Abstract
Neuronal activity in the frontal eye field (FEF) identifies locations of behaviorally important objects for guiding attention and eye movements. We recorded neural activity in the FEF of monkeys trained to manually turn a lever towards the location of a pop-out target of a visual search array without shifting gaze. We examined whether the reliability of the neural representation of the salient target location predicted the monkeys’ accuracy of reporting target location. We found that FEF neurons reliably encoded the location of the target stimulus not only on correct trials but also on error trials. The representation of target location in FEF persisted until the manual behavioral report but did not increase in magnitude. This result suggests that, in the absence of an eye movement report, FEF encodes the perceptual information necessary to perform the task but does not accumulate this sensory evidence towards a perceptual decision threshold. These results provide physiological evidence that, under certain circumstances, accurate perceptual representations do not always lead to accurate behavioral reports and that variability in processes outside of perception must be considered to account for the variability in perceptual choice behavior.
Keywords: decision, Macaca mulatta, perception, physiology, vision
Introduction
In perceptual decision tasks, experimenters rely on a behavioral report to infer a subject’s perceptual state. However, sensory-guided behaviors are the result of at least two independent selective processes, the selection of which stimulus to act upon and the selection of which action to make (Sternberg, 1969; Allport, 1987; Meyer et al., 1988; Pashler, 1991; Holroyd et al., 2005). Perceptual decision errors are generally attributed to variability in perceptual processing (Green & Swets, 1966; Ratcliff & Rouder, 1998; Gold & Shadlen, 2007). However, studies have shown that variability in choice behavior could also be a result of variability in processes unrelated to the brain’s representation of the stimulus (Norman, 1981; Neuringer, 2002). In this study we provide physiological evidence that variability in choice behavior in an easy sensorimotor decision task cannot be attributed solely to variability in the perceptual representations in the brain.
Neurophysiological studies conducted in brain regions involved in decision making [e.g. frontal eye field (FEF), lateral intraparietal cortex (LIP) and superior colliculus (SC)] indicate that developing motor commands represent the accumulation of sensory evidence to a decision threshold (Gold & Shadlen, 2000, 2003, 2007; Ratcliff et al., 2003, 2007). In visual search tasks requiring monkeys to identify the location of a target stimulus with an eye-movement, neurons in the FEF form an explicit perceptual representation of target location and exhibit a corresponding presaccadic growth of activity leading to a gaze shift toward the target (Schall & Thompson, 1999). The presence of a perceptual representation of target location and the corresponding eye movement plan suggests a direct coupling of perceptual processing to motor processing. However, because both processes are ongoing and superimposed in the FEF, it is difficult to interpret how variability in perceptual and motor processing affects variability in choice behavior. This difficulty extends to all brain regions where perceptual and motor processing occur together (e.g. LIP and SC).
A number of physiological studies have demonstrated the dissociation between perceptual and motor processing in FEF (Murthy et al., 2001; Sato & Schall, 2003; Juan et al., 2004). We recently showed in monkeys performing covert visual search tasks while maintaining fixation that visually responsive FEF neurons signal the location of a behaviorally relevant target in the search array without saccades or any physiological or behavioral evidence of saccade planning (Thompson et al., 2005b). Here we show that neurons in FEF accurately identify the correct location of the target stimulus even when the monkeys’ overt choice behavior (a manual lever turn) does not. In other words, the monkeys’ behavioral report of target location does not reliably reflect the brain’s representation of target location. This result provides physiological evidence that additional influences outside the perceptual system are necessary to account for the overt behavior that the monkey uses to report the percept. Some of these findings have been presented in abstract form (Trageser et al., 2006).
