Abstract
Whether Kinase Suppressor of Ras1 (KSR1) is an active kinase that phosphorylates c-Raf-1 or a scaffold that coordinates signaling along the Ras/ERK1 signaling module is actively debated. In this study, we generated a monoclonal antibody against a c-Raf-1 peptide containing phosphorylated Thr269, the putative target for KSR1 kinase activity. We show that this antibody detects Thr269-phosphorylated c-Raf-1 in A431 cells upon epidermal growth factor (EGF) stimulation, preceding MEK1 activation. Furthermore, this antibody detects in vitro phosphorylation of FLAG-c-Raf-1 and kinase-dead FLAG-c-Raf-1(K375M) by immunopurified KSR1, but fails to detect phosphorylation of FLAG-c-Raf-1(K375M/T269V), engineered with a Thr269 to valine substitution. To provide unequivocal evidence that KSR1 is a legitimate kinase, we purified KSR1 to homogeneity, confirmed by mass spectrometry, renatured it in gel, and demonstrated that it phosphorylates BSA-conjugated c-Raf-1 peptide at Thr269. These studies add to emerging data validating KSR1 as a kinase that phosphorylates c-Raf-1.
Keywords: KSR, c-Raf-1, MAPK, Scaffold, EGF
INTRODUCTION
Kinase Suppressor of Ras1 (KSR1) was originally identified in D. melanogaster and C. elegans as a positive modulator of Ras/MAPK signaling either upstream of or parallel to Raf [1]. In C. elegans, KSR-1 is dispensable for normal development and adult worm function, but required for gain-of-function signaling through mutated RAS-1 or LET-23, the epidermal growth factor receptor (EGFR) homolog [1]. Murine and human KSR1 orthologs were subsequently isolated with high sequence identity suggesting KSR1 signaling is evolutionarily conserved. As in C. elegans, KSR1 inactivation has little, if any, impact on Ras function in normal mammalian development or physiology, but rather KSR1 appears specifically engaged when demand for Ras function is increased, such as via activating point mutations or enhanced upstream signaling through hyperactivated tyrosine kinase receptors [2]. Consistent with this observation, mice lacking KSR1 develop and reproduce normally, and manifest a normal lifespan [3]. However, ksr1−/− mice develop an unusual disorganized hair follicle phenotype also found in EGFR knockout mice, indicating that as in C. elegans, EGFR, Ras, and KSR1 are on the same signaling pathway [2]. Moreover, papilloma formation in Tg.AC mice, resulting from skin-specific v-Ha-ras expression, is abrogated in a ksr1−/− background, indicating v-Ha-ras-mediated skin cancer, signaled through the Raf-1/MAPK cascade, requires KSR1 [2]. These results suggest KSR1 may represent a therapeutic target for Ras/MAPK signaling in some human cancers.
KSR1 is composed of five domains, termed CA1–CA5, the first four of which are N-terminal [1]. CA1 is unique to KSR1, and conserved in most KSR1 orthologs. Its function awaits characterization. CA2 is a Src homology 3 (SH3) recognition site and CA3 a Cys-rich domain similar to the lipid-binding domain of protein kinase C. CA4 is a Ser/Thr-rich region resembling the CR2 domain of c-Raf-1. The C-terminal CA5 region contains the 11 conserved kinase subdomains found in all kinases. However, it is not clear whether KSR1 might be a Tyr or Ser/Thr kinase. The sequence YI[4]APE in subdomain VIII, conserved among Ser/Thr kinases, is present in all KSR genes cloned so far, yet C. elegans and D. melanogaster KSR1 also contain the sequence HKDLR indicative of Tyr kinases [1]. Human and murine KSR1 have an Arg in kinase subdomain II instead of the conserved Lys normally involved in ATP binding. As Arg for Lys substitutions typically inactivate kinase function, significant doubt was expressed soon after the discovery of KSR1 as to whether it was a kinase at all [5–8], as opposed to a scaffold protein coordinating elements of the Ras/Raf/MAPK module.
In quiescent cells, KSR1 is phosphorylated on Ser297 by an unknown kinase and on Ser392 by C-TAK1, creating docking sites for 14-3-3. The 14-3-3–KSR1 interaction sequesters KSR1 and MEK1, to which it is constitutively bound, in the cytoplasm [9]. Both KSR1 and its target, c-Raf-1, are also constitutively bound to the PP2A core enzyme (subunits A+C)[10]. Impedes mitogenic signal propagation (IMP) also interacts with the KSR1 N-terminus, maintaining KSR1 inactive [11]. During growth factor stimulation (such as via EGF), the KSR1/PP2A and c-Raf-1/PP2A complexes acquire the PP2A regulatory B subunit, and the PP2A holoenzyme dephosphorylates KSR1 Ser392 and c-Raf-1 Ser259, leading to partial release of 14-3-3 from each protein [10]. For KSR1, this step exposes a MAPK binding site, while for c-Raf-1 it confers activated Ras binding at the plasma membrane. In addition, Ras-GTP binds IMP, relieving KSR1 inhibition [11]. KSR1 dissociation from IMP and displacement of 14-3-3 on Ser392 leads to rapid plasma membrane translocation of KSR1, localizing MEK1 and MAPK to membrane-bound c-Raf-1. This series of events, and the inability of multiple groups to observe KSR1 kinase activity toward c-Raf-1, has led to the predominating position in the field that KSR1 functions as a scaffold protein only [6,8,12]. In this model, KSR1 appears to coordinate signaling through the c-Raf-1/MEK/MAPK module, but has no other direct impact on c-Raf-1 activation.
