Abstract
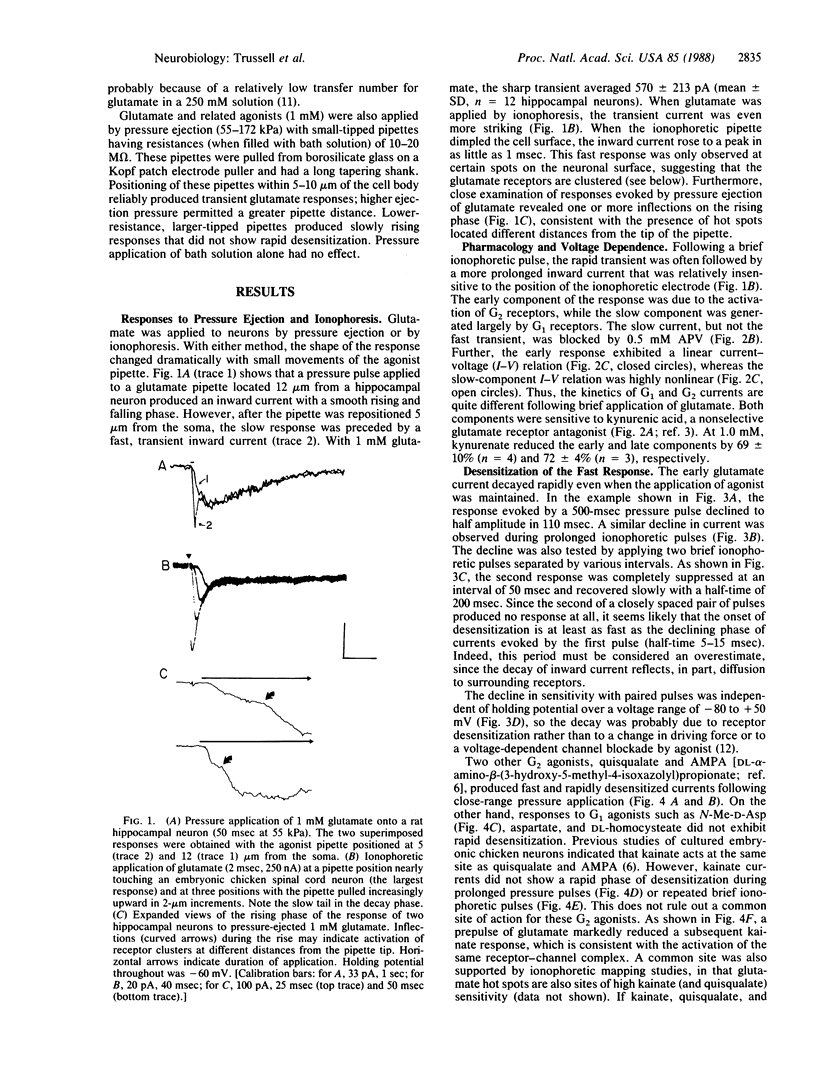
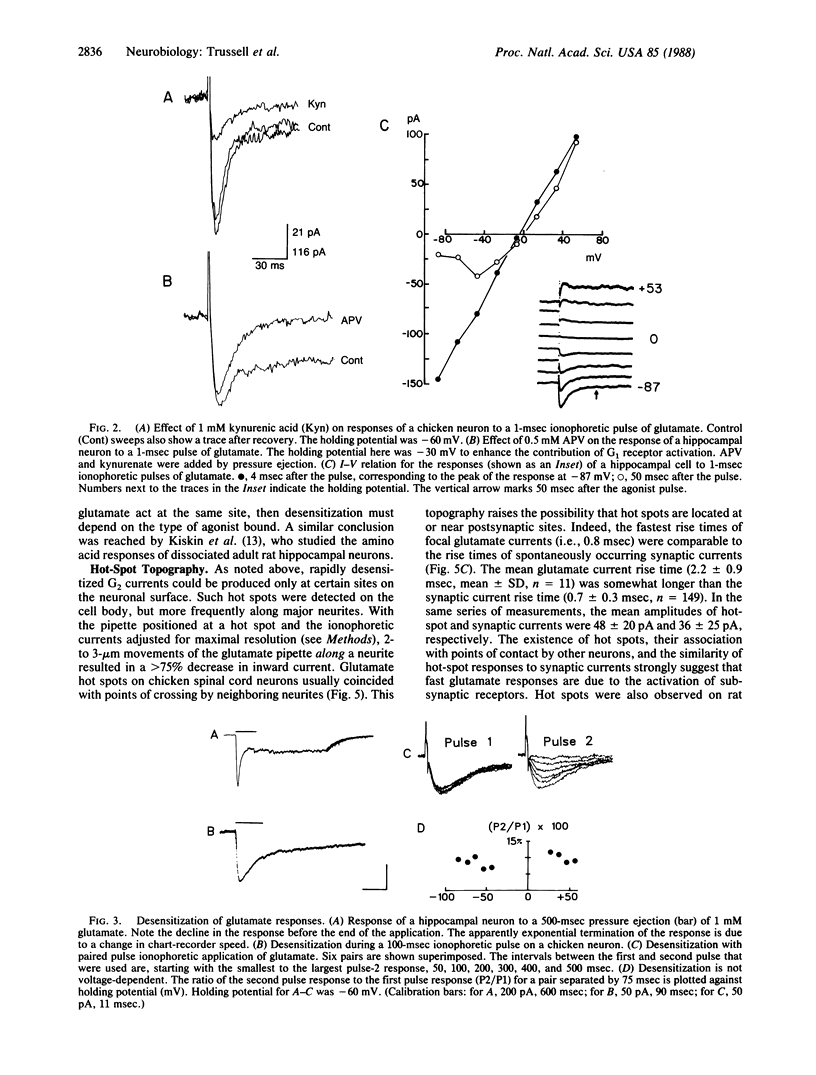
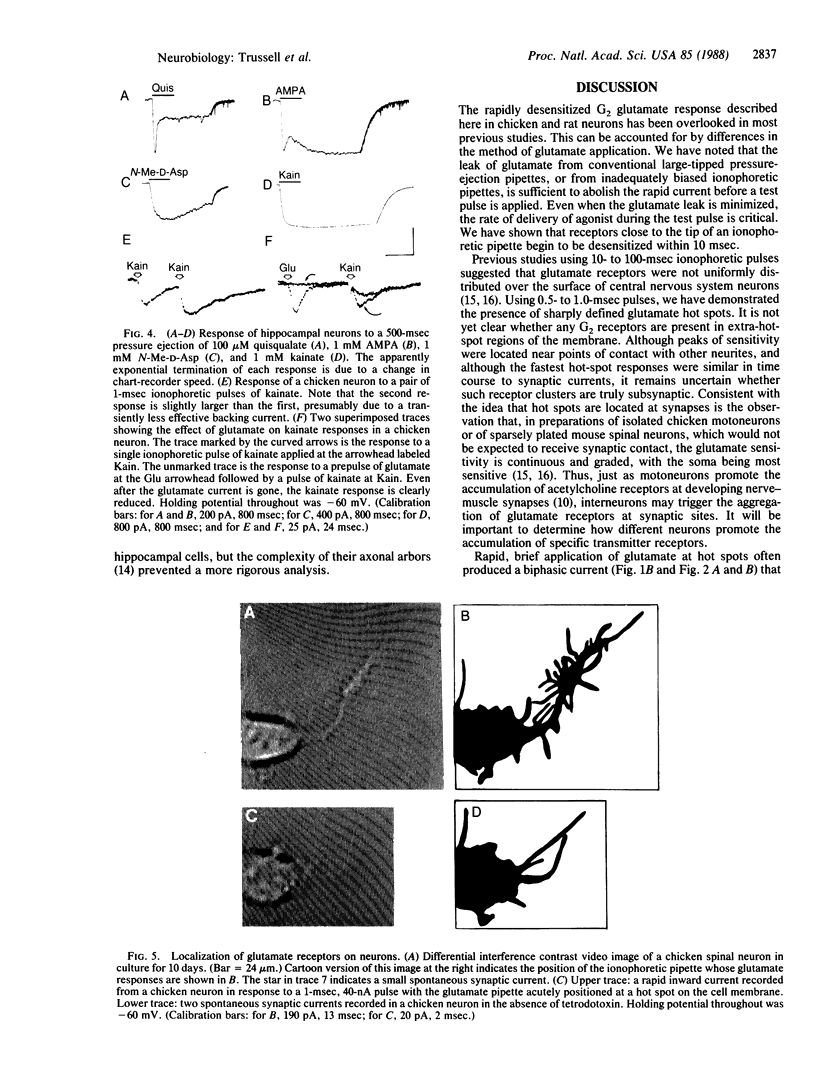
We have examined glutamate receptor desensitization in voltage-clamped embryonic chicken spinal cord neurons and postnatal rat hippocampal neurons maintained in culture. Rapid currents that rose in 0.8-3.6 msec were evoked when glutamate was ionophoresed with 0.5- to 1.0-msec pulses. With prolonged pulses or brief, repetitive pulses, glutamate-evoked currents decayed rapidly in a manner that was independent of holding potential. A similar desensitization occurred following close-range pressure ejection of glutamate. The rapid, desensitizing glutamate current exhibited a linear current-voltage relation and it was not blocked by 2-amino-5-phosphonovalerate, suggesting that it was mediated by N-methyl-D-aspartate-insensitive (G2) receptors. Desensitization of G2 receptors may be agonist-dependent: currents evoked by kainate, a selective G2 agonist, did not decay, whereas prior application of glutamate did reduce the size of kainate responses. The appearance of the rapid current depended critically on the position of the ionophoretic pipette. Such glutamate-receptor "hot spots" often corresponded to points of contact with neighboring neurites, which raises the possibility that they are located at synapses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cowan W. M. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979 Oct 1;187(3):469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Neyton J. Quisqualate and L-glutamate inhibit retinal horizontal-cell responses to kainate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1837–1841. doi: 10.1073/pnas.82.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. doi: 10.1016/0304-3940(86)90360-5. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Leonard R. J., Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1986 May;83(9):3022–3026. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Characterization of excitatory amino acid receptors expressed by embryonic chick motoneurons in vitro. J Neurosci. 1986 Nov;6(11):3275–3283. doi: 10.1523/JNEUROSCI.06-11-03275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. J., Fischbach G. D. Isolation of embryonic chick motoneurons and their survival in vitro. J Neurosci. 1986 Nov;6(11):3265–3274. doi: 10.1523/JNEUROSCI.06-11-03265.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. III. Neuronal chemosensitivity and its relationship to synaptic activity. J Neurophysiol. 1977 Sep;40(5):1163–1177. doi: 10.1152/jn.1977.40.5.1163. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachová V., Vyklický L., Vyklický L., Jr, Vyskocil F. The action of excitatory amino acids on chick spinal cord neurones in culture. J Physiol. 1987 May;386:425–438. doi: 10.1113/jphysiol.1987.sp016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]