Materials and methods
Data collection
The data were collected from two monkeys (Macaca mulatta), weighing 8 kg (monkey S) and 7 kg (monkey B). The National Eye Institute Animal Care and Use Committee approved all surgical and experimental protocols and these protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sterile surgery was performed under isoflurane anesthesia (1–2%) to place a head-holding device and a scleral search coil. A craniotomy over FEF was performed and a plastic recording chamber was secured to the skull using dental acrylic and ceramic screws. Post-surgically, monkeys were monitored closely and given antibiotics and analgesics as needed. They were given 8 weeks to recover before they began behavioral training. In each recording session, a single tungsten microelectrode (Frederick Haer, Bowdoin, ME) was inserted into the monkeys’ FEF through a stainless steel guide tube supported by a plastic grid placed inside the recording chamber. Electrodes were driven by a motorized microdrive under computer control. We used a computer-based data acquisition system (Plexon, Dallas, TX, USA) to digitize and save action potential waveforms. We often simultaneously recorded two or three units on one electrode and we sorted these offline and analyzed them individually. We confirmed the location of the recording sites in the rostral bank of the arcuate sulcus histologically in monkey S following his unexpected death from an unknown cause, and by magnetic resonance imaging in monkey B. Monkey B is still alive and is participating in a different experiment.
Behavioral training and task
Visual stimulus presentation and behavioral monitoring were computer controlled (Thompson et al., 2005b). The monkeys were acclimatized to head restraint and trained in the behavioral tasks using operant conditioning with positive reinforcement. The monkeys performed a memory-guided saccade task which was used to characterize the neurons as visual, visuomovement or movement and to determine the spatial extent of the neurons’ receptive fields (RFs; Bruce & Goldberg, 1985). Following the memory-guided saccade task, the monkeys performed a covert visual search task illustrated in Fig. 1. We trained the monkeys to report whether the color singleton target of the search array was on the left or right side of the fixation spot by turning a lever in one of two directions (left or right). The monkeys were trained in the tasks 5 or 6 days a week for ~2 months before beginning neurophysiological recordings. Prior to this experiment, monkey S was experimentally naïve; he was not trained in any other task. Monkey B was involved in one other study in which he was trained to make a saccade to a luminance-defined target (Zhou & Thompson, 2008). This was the first visual search task the monkeys learned, and in their training history the monkeys were never rewarded for making a saccade to the target of a search array. The stimuli were green and red discs adjusted to be isoluminant as measured with a Minolta CA-100 spectrophotometer. The target could be either green or red, but within a block of trials the color of the target and distractors did not change. The monkeys performed the two complimentary search tasks equally well (e.g. red among green and green among red), and the behavioral and neurophysiological results were the same (Thompson et al., 2005b). Once the monkey grasped the lever and held it in the vertical position, a central cross was illuminated. After fixating the central cross for a random interval (400–800 ms), we randomly presented the target at six isoeccentric locations spaced equally around the fixation cross. Distractors occupied the remaining locations. Each of the stimuli subtended 1.5° of visual angle, and we adjusted the eccentricity of the stimuli so that at least one of the stimulus locations was inside the RF of the neuron. While waiting for the appearance of the search array the monkeys rarely moved the lever. If the lever deviated from the vertical position by 15° before the onset of the search array, the trial was aborted. We rewarded the monkeys for holding the lever in a vertical position, maintaining fixation on the central cross and then making the correct lever turn (> 15° from vertical) within 2 s after search array presentation; in practice, the monkeys nearly always turned the lever to the limit of 35° from vertical. We aborted the trial immediately and extinguished the visual stimuli if the monkey broke fixation on the central cross, released the lever or made an incorrect lever turn. A correct response was a lever turn in the same direction as the target stimulus relative to the fixation cross. We gave the reward after a correct lever turn; however, the search array remained on for an additional 250–500 ms to probe for latent saccade plans (Thompson et al., 2005b). The intertrial interval was at least 500 ms on both correct trials and error trials. Longer intertrial intervals occurred when the monkeys did not maintain gaze at the central location between trials or when the lever was not held in the vertical position between trials.