The minority opinion in the field is that mammalian KSR1 is a Ser/Thr kinase that transphosphorylates human c-Raf-1 at Thr269 conferring c-Raf-1 kinase activation [13]. Investigations supporting this position show that immunoprecipitated KSR1 when washed extensively to the point where it is the only protein observed after SDS-PAGE by silver staining, is capable of phosphorylating recombinant c-Raf-1 [14–19]. The suggestion that KSR1 activity was transphosphorylating rather than enhancing c-Raf-1 intrinsic autokinase activity was further supported by transphosphorylation of a kinase-inactive c-Raf-1 substrate (c-Raf-1-K375M). Furthermore, not only can immunopurified KSR1 phosphorylate c-Raf-1 in vitro but it also transactivates c-Raf-1 towards MEK1, as measured by direct MEK1 phosphorylation and MEK-induced ERK/MAPK activation [14–16,18–20]. Moreover, mutation of two conserved Asp residues (D683 and D700) required for ATP catalysis and phosphotransfer to Ala, a classic approach to generate a dead kinase, abolished both the transphosphorylating and transactivating activity of KSR1 isolated from multiple cell lines [14–21]. Despite this evidence, it has been argued that the KSR1 proteins isolated to date are contaminated with a small but highly active pool of c-Raf-1, MEK and/or other kinases capable of instigating the signaling observed in vitro [3,5,7,9,12,22].
The current investigations address this issue directly by generating a monoclonal antibody specific for Thr269 of c-Raf-1, and using this reagent to demonstrate that this putative KSR1 phosphorylation site on c-Raf-1 is indeed phosphorylated in intact cells upon EGFR activation. Furthermore, we have purified KSR1 to homogeneity as measured by mass spectrometry and then renatured it in gel. The renatured protein phosphorylates a peptide derived from the region surrounding Thr269of c-Raf-1, providing unambiguous evidence that KSR1 is a Thr kinase.
MATERIALS AND METHODS
Conjugation of phosphorylated and non-phosphorylated peptide to maleimide-activated BSA
Phosphorylated Thr269 c-Raf-1 peptide (VHMVSTpTLPVDSRMC) and non-phosphorylated (VHMVSTTLPVDSRMC) c-Raf-1 peptide were conjugated to BSA using the Imject Maleimide Activated BSA Kit (Pierce) according to manufacturer’s instructions. Briefly, peptides were mixed with an excess (1:5) of BSA-Sulpho-SMCC sulfosuccinimidyl 4-(N-maleimidomethyl)- cyclohexane-1-carboxylate (sulfo-SMCC) cross-linker in buffer containing 0.1M sodium phosphate, 0.15M NaCl, pH 7.2. After 1h at room temperature, BSA-conjugated peptides were purified by Dextran desalting column (Pierce).
Anti-phospho-peptide ELISA
To determine the optimal BSA-Thr269 phospho-peptide or BSA-non-phospho-peptide concentration with which to coat ELISA plates, we evaluated whether 25, 50, 100 or 250µg BSA-conjugated peptide/well would deliver the best signal to noise ratio. 25µg conjugated BSA-Thr269 phospho-peptide/well was optimal and utilized throughout for the ELISA.
Generation and purification of monoclonal anti-pThr269 antibody
A monoclonal antibody (mAb) specific for human-c-Raf-1 phospho-Thr269 was generated by immunizing Balb/c mice 4× at 3-week intervals with KLH-conjugated phospho-peptide covering human amino acids 263–276 (VHMVSTpTLPVDSRMC). Immunogen was emulsified in an equal volume of TiterMAX® (Cytogen, Inc) adjuvant and 50µg injected subcutaneously. Mice were bled 7 days post injection, and sera tested for anti-phosphopeptide antibodies by ELISA. For fusion with myeloma cells, the mouse with the highest Ab titer was primed with 25µg of phospho-peptide conjugated to OVA and the spleen harvested 4 days later. Splenocytes were prepared, mixed at a 5:1 ratio with SP/2-Ag14 (ATCC, CRL-1581) and fused using a polyethylene glycol (PEG 1500, Roche)/centrifugation-based method. Fusion products recovered overnight, and were selected in 25–96 well plates using media containing HAT (Sigma). Eight days later, there were on average 0.8 colonies/well and the fusion was fed for the first time. ELISA screening of media began on day 11 against BSA-phospho- and BSA-non-phospho-peptide conjugates, with all positives re-screened after expanding the culture. Repeat positives were tested by western blot using BSA conjugates as protein source. Antibody was purified by using HiTrap IgM purification HP, 1ml affinity column (GE Health care).
KSR1 Renaturation
Myc–KSR1 was immunoprecipitated using anti-Myc antibody from 20mg overexpressing COS-7 cell lysate, and beads washed 15× with lysis buffer containing 1M NaCl. Immunoprecipitated KSR1 was electrophoresed on an 8% SDS gel, and renatured as per calmodulin-dependent protein kinase II [23]. Briefly, the gel was washed with two changes of wash buffer (50mM Tris, pH 7.4, 5mM 2-mercaptoethanol) containing 20% isopropylalcohol at room temperature for 1h, and once in wash buffer without isopropyl alcohol for 1h. Denaturation was accomplished by incubating the gel in two changes of 6M guanidine HCl in wash buffer for 1h each. Renaturation was accomplished by incubating the gel overnight at 4°C in wash buffer containing 0.04% Tween-20.
Phosphorylation of c-Raf-1 peptide by in-gel renatured KSR1
The 110 kDa band corresponding to purified KSR1 was visualized with amido black, destained, excised, crushed in an eppendorf tube using a glass rod, and incubated with 2µg BSA-conjugated c-Raf-1 non-phospho-peptide in 80µl of kinase reaction mixture (25mM HEPES, pH 7.4, 10mM MgCl2, 0.5mM EGTA, and 5mM NaF) containing 100µM cold ATP. Controls received blank gel pieces. BSA-conjugated c-Raf-1 peptide was resolved by 8% SDS-PAGE and phosphorylation status interrogated using the mAb specific to c-Raf-1 phospho-Thr269 as described in Supplementary Methods.
