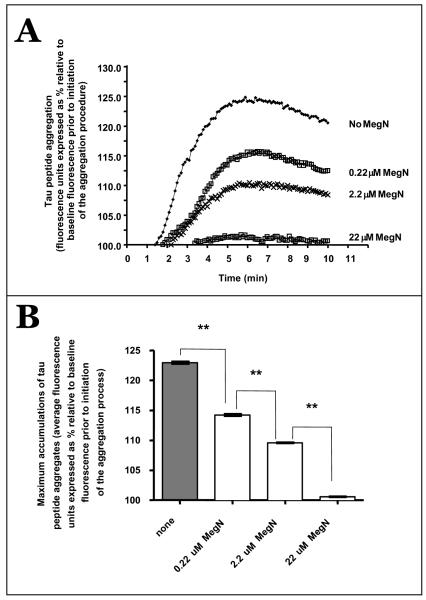
The kinetics of Ac-306 VQIVYK 311 tau peptide aggregation in the absence or presence of Meganatural-Az® GSPE. Ac-306VQIVYK311 tau peptides were aggregated in the absence (No MegN) or presence of varying doses (0.22–22 μM MegN) of Meganatural-Az® GSPE. (A) Accumulations of aggregated tau as a function of time were assessed by measuring ThS fluorescence. Concentrations of the GSPE at 0.22 μM, 2.2 μM and 22 μM correspond to, respectively, 1:10, 1:1, and 10:1 molar ratios of GSPE relative to tau peptides. (B) Maximum accumulation of tau aggregates, calculated as average fluorescent unit from 6–10 min. Bar graphs represent means ± SEM values. One-way ANOVA, p < 0.0001; **p < 0.001, Tukey post hoc pair analysis for no MegN vs. 0.22 μM MegN, 0.22 vs. 2.2 μM MegN, 2.2 vs. 22 μM MegN. Abbreviation: MegN, Meganatural-Az® GSPE.
We then determined the maximum accumulations of aggregated tau peptide by assessing the mean fluorescence emission in the absence or in the presence of Meganatural-Az® GSPE and found that addition of 0.22 to 22 μM of the GSPE significantly interferes with aggregations of the tau peptide in a dose-dependent manner (one-way ANOVA, p < 0.0001); the calculated mean fluorescent emission in the absence of the GSPE is 122.9 ± 1.3 units, compared to the calculated mean fluorescent emissions of 114.2 ± 1.1, 109.6 ± 0.6 and 100.6 ± 0.3 units, respectively, in the presence of 0.22, 2.2 and 22 μM Meganatural-Az® GSPE (Fig. 2B).
Interestingly, we found each step-wise increase in the levels of Meganatural-Az® GSPE resulted in significant incremental reductions in ThS fluorescence (Tukey post hoc pair analysis, p < 0.001 for no Meganatural-Az® versus 0.22 μM of Meganatural-Az, 0.22 versus 2.2 μM of Meganatural-Az®, and 2.2 versus 22 μM of Meganatural-Az® (Fig. 2B). Moreover, detectable reduced accumulations of tau peptide aggregates were observed at a low content of 0.22 μM of the GSPE, which corresponds to a molar ratio of 1:10 GSPE relative to tau peptides. Tau peptide aggregation was completely inhibited at 22 μM of GSPE where the GSPE was present in 10:1 excess molar ratio relative to tau peptides (Fig. 2A, B). Collectively, our observation suggested that Meganatural-Az® GSPE may interfere with the aggregation of tau proteins into oligomeric paired helical filaments.
Meganatural-Az® GSPE dissociates pre-formed tau aggregates
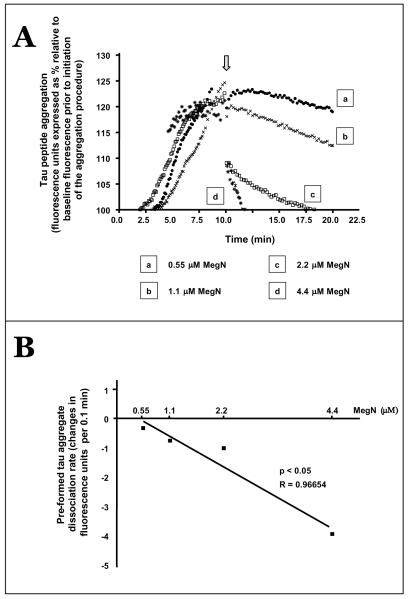
We found that Meganatural-Az® GSPE is capable of dissociating preformed Ac-306VQIVYK311 tau peptide aggregates. In particular, the addition of 1.1 μM of the GSPE, corresponding to a molar ratio of 1:1 GSPE relative to tau peptides, was able to reduce the content of preformed tau peptide aggregates, which was reflected by decreasing amounts of ThS-positive tau aggregates as a function of time (Fig. 3A). As expected, parallel studies using higher concentrations of the GSPE (2.2 μM, and 4.4 μM GSPE, corresponding to, respectively, molar ratios of 1:1, and 2:1 GSPE relative to tau peptides) also promoted dissociations of preformed tau peptide aggregates (Fig. 3A).