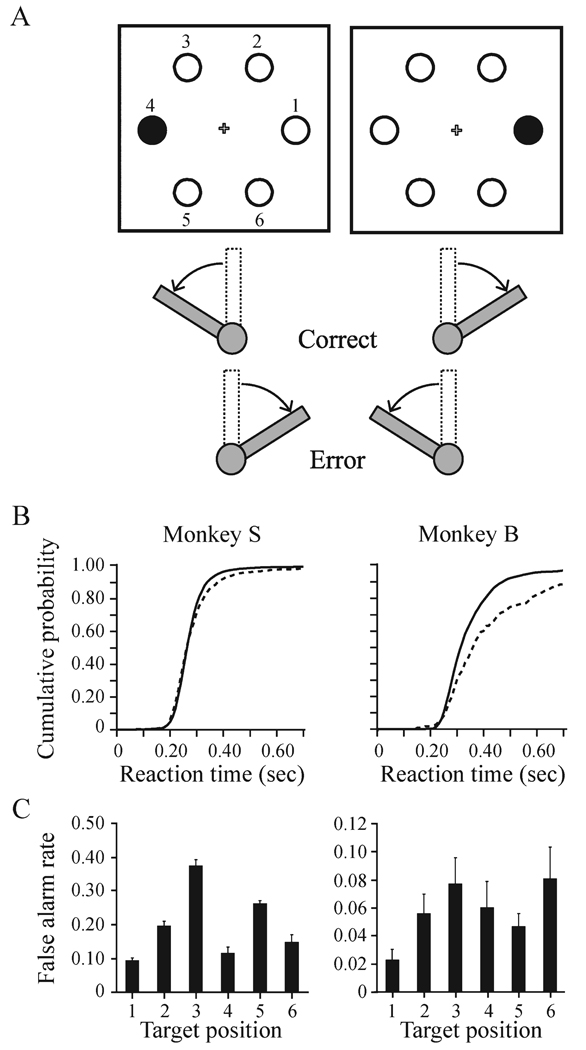
FIG. 1.
(A) Covert visual search task. After the monkey grasped the lever in the vertical position, a small fixation cross appeared. After fixating the cross for a random interval (400–800 ms), a search array appeared in which one of the stimuli was different. The monkey was rewarded for turning the lever, either left or right, in the direction of the different-colored stimulus in relation to the fixation cross. Maintained fixation was required throughout the trial. The stimuli are numbered 1–6 in the left panel as a reference for the performance at each location plotted in C. (B) Cumulative distributions of reaction times for correct (thick line) and error (thin line) trials for monkey S (left) and monkey B (right). Monkeys displayed longer reaction times on error trials. (C) The proportion of lever-turn errors committed by monkey S (left) and monkey B (right) at each target location labeled in A. Results are expressed as mean ± SEM across recording sessions.
Data analysis
Lever position and eye position were sampled at 1 kHz. Saccades were detected by using a computer algorithm that searched for elevated eye velocity (> 20° / s). Saccade initiations and terminations then were defined as the beginnings and ends of the monotonic changes in eye position that lasted for at least 10 ms. A lever turn was defined as a turn > 15° from vertical. The beginning and end of each lever turn were defined as the beginning and end of the monotonic change in lever position before and after the 15° threshold was reached. The time of the beginning of the lever turn on each trial was used as the reaction time for that trial.
Spike times were converted to continuous spike density functions with a kernel that projects activity forward in time and approximates an EPSP (Thompson et al., 1996). We identified the neurons that represented the location of the singleton target of the search array by generating area under the receiver operating characteristic (ROC) curves at 1-ms intervals from the spike density functions on single trials aligned on search array presentation and aligned on the lever turn initiation. This method has been described previously in detail (Thompson et al., 1996, 2005a). For each neuron, ROC areas were used to compare the distribution of activity on trials in which the target was presented in the RF to the distribution of activity on trials in which the target was in the hemifield opposite the RF and only distractors were presented in the RF. By convention, ROC area values > 0.50 indicate that the activation on the trials in which the target was in the RF was greater than on trials in which the distractor was in the RF and corresponds to the probability that an ideal observer would correctly distinguish target- from distractor-related activity. We included only those neurons whose ROC area values reached 0.70 before the median lever turn reaction time of the recording session.