RESULTS and DISCUSSION
An antibody that detects phospho-Thr269 residue on c-Raf-1
Previous studies using c-Raf-1 mutant proteins indicated that KSR1 phosphorylates c-Raf-1 on the Thr269 residue, conferring c-Raf-1 activation [13]. To provide an approach for direct detection of c-Raf-1 phosphorylation at Thr269, a monoclonal antibody (MAb) specific for human-c-Raf-1 phospho-Thr269 was generated by immunizing Balb/c mice with a KLH-conjugated phospho-peptide encompassing human c-Raf-1 amino acids 263–276. Sera, prepared from mice 7 days post-immunization, displayed immunoreactivity against both phospho- and non-phospho-peptide by ELISA (Fig. 1A, upper panel). Hybridomas, generated from the mouse displaying the highest titer immunoreactivity, were re-screened by ELISA against phospho- and non-phospho-peptides, and of 3000 clones analyzed 5 displayed specific phospho-peptide immunodetection. All positive clones were subsequently screened by western blot analysis using BSA-conjugated peptides as protein source, and a single clone expressing high titer IgM specific for phospho-peptide was identified (Fig. 1A, lower panel). Antibody was purified using a thiophilic adsorption/desalting column for further experimentation.
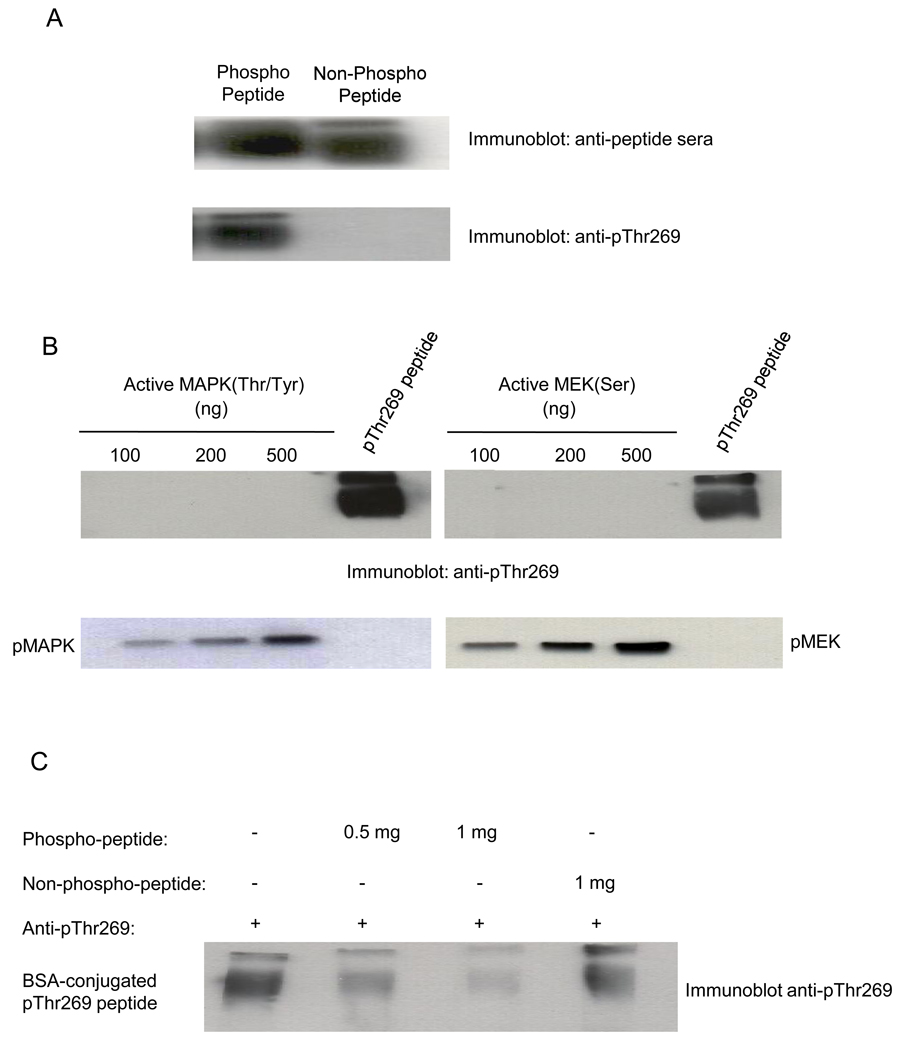
Fig. 1. Anti-pThr269 antibody specifically recognizes the phospho-Thr residue of c-Raf-1 peptide conjugated to BSA.
(A) Anti-pThr269 antibody reacts specifically with c-Raf-1 phospho-peptide. Peptides containing either phosphorylated or non-phosphorylated c-Raf-1 Thr269 residue were cross-linked to BSA using Imject Maleimide Activated BSA Kit. 1µg of each conjugated peptide, separated on 8% SDS-PAGE gel, was subjected to western blotting using anti-peptide sera and anti-pThr269 monoclonal antibody. (B) Anti-pThr269 antibody does not recognize active MAPK/ERK1 phosphorylated at Thr202 and Tyr204 residues, or active MEK1 phosphorylated at Ser217/221 residues. Lower panels show levels of MAPK/ERK1 and MEK1, respectively, by western blotting. (C) Anti-pThr269 Ab is neutralized specifically by pThr269 peptide. Anti-pThr269 Ab was pre-incubated either without peptide, or with 500ng or 1mg phospho-peptide, or 1mg non-phospho-peptide (representing a 1–2 log molar excess of peptide compared to antibody) at 4°C for 1h prior to the western blotting of BSA-conjugated pThr269 peptide. Data represent 1 of 3 similar experiments for (A–C).
To examine cross reactivity of anti-c-Raf-1 pThr269 mAb with other proteins of the MAPK cascade known to be phosphorylated at Thr, Tyr and Ser residues, western blot analysis was performed with increasing amounts of active Thr202/Tyr204-phosphorylated ERK1/MAPK [24], and active Ser217/221-phosphorylated MEK1 [25]. The upper panels of Fig. 1B show that while anti-pThr269 mAb recognizes BSA-bound c-Raf-1 phospho-Thr269 peptide, it fails to recognize phosphorylated ERK1/MAPK or MEK1. In contrast, commercially-available anti-Thr202/Tyr204 mAb or anti-Ser217/221 mAb, recognize their cognate targets but not BSA-bound c-Raf-1 phospho-Thr269 peptide (lower panels, respectively). Furthermore, pre-incubation of anti-pThr269 mAb with c-Raf-1 phospho-peptide but not non-phospho-peptide neutralized immunodetection of BSA-bound c-Raf-1 phospho-Thr269 peptide by western blot (Fig. 1C). These studies provide additional evidence for specificity of our anti-pThr269 mAb.