Fig. 3.
Meganatural-Az® GSPE treatment induces dissociation of preformed tau aggregates. Ac-306VQIVYK311 tau peptides were aggregated in the absence of Meganatural-Az® GSPE to allow accumulations of tau peptide aggregates. Varying levels of Meganatural-Az® GSPE (0.55–4.4 μM) were added at 10 min, as indicated by an arrow point. (A) Changes in the levels of fluorescent emissions from ThS-positive tau aggregates in response to additions of MegN were assessed for an additional 10 min. Concentrations of GSPE at 0.55 μM, 1.1 μM, 2.2 μM and 4.4 μM correspond to, respectively, 1:4, 1:2, 1:1 and 2:1 molar ratios of GSPE relative to tau peptides. (B) Preformed tau aggregate dissociation rates as a function of MegN concentrations. Preformed tau aggregate dissociation rates in response to MegN additions were calculated by linear regression analysis of ThS fluorescence emissions recorded from the time of MegN addition (10 min) to either the end of the assay (20 min, for 0.55 and 1.1 uM MegN) or until fluorescence emissions reached baseline levels (18.6 min. and 12.2 min., respectively, for 2.2 and 22 μM MegN). Straight lines represent best linear regression fit. Abbreviation: MegN, Meganatural-Az® GSPE.
To further explore the dose-response efficacy of preformed tau peptide disassociation, we calculated the dissociation rate of tau aggregates in the presence of varying concentrations of the GSPE by linear regression analysis of ThS fluorescent emission measurements. We found that the dissociation rate of aggregated tau peptides, reflected by reduced ThS fluorescent emission, directly correlated with the concentration of this GSPE (Pearson R = 0.96654, p < 0.05) (Fig. 3B). Thus, the addition of increasing concentrations of Meganatural-Az® GSPE, from 1.1 to 4.4 μM, promoted the dissociation of preformed tau peptide aggregates in a dose-dependent manner (Fig. 3B).
DISCUSSION
A key molecular mechanism implicated in diverse neurodegenerative diseases is protein misfolding, resulting in pathologic aggregation and accumulation of proteins in the brain [1,4]. Compelling evidence strongly supports the hypothesis that accumulation of misfolded proteins leads to synaptic dysfunction, brain damage, and disease. However, the mechanism by which protein misfolding and aggregation trigger neurodegeneration and the identity of the neurotoxic structure are still unclear. Recent evidence indicates that certain grape-derived dietary compounds – in particular, certain polyphenolic compounds enriched in grape derived polyphenols – may interfere with abnormal protein folding, thereby reducing the accumulation of neurotoxic proteins.
Misfolding of the microtubule-associated protein tau, leading to the aggregation of tau into paired helical filaments that are ultimately deposited as NFTs, is a key neuropathologic feature among tauopathies [4]. Based on our recent observation that a particular grape seed polyphenolic extract, namely Meganatural-Az, GSPE attenuates the generation and stability of misfolded proteins [5,6], we hypothesized that this GSPE might also attenuate the tau protein misfolding that leads to the generation of tau aggregates critical for the initiation and progression of neurodegeneration and/or cognitive dysfunctions in tauopathies.
In this study, we used aggregations of synthetic Ac-306VQIVYK311 tau peptide as an in vitro model system to assess the potential role of Meganatural-Az® GSPE in preventing and/or treating tau-associated neurodegenerative disorders by interfering with the generation and/or stability of aggregated tau filament deposits in the brain.
We recently found that Meganatural-Az® GSPE inhibited the aggregation of AD-type Aβ peptides [6]. In the current study, we demonstrate that the Ac-306VQIVYK311 tau peptide is also a substrate for the anti-oligomerization activity of GSPE. In particular, we found that GSPE is capable of inhibiting aggregations of the tau peptide into filaments, and dissociating pre-formed tau aggregates. Collectively, our observation suggests that interactions of Meganatural-Az® GSPE with tau may attenuate the accumulation of tau aggregate deposits, a key neuropathologic feature among multiple tau-associated neurodegenerative disorders.
We recently demonstrated that Meganatural-Az® GSPE is bioavailable at the organism level and exerts an anti-oligomerization activity in experimental model systems. In particular, we established that dietary supplementation with Meganatural-Az® GSPE reduces aggregations of Aβ peptides into high molecular weight Aβ oligomeric species in the brain of a transgenic mouse model of AD [6].
Based on in vivo bioavailability of Meganatural-Az® GSPE [6] and in vitro evidence from this study suggesting that GSPE mechanistically interferes with the generation of tau aggregates, we hypothesized that applications of this GSPE might be effective as a preventive measure to attenuate the onset of tauopathies by interfering with the generation of abnormal tau aggregates. In addition, based on our in vitro evidence that Meganatural-Az® GSPE effectively dissociates pre-formed tau peptide aggregates, it might be possible to develop this GSPE as a therapy for treating tau-associated neurodegenerative disorders.