Results
We obtained neuronal recordings from the FEF of two monkeys trained to perform a two-choice pop-out visual search task. The monkeys identified the location of a salient oddball target in the presence of distractors and indicated their choice overtly by turning a lever either to the left or to the right in the direction of the target stimulus relative to the fixation point. The stimulus randomly appeared at one of six possible locations in the search array (Fig. 1A). For a trial to count as valid, the monkey was required to grasp the lever and maintain it at a center position, fixate the central spot (400–800 ms), wait for the search array to appear and, while maintaining fixation on the central spot, turn the lever to report the target location. We required the monkeys to perform this sequence of behaviors to keep them engaged in the task during the completion of a valid trial. We discarded invalid trials in which the monkeys broke fixation or released the lever before the lever turn. We analyzed only valid correct and error trials. The monkeys’ overall performance accuracy (PA) on valid trials was 82.2% correct for monkey S and 93.8% correct for monkey B.
The monkeys’ less-than-perfect performance in this task was not due to a speed–accuracy tradeoff. The reaction times on error trials were longer than on correct trials (t-test: monkey S, P < 0.001; monkey B, P < 0.001). Figure 1B shows the cumulative distributions of reaction times on correct and error trials for the two monkeys. The overall reaction times measured from the presentation of the search array to the beginning of the lever turn averaged 281.3 ± 80.3 ms (mean ± SD; median = 267.6 ms) for monkey S (correct trials: 278.8 ± 70.4 ms, median = 268.0 ms; error trials: 292.4 ± 113.1 ms, median = 265.8 ms) and 356.4 ± 151.7 ms (median = 318.7 ms) for monkey B (correct trials: 350.0 ± 122.8 ms, median = 317.2 ms; error trials: 473.2 ± 393.9 ms, median = 364.4 ms).
Lack of motivation also cannot explain the errors because the monkeys were required to perform a specific sequence of behaviors requiring a degree of coordination and concentration. An alternative explanation for the monkeys’ behavioral performance is that they had not fully learned the association between the target positions and the correct lever-turn behavior. To test this we calculated the average percentage of errors committed as a function of the target position across all of the recording sessions. Both monkeys performed above chance levels at all target positions. In addition, both monkeys committed errors at all positions, indicating that there was no general tendency for a particular position to induce errors to the exclusion of other positions (Fig. 1C).
Monkey S did make significantly more errors (one-way anova F83 = 6.98, P < 0.001) when the target was located in the upper-left (post hoc Tukey test: 0.37 ± 0.13) and lower-left (Tukey test: 0.26 ± 0.15) positions. The performance accuracy at these locations was significantly greater than the chance level of 50% (mean percentage correct: upper-left, 62.5%; t-test, t13 = 3.606, P = 0.003; lower-left, 73.8%; t13 = 6.096, P < 0.001). This indicates that the monkey understood the rules of the task for these locations. Furthermore, the monkey’s ability to accurately associate the target stimulus with the correct lever-turn at all the other target positions (both left and right) strongly suggests that the monkey understood the task. The performance of monkey B did not vary significantly as a function of target position (one-way anova, F71 = 1.77, P = 0.131).
In a previous report we analyzed gaze behavior on correct trials and showed that there was no evidence of latent saccade planning during the covert visual search task (Thompson et al., 2005b). We performed the same analysis of gaze behavior on error trials to test the possibility that there was latent saccade planning on error trials. After an incorrect lever turn, the monkeys nearly always maintained fixation at the center of the screen, awaiting the beginning of the next trial that started 500 ms later. By 500 ms after the removal of all visual stimuli following the incorrect lever turn, monkey S continued to maintain fixation at the central position after 90% of trials and monkey B after 75% of trials. Furthermore, when either monkey made a saccade it did not tend to land near the target of the search array. Post-trial saccades landed within 7° of the target after 2.0% of trials for monkey S and after 2.6% of trials for monkey B. Thus, in agreement with our previous analysis of saccade activity on correct trials (Thompson et al., 2005b), we found no behavioral evidence of saccade planning on error trials.