EGF treatment enhances phosphorylation of endogenous c-Raf-1 at Thr269 in A431 cells
Using A431 cells, which express abundant EGF receptors [26], we examined low (“physiologic”; 1ng/ml) and high “pharmacologic” dose (50ng/ml) EGF induction of c-Raf-1 phosphorylation at Thr269 using anti-pThr269 mAb. Fig. 2A shows that unstimulated cells displayed minimal c-Raf-1 Thr269 phosphorylation, increased upon treatment with 1ng/ml or 50ng/ml EGF. Complementary Raf kinase activity assays are shown in Fig. S1. Similarly, wild-type murine embryonic fibroblasts (MEFs) displayed Thr269 phosphorylation upon EGF stimulation, absent in c-Raf-1−/− MEFs (Fig. S2), confirming c-Raf-1 as a target recognized by our anti-pThr269 mAb. In contrast, A-Raf, which contains a TTAP motif at position 224 nearly identical to the TTLP motif found in c-Raf-1 (the second Thr being Thr269), is not recognized by our anti-pThr269 Ab (Fig. S3). These latter results are consistent with either A-Raf not serving as a target for phosphorylation at Thr224, or with a high level of specificity of our antibody.
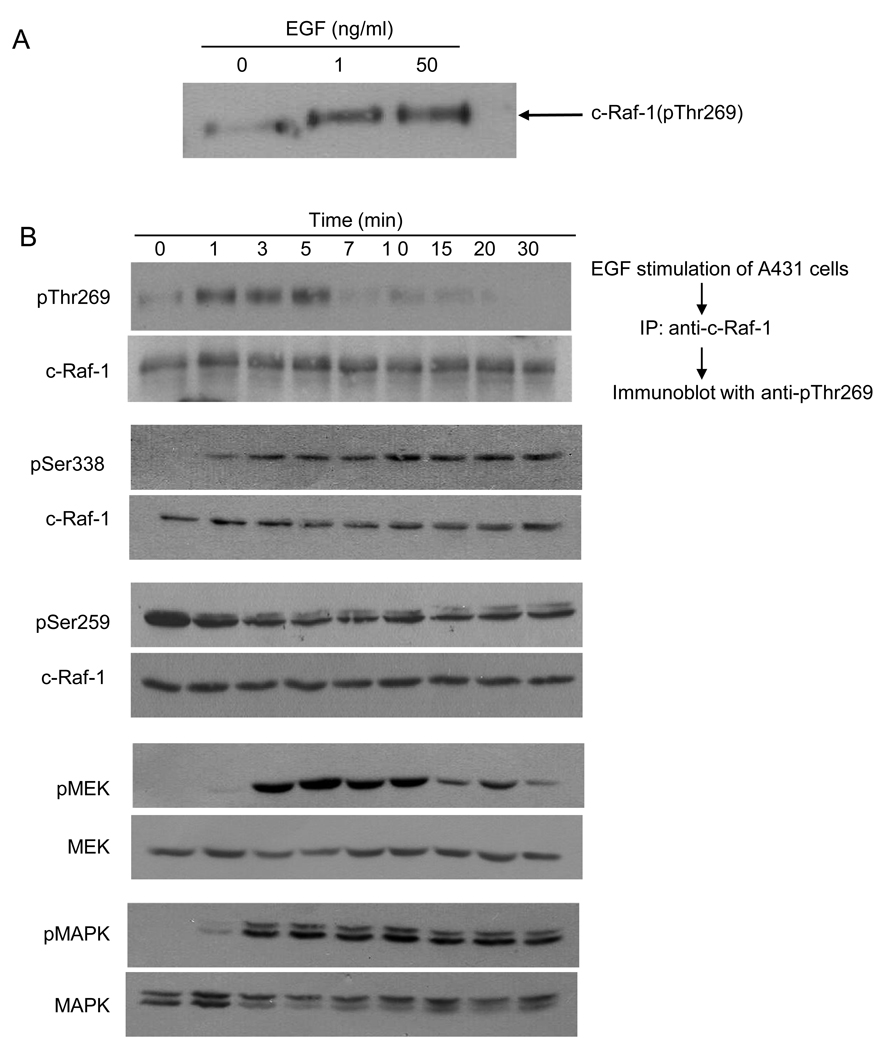
Fig. 2. EGF treatment enhances phosphorylation of endogenous c-Raf-1 at Thr269.