We found that low levels of Meganatural-Az® GSPE, at molar ratios less than the concentration of the Ac-306VQIVYK311 tau peptide, effectively reduced aggregations of the tau peptide while promoting the dissociation of preformed tau aggregates. This suggests that treatments with low dosages of Meganatural-Az® GSPE might be sufficient to provide beneficial disease-modifying bioactivities in tau-associated neurodegenerative disorders. Future in vivo studies using animal models of tauopathies will identify the most appropriate doses of Meganatural-Az® GSPE to test for preventive and/or therapeutic efficacy in humans.
Meganatural-Az® GSPE comprises of catechin and epicatechin in monomeric, dimeric oligomeric, and polymeric forms. In ongoing studies, we are identifying the specific polyphenolic component(s) responsible for anti-tau aggregation bioactivity of the GSPE. Hasegawa [11] reported that epicatechin-3-gallate, which is structurally related to catechin, inhibits tau filament formation in vitro. Thus, it is possible that catechin in the Meganatural-Az® GSPE might contribute to anti-tau aggregation bioactivity.
Evidence suggests that Meganatural-Az® GSPE is highly tolerable and safe in humans. A recent report found that a long-term oral application of up to 300 mg/day of this GSPE is not associated with observable aversive response [9]. In animal modeling systems, long-term treatment with up to 200 mg/day has been shown to be tolerable and safe [6]. Translating drug dosage from mice to an equivalent drug dosage in humans, using a United States Food and Drug Administration criterion that factors in body surface area in calculating equivalent drug dosages across species [human equivalent dose in mg/kg = animal dose in mg/kg × (animal weight in kg/human weight in kg)0.33] [12], we calculated that 200 mg/day of Meganatural-Az® GSPE in mice is equivalent to 1 g/day of the GSPE in humans. Thus, we anticipate that dosages of Meganatural-Az® GSPE, higher than 300 mg/day might be applicable in humans.
In conclusion, the demonstrated bioavailability as well as safety and tolerability of Meganatural-Az® GSPE, in conjunction with evidence from this study implicating the efficacy of this GSPE to modulate tau-mediated neuropathologic mechanisms, supports development of Meganatural-Az® GSPE for the prevention and/or treatment of tau-associated neurodegenerative disorders.
ACKNOWLEDGMENTS
These studies were supported by Research Funding from Polyphenolics, Inc. to GMP, 1PO1 AT004511-01 Project 1 to LH and 1PO1 AT004511-01 Project 3 to GMP, MERIT Review grant from Dept. of Veterans Affairs to GMP, and James J. Peters VA GRECC Program to GMP. We thank Drs. Hanna Ksiezak-Reding and Don Scott for their editorial comments in the preparation of this manuscript.
References
- [1].Sorrentino G, Bonavita V. Neurodegeneration and Alzheimer’s disease: the lesson from tauopathies. Neurol Sci. 2007;28:63–71. doi: 10.1007/s10072-007-0789-x. [DOI] [PubMed] [Google Scholar]
- [2].Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Binder LL, Frankurter A, Reuhun LL. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mi K, Johnson GV. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2006;3:449–463. doi: 10.2174/156720506779025279. [DOI] [PubMed] [Google Scholar]
- [5].Ono K, Condron M, Ho L, Wang J, Zhao W, Pasinetti GM, Teplow DB. Effects of grape seed-derived polyphenols on amyloid β-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang J, Ho L, Zhao W, Ono K, Rosensweitg C, Chen C, Humala N, Teplow DB, Pasinetti GM. Grape derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Siva B, Edirisinghe I, Randolph J, Steinberg F, Kappagoda T. Effect of polyphenolics extracts of grape seeds (GSE) on blood pressure (BP) in patients with metabolic syndrome (MetS) FASEB J. 2006;20:A305. [Google Scholar]
- [8].Lu B, Robinson M. Effect of a grape seed extract on blood pressure in subjects with pre-hypertension. FASEB J. 2007;21:750.33. [Google Scholar]
- [9].Bentivegna SS, Whitney KM. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem Toxicol. 40:1731–1743. doi: 10.1016/s0278-6915(02)00155-2. 2992. [DOI] [PubMed] [Google Scholar]
- [10].Goux WJ, Kopplin L, Nguyen AD, Leak K, Rutkofsky M, Shanmuganandam VC, Sharma D, Inouye H, Kirshner DA. The formation of straight and twisted filaments from short tau peptides. J Biol Chem. 2004;279:26868–26875. doi: 10.1074/jbc.M402379200. [DOI] [PubMed] [Google Scholar]
- [11].Hasegawa M. Biochemistry and molecular biology of tauopthies. Neuropathology. 2006;26:484–490. doi: 10.1111/j.1440-1789.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- [12].United States Food and Drug Administration Guidance for Industry and Reviews: Estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. 2005 http://www.fda.gov/cber/gdlns/dose.htm.