In the following section, we compare the neuronal activity when the target stimulus landed in the FEF neurons’ RF to the activity when distractor stimuli landed in the RF during the visual search task and relate this difference in activity to the monkeys’ behavioral choice of target location. For monkey S, recordings were made in the left FEF and the neuronal RFs were in the right (contralateral) visual hemifield. It is important to keep this in mind when considering the neural data presented for monkey S; the target positions associated with an increased percentage of errors in monkey S were located in the left (ipsilateral) visual hemifield, and are not included in the neural activity analysis.
FEF neurons reliably signaled the target location regardless of choice behavior
We recorded the activity of 43 FEF neurons (26 from monkey S and 17 from monkey B) that formed an explicit representation of target location before the lever turn on correct trials regardless of whether the target was red or green. All neurons in this study displayed either purely visual- or a combination of visual- and movement-related activity in a memory-guided saccade task; neurons with purely movement-related activity are not active in the covert visual search task (Thompson et al., 2005b), and therefore are not included in this study. The data on correctly performed trials recorded from monkey S were described in a previous report (Thompson et al., 2005b). Figure 2 depicts the averaged activity of single FEF neurons recorded from monkey S (Fig. 2, top left) and monkey B (Fig. 2, top right) on trials in which the monkey reported correctly the location of the target during a typical recording session. Initially, these neurons responded similarly to the appearance of either the target (thick line) or the distractor (thin line) in their RF. Later in the trial, the activity when the target was in their RF became elevated relative to when a distractor was in their RF. All descriptions of distractor-related activity refer to stimulus arrangements in which only distractors were in the RF and the target was outside the RF and at one of the locations in the opposite hemifield.
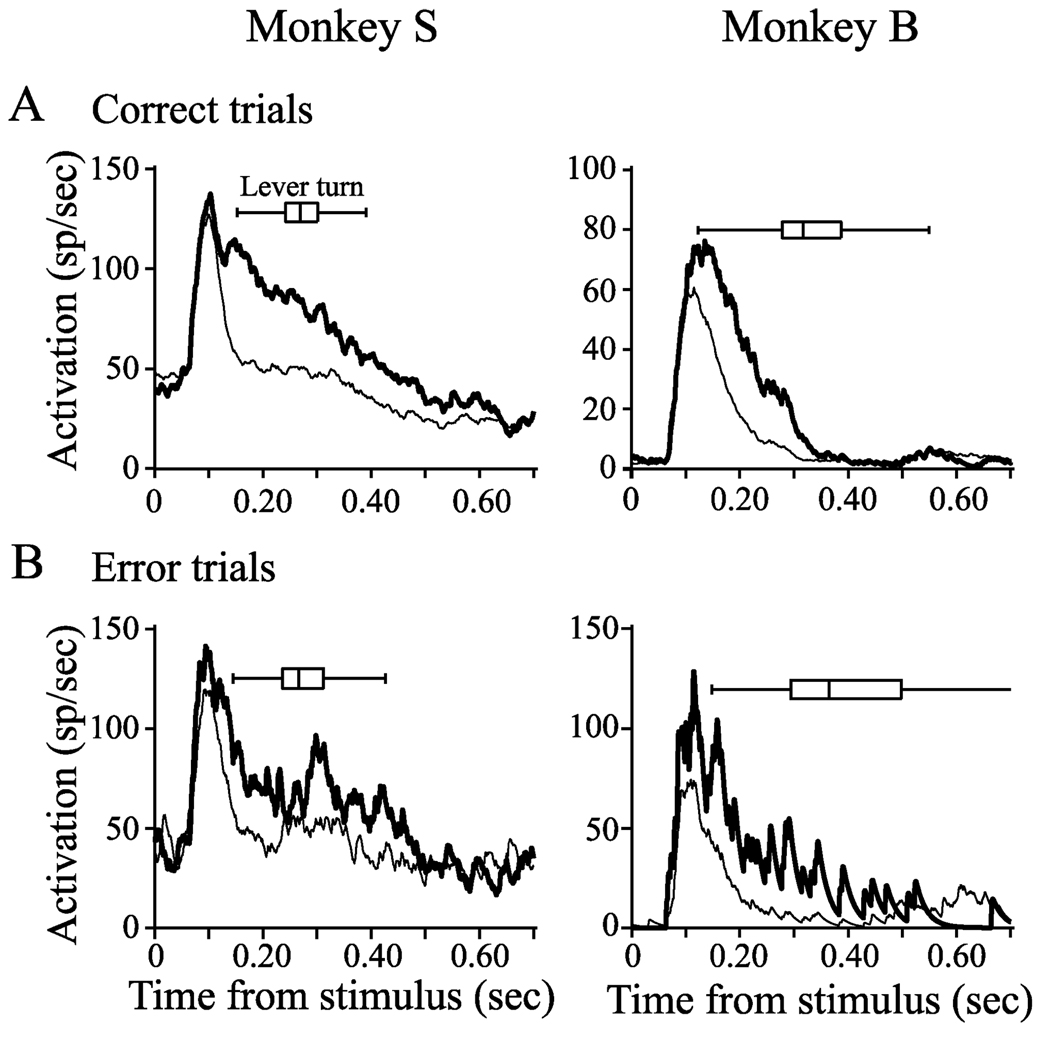