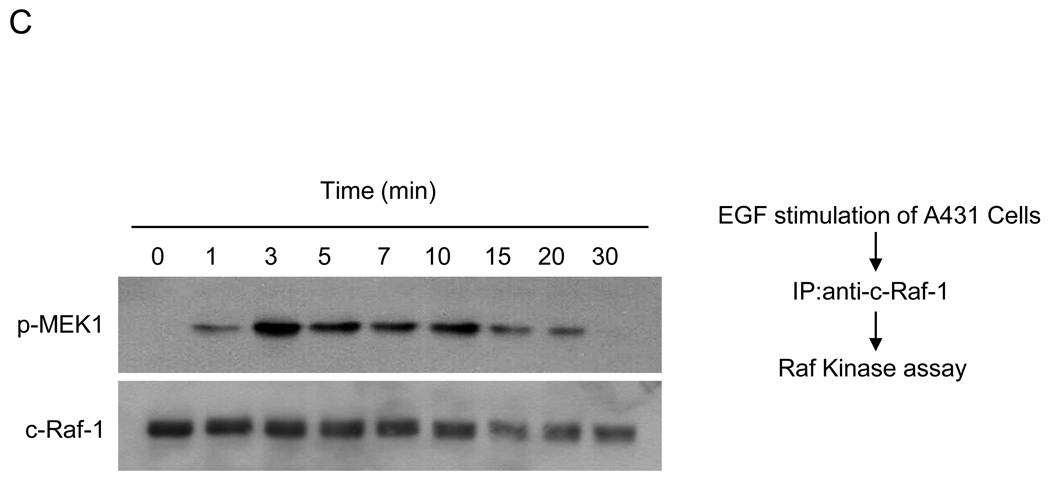
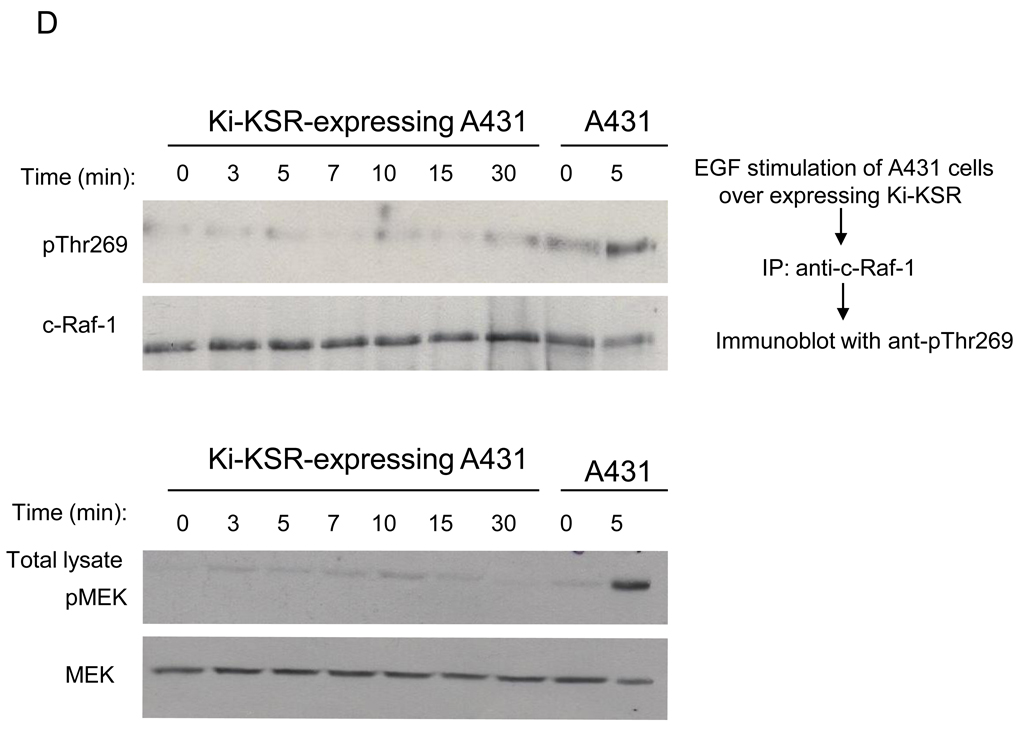
(A) Effect of low and high dose EGF treatment. 24h post serum starvation, A431 cells were treated with 1ng/ml or 50ng/ml EGF for 5min at 37°C, and endogenous c-Raf-1, immunoprecipitated from 2mg cell lysates using agarose-conjugated anti-c-Raf-1 antibody at 4°C for 16h, was resolved by 8% SDS-PAGE and phosphorylation detected by western blotting using anti-pThr269 antibody. (B) Phosphorylation of c-Raf-1 at Thr269 precedes activation of MEK1 in A431 cells. A431 cells were treated with 10ng/ml EGF for the indicated times. The phosphorylation level of endogenous c-Raf-1 (upper panel, upper lane) at Thr269 was analyzed as in (A), while Raf-1 phosphorylation at Ser338 and Ser259, and MEK1 and MAPK phosphorylation were analyzed using 50µg total cell lysate as in Supplementary Methods. Equal loading was confirmed by probing with anti-Raf-1, anti-MEK and anti-MAPK antibody. (C) 24h post serum starvation, A431 cells were treated with 10ng/ml EGF for the indicated time at 37°C, and 500µg cell lysates were subjected to immunoprecipitation using agarose-conjugated anti-c-Raf-1 antibody overnight at 4°C. Raf-1 activity assay was performed as described in Supplementary Method using MEK1 as substrate (upper panel). Loading controls are shown in the lower panel. (D) Kinase inactive KSR1 does not phosphorylate c-Raf-1. At 24h post serum starvation, A431 cells stably-expressing dominant negative kinase inactive KSR1(D683A/D700A) were treated with 10ng/ml EGF at 37°C. At the indicated times, cells were lysed and 2mg cell lysate subjected to immunoprecipitation using agarose-conjugated anti-c-Raf-1 antibody. c-Raf-1 phosphorylation (upper panel, upper lane) was measured as in Fig. 2A. Untransfected A431 cells were used as positive control for c-Raf-1 phosphorylation. Upper panel, lower lane shows c-Raf-1 loading levels. Lower panel, upper lane shows impact of stable expression of kinase inactive KSR1 in A431 cells on EGF-induced MEK phosphorylation. MEK phosphorylation was determined as in Fig. 2B. Loading controls are shown in lower lanes of each panel. Data represent 1 of 3 similar experiments for (A–D).
EGF-induced Thr269 c-Raf-1 phosphorylation in A431 cells was time dependent, maximal at 1min (Fig. 2B upper panel, upper lane), and sustained for 5min before returning to baseline. Peak c-Raf-1 Thr269 phosphorylation correlated with the onset of c-Raf-1 Ser338 phosphorylation and dephosphorylation of Ser259, events known to regulate c-Raf-1 activation [27,28]. While c-Raf-1 Thr269 phosphorylation occurred concomitant with increased c-Raf-1 kinase activity (Fig. 2C), it preceded MEK and MAPK phosphorylation (compare first, fourth and fifth sets of panels Fig. 2B). Furthermore, a kinase-inactive dominant negative KSR1 mutant abolished both EGF-induced c-Raf-1 Thr269 phosphorylation (Fig. 2D upper panel, upper lane) and EGF-induced MEK phosphorylation (Fig. 2D lower panel, upper lane). Note that empty vector had no effect on EGF-induced c-Raf-1 Thr269 phosphorylation or MEK phosphorylation (data not shown).