FIG. 2.
Representative examples of individual visually responsive, spatially selective FEF neurons recorded from (A) monkey S and (B) monkey B during the covert visual search task on correct trials (top row) when the monkey turned the lever toward the target and on error trials (bottom row) when the monkey turned the lever away from the target. Thick lines represent activity on trials in which the target was presented in the RF and thin lines represent activity on trials in which a distractor was presented in the RF. Examples represent the averaged activity during a single recording session. The greater variability on error trials compared to correct trials is due to fewer trials for averaging. Box plots indicate the median, quartiles and range of lever-turn reaction times.
Although pop-out visual search tasks are perceptually easy (Nothdurft, 2002), the monkeys made lever turn errors on a fraction of trials (Fig. 1C). Figure 2 (bottom row) plots the activity recorded on error trials of the same neurons described above. The neurons accurately signaled the presence of the target in their RF, just as they did on correct trials. As a population, FEF neurons from both monkeys exhibited significant target selection on both correct and error trials (Fig. 3). In Fig. 3 the activity is plotted aligned on search array presentation (Fig. 3A) and on the initiation of the lever turn (Fig. 3B). It is noteworthy that the activity on trials in which the target was in the RF decreased before the lever turn. This differs significantly from previous reports of FEF activity using reaction-time tasks with eye movements. When saccades are used as the behavioral report there is a pre-saccadic growth of activity that has been associated with the accumulation of sensory evidence (Gold & Shadlen, 2007) and saccade preparation (Hanes & Schall, 1996; Hanes et al., 1998; Schall & Thompson, 1999). The lack of pre-motor buildup of activity in this task, which does not involve eye movements, suggests that the spatially selective response represents the sensory evidence of target location but not the accumulation of evidence towards a perceptual decision or motor execution threshold.