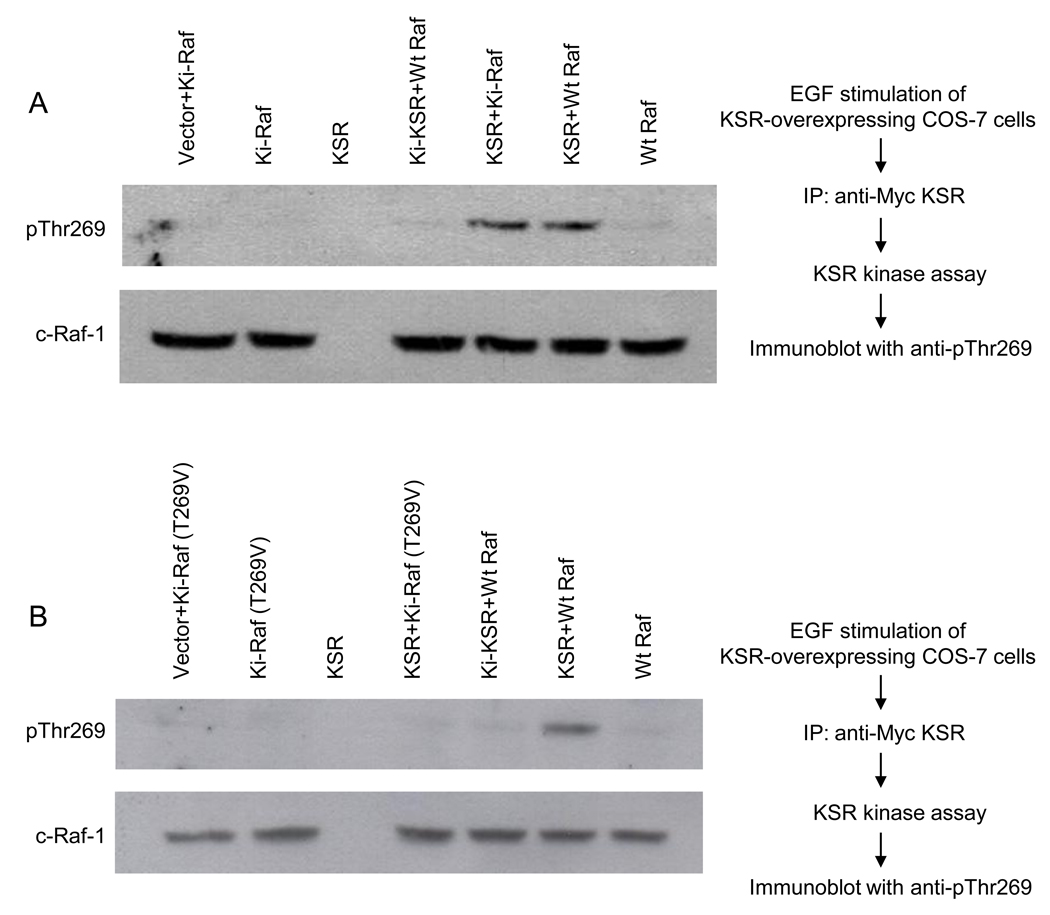
KSR1 phosphorylates purified recombinant kinase-defective FLAG-c-Raf-1(K375M) and wild-type FLAG-c-Raf-1 at Thr269 in vitro
To show that KSR1-mediated c-Raf-1 phosphorylation occurs via transphosphorylation rather than increased c-Raf-1 autophosphorylation, kinase-dead FLAG-c-Raf-1(K375M) was used as target in our KSR1 kinase assay. For these studies, FLAG-c-Raf-1 and FLAG-c-Raf-1(K375M) were purified from COS-7 cells by immunoaffinity chromatography, and immune complexes were washed 15× with NP-40 lysis buffer containing 1M NaCl. While kinase defective c-Raf-1 displayed no autophosphorylation, wild-type c-Raf-1 showed minimal autophosphorylation (Fig. 3A, upper panel). Incubation of kinase-active or kinase-defective c-Raf-1 with kinase-active KSR1 resulted in Thr269 phosphorylation, whereas kinase-inactive KSR1(D683A/D700A; Ki-KSR1) did not yield Thr269 phosphorylation. These studies indicate that KSR1 induces Thr269 phosphorylation of c-Raf-1 by transphosphorylation. Consistent with this observation substitution of Thr269 with a valine residue in kinase-defective FLAG-c-Raf-1(T269V/K375M) resulted in abrogation of KSR1-induced phosphorylation of c-Raf-1 polypeptide as detected using anti-pThr269 mAb (Fig. 3B, upper lane)
Fig. 3. EGF-stimulated KSR1 enhances phosphorylation of purified recombinant c-Raf-1 at Thr269 in vitro.
COS-7 cells, transfected with mouse Myc-KSR1, were treated with 10ng/ml EGF for 3min at 37°C. Myc-KSR1 was immunoprecipitated from 1mg cell lysate, purified to near homogeneity, and employed to phosphorylate human FLAG-c-Raf-1 as described in Supplementary Methods. (A) KSR1 phosphorylates kinase inactive Ki-c-Raf-1(K375M). Immunoprecipitated KSR1 was incubated in a reaction mixture containing 100µM ATP and either purified FLAG-c-Raf-1 or FLAG-Ki-c-Raf-1(K375M), while Ki-KSR1 reactions contained Wt c-Raf-1. After 60min incubation, phosphorylated c-Raf-1 proteins were resolved by 8% SDS-PAGE and detected by western blotting using anti-pThr269 antibody. Lower panel shows c-Raf-1 loading levels. (B) KSR1 does not phosphorylate Ki-c-Raf-1 substituted at Thr269 with valine. These studies, using Ki-c-Raf-1(T269V/K375M) as substrate, were performed as in (A). Data depict 1 of 3 experiments for (A–B).
KSR1, purified to homogeneity, retains kinase activity
As a number of MAPK signaling proteins including c-Raf-1, MEK1 and at times ERK/MAPK are bound to KSR1, even with the most extensive washing procedures it has been difficult to provide unequivocal evidence that KSR1 itself, rather than a contaminating kinase, manifests kinase activity, in addition to its scaffolding function. To further address this issue, KSR1 was immunoprecipitated from COS-7 cells overexpressing Myc-KSR1 using anti-Myc antibody, washed multiple times in high salt buffer, resolved by SDS-PAGE, and analyzed for extent of purity by matrix-assisted laser desorption/ionization reflectron time-of-flight (MALDI-reTOF) mass spectrometry (MS) (UltraFlex TOF/TOF) as described in Supplementary Methods. Specifically, immunopurified KSR1 was resolved by 8% SDS-PAGE, stained with coomassie blue (Fig. S4), the band containing purified KSR1 was excised, digested with trypsin, batch purified on a reversed-phase micro-tip, and resulting peptide pools were individually analyzed by MALDI-reTOF. All 41 peptides sequence covering 356 out of 873 amino acids (40.78 %) were derived from KSR1 (Fig. S5).
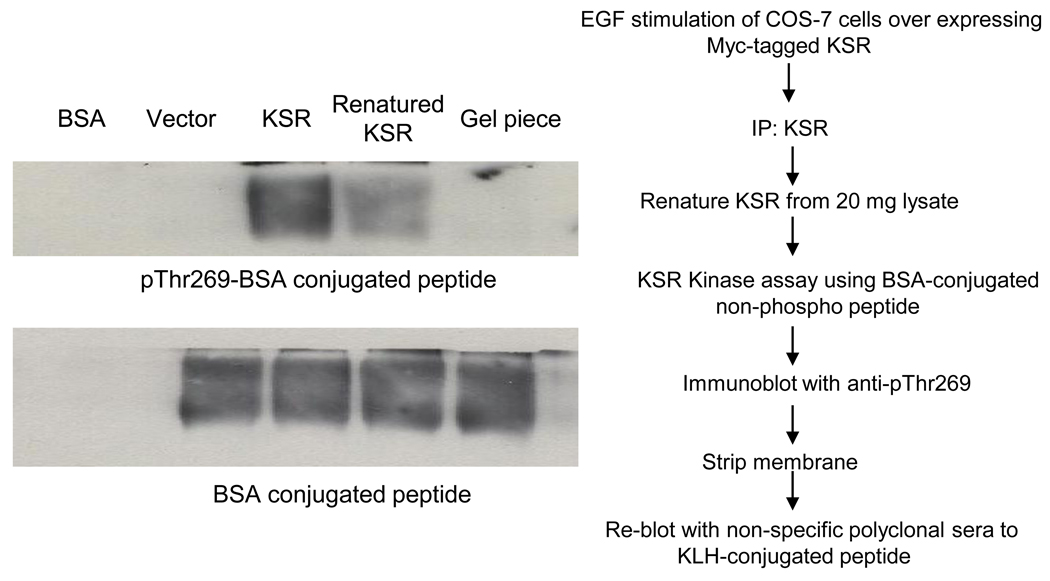
KSR1, purified to apparent homogeneity was subsequently renatured in-gel as described in “Materials and Methods”. Renaturation is a technique that allows restoration of kinase activity, albeit at a low level, and has been extensively used to identify kinase activity of proteins separated by SDS-PAGE. For these studies, the gel slice containing KSR1 purified to homogeneity and renatured, was crushed and incubated with BSA-conjugated c-Raf-1 peptide containing Thr269 in a solution containing 100µM ATP. Incubation of BSA alone or BSA-conjugated non-phospho-peptide with immunoprecipitates from cells expressing vector only did not result in anti-pThr269 mAb-detectable c-Raf-1 peptide phosphorylation by western blot (Fig. 4). In contrast, renatured FLAG-KSR1, immunopurified from 20mg of EGF (10ng)-stimulated COS-7 cell lysates (Fig. 4, lane 4), but not from lysates expressing the catalytic subunit of KSR1 (AAs 542–831 designated ΔN KSR) (Fig. S6), induced significant c-Raf-1 peptide phosphorylation. A densitometric comparison to KSR1 immunoprecipitated from 2mg EGF-stimulated COS-7 cell lysate used to directly phosphorylate c-Raf-1 (not renatured) showed that renaturing KSR1 recovered 4.1% of native KSR1 kinase activity (compare lanes 3 and 4, Fig. 4). In contrast, SDS-acrylamide gel pieces without protein do not show any phosphorylation confirming that peptide phosphorylation by renatured KSR1 was specific. These results strongly support KSR1 as a kinase that phosphorylates the Thr269 residue of c-Raf-1.
Fig. 4.
KSR1, purified to homogeneity as defined by mass spectroscopy, when renatured phosphorylates c-Raf-1 peptide. Immunopurified Myc-KSR1, purified from COS-7 cells as described in Materials and Methods, was subjected to 8% SDS-PAGE, stained using amido black, and renatured in gel as in Materials and Methods. The renatured KSR1 band was excised from the gel, crushed in an eppendorf tube using a glass rod, and used to phosphorylate BSA-conjugated c-Raf-1 peptide containing Thr269 residue. Phosphorylation was detected by western blotting using anti-pThr269 antibody, and quantified by densitometry using Imagej 1.37v (NIH, USA). Data represent 1 of 3 experiments.
While the current studies attempt to resolve a debate regarding whether KSR1 has kinase activity, they underscore a mechanistic issue as to the nature of the KSR1 catalytic activity. In this regard, KSR1 joins a recently-described small group of kinases including protein kinase C-i, the Cak-1 cyclin-dependent kinase-activating kinases and WNK (for with no lysine) that coordinate ATP in the absence of the conserved Lys in subdomain II. Despite the fact that human KSR1 has an Arg at position 637 (mouse position 589) rather than a Lys, Morrison and co-workers showed it is still capable of binding ATP analogs [22]. Further, restoration of Lys to that position had little impact on biological activity [29], nor did mutation of the canonical Lys in C. elegans KSR-1 to Arg block biological activity [8]. While these latter studies were interpreted as evidence that KSR-1 is a scaffold protein, they do not rule out the possibility that KSR orthologs coordinate ATP by a novel mechanism. At least for the mammalian KSR1 homologs, there is no obvious Lys in the ATP binding pocket that might subserve this function. Hence the question of how KSR1 coordinates ATP will most likely have to await more definitive structural studies.