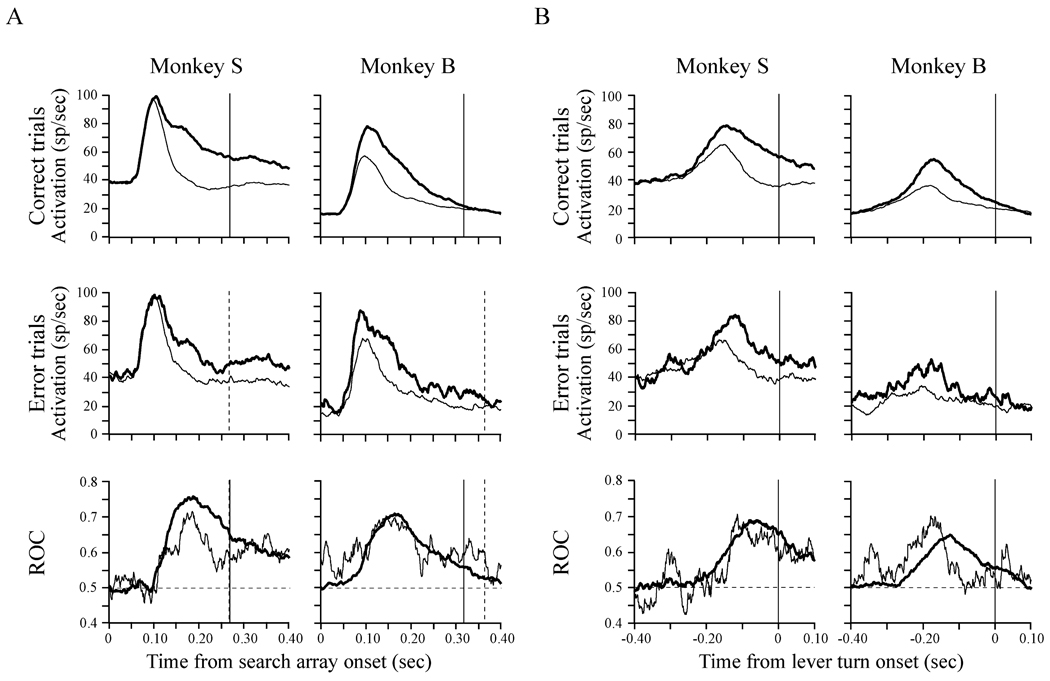
FIG. 3.
Population average activity and average ROC area values as a function of time on correct and error trials for monkey S and monkey B aligned on (A) stimulus array onset and (B) the initiation of the lever turn. In the average activity plots, thick lines represent average activity on trials in which the target was presented in the RF and thin lines represent average activity on trials in which a distractor was presented in the RF. The ROC areas plotted as a function of time (bottom) quantify the magnitude of the spatial selection on correct trials (thick lines) and error trials (thin lines). Vertical lines in the stimulus-aligned plots (A) indicate median reaction time on correct trials (solid line) and error trials (dotted line).
To quantify the magnitude of the target selection we employed an ROC analysis, a nonparametric method of calculating the difference between target- and distractor-related activity normalized to values between 0 and 1 (see Materials and methods). A value of 0.50 indicates that the activity distributions representing the target and the distractors are the same. A value of 1.0 indicates that the two distributions are completely nonoverlapping, that the neuron selected the target with 100% accuracy. The goal of this study is to compare the magnitude of target selection on correct trials to that on error trials. For a neuron to be included in the analysis it had to exhibit a spatially selective response on correct trials, which we defined as reaching an ROC area of 0.70 or greater before the median reaction time of the recording session.
The bottom row of Fig. 3 shows the average ROC areas from correct (thick lines) and error (thin lines) trials of all neurons recorded in the two monkeys, plotted as a function of time. Because reaction times are variable across trials, the neural activity was aligned on search array onset (Fig. 3A) and on lever turn onset (Fig. 3B). Initially the ROC area hovered around 0.5, indicating that the sensory evidence embedded in the FEF signal was ambiguous. However, over time the ROC area identified the pop-out target on correct and error trials and maintained a value > 0.5 until the lever turn report of target location. The average ROC area on both correct and error trials in both monkeys peaked between 150 and 200 ms following the presentation of the search array and then decreased before the lever turn (illustrated by the search array aligned data). Importantly, the lever turn-aligned ROC plots clearly show that there was no pre-motor increase in the magnitude of selectivity.