In sum, in these studies we have generated a monoclonal antibody against the putative KSR1 phosphorylation site on c-Raf-1 and shown this site to be phosphorylated in intact cells upon EGF stimulation. Furthermore, this site is phosphorylated directly by highly-purified KSR1 preparations, and by in-gel renatured kinase, shown to be a purified protein by mass spectrometry. These studies provide additional support for the assertion that KSR1 has intrinsic kinase activity, in addition to scaffold function.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by NIH grants RO1 CA42385 (R.K.), and RO1 CA105125 (A. H-F.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Claperon A, Therrien M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene. 2007;26:3143–3158. doi: 10.1038/sj.onc.1210408. [DOI] [PubMed] [Google Scholar]
- 2.Lozano J, Xing R, Cai Z, et al. Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res. 2003;63:4232–4238. [PubMed] [Google Scholar]
- 3.Nguyen A, Burack WR, Stock JL, et al. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol Cell Biol. 2002;22:3035–3045. doi: 10.1128/MCB.22.9.3035-3045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez C, Jones DR, Rodriguez-Viciana P, et al. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. Embo J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacace AM, Michaud NR, Therrien M, et al. Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol. 1999;19:229–240. doi: 10.1128/mcb.19.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaud NR, Therrien M, Cacace A, et al. KSR stimulates Raf-1 activity in a kinase-independent manner. Proc Natl Acad Sci U S A. 1997;94:12792–12796. doi: 10.1073/pnas.94.24.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therrien M, Michaud NR, Rubin GM, et al. KSR modulates signal propagation within the MAPK cascade. Genes Dev. 1996;10:2684–2695. doi: 10.1101/gad.10.21.2684. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Sundaram M, Zhang Y, et al. Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol Cell Biol. 1999;19:5523–5534. doi: 10.1128/mcb.19.8.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller J, Ory S, Copeland T, et al. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell. 2001;8:983–993. doi: 10.1016/s1097-2765(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 10.Ory S, Zhou M, Conrads TP, et al. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol. 2003;13:1356–1364. doi: 10.1016/s0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- 11.Matheny SA, Chen C, Kortum RL, et al. Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature. 2004;427:256–260. doi: 10.1038/nature02237. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Fantl WJ, Harrowe G, et al. Regulation of the MAP kinase pathway by mammalian Ksr through direct interaction with MEK and ERK. Curr Biol. 1998;8:56–64. doi: 10.1016/s0960-9822(98)70020-x. [DOI] [PubMed] [Google Scholar]
- 13.Kolesnick R, Xing HR. Inflammatory bowel disease reveals the kinase activity of KSR1. J Clin Invest. 2004;114:1233–1237. doi: 10.1172/JCI23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan F, John SK, Polk DB. Kinase suppressor of Ras determines survival of intestinal epithelial cells exposed to tumor necrosis factor. Cancer Res. 2001;61:8668–8675. [PubMed] [Google Scholar]
- 15.Yan F, John SK, Wilson G, et al. Kinase suppressor of Ras protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004 doi: 10.1172/JCI21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–969. [PubMed] [Google Scholar]
- 17.Zhang Y, Yao B, Delikat S, et al. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 18.Xing HR, Lozano J, Kolesnick R. Epidermal growth factor treatment enhances the kinase activity of kinase suppressor of Ras. J Biol Chem. 2000;275:17276–17280. doi: 10.1074/jbc.C900989199. [DOI] [PubMed] [Google Scholar]
- 19.Yin X, Zafrullah M, Lee H, et al. A Ceramide-binding C1 Domain Mediates Kinase Suppressor of Ras Membrane Translocation. Cell Physiol Biochem. 2009;24:219–230. doi: 10.1159/000233248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Studzinski GP. Kinase suppressor of RAS (KSR) amplifies the differentiation signal provided by low concentrations 1,25-dihydroxyvitamin D3. J Cell Physiol. 2004;198:333–342. doi: 10.1002/jcp.10443. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Studzinski GP. Phosphorylation of raf-1 by kinase suppressor of ras is inhibited by "MEK-specific" inhibitors PD 098059 and U0126 in differentiating HL60 cells. Exp Cell Res. 2001;268:294–300. doi: 10.1006/excr.2001.5292. [DOI] [PubMed] [Google Scholar]
- 22.Muller J, Cacace AM, Lyons WE, et al. Identification of B-KSR1, a novel brain-specific isoform of KSR1 that functions in neuronal signaling. Mol Cell Biol. 2000;20:5529–5539. doi: 10.1128/mcb.20.15.5529-5539.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kameshita I, Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 24.Payne DM, Rossomando AJ, Martino P, et al. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) Embo J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alessi DR, Saito Y, Campbell DG, et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. Embo J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King IC, Sartorelli AC. The relationship between epidermal growth factor receptors and the terminal differentiation of A431 carcinoma cells. Biochem Biophys Res Commun. 1986;140:837–843. doi: 10.1016/0006-291x(86)90710-2. [DOI] [PubMed] [Google Scholar]
- 27.Diaz B, Barnard D, Filson A, et al. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17:4509–4516. doi: 10.1128/mcb.17.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubicek M, Pacher M, Abraham D, et al. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002;277:7913–7919. doi: 10.1074/jbc.M108733200. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto T, Stewart S, Han M, et al. The kinase suppressor of Ras (KSR) modulates growth factor and Ras signaling by uncoupling Elk-1 phosphorylation from MAP kinase activation. Embo J. 1998;17:1717–1727. doi: 10.1093/emboj/17.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.