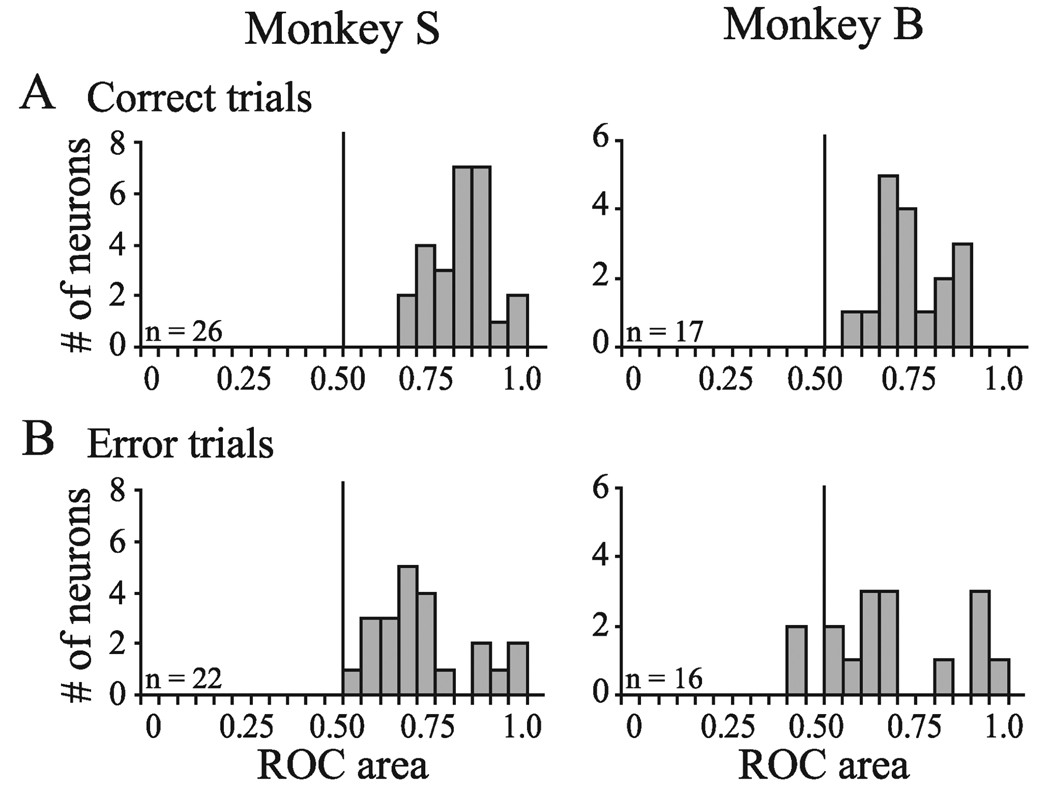
Figure 4 shows the distributions of the average ROC areas measured between 125 and 225 ms following the onset of the search array for all neurons on correct and error trials. This time interval was chosen because it captured the spatially selective activity of FEF neurons before the initiation of the lever turn. We included only those neurons for which we were able to obtain at least five trials for each trial condition. For both monkeys, all of the neurons had ROC areas > 0.50 on correct trials (monkey S: mean = 0.82; t-test, t25 = 20.3, P < 0.001; monkey B: mean = 0.74; t16 = 11.2, P < 0.001) and all but two neurons in monkey B had ROC areas > 0.50 on error trials (monkey S: mean = 0.72; t-test, t21 = 7.4, P < 0.001; monkey B: mean = 0.68; t15 = 4.1, P < 0.001). There was no evidence for an activity bias prior to the presentation of the search array. Baseline ROC areas before the visual response, measured as the average ROC area for the 30 ms after the search array presentation, did not differ from 0.50 for either correct (monkey S: mean = 0.49; t-test, t25 = −0.777, P = 0.444; monkey B: mean = 0.51; t16 = 0.759, P = 0.459) or error (monkey S: mean = 0.51; t-test, t21 = 0.307, P = 0.762; monkey B: mean = 0.58; t15 = 1.52, P = 0.148; data not shown) trials.
FIG. 4.
Average ROC areas measured between 125 and 225 ms following the search array for the FEF neurons recorded in monkey S and monkey B on (A) correct and (B) error trials. All ROC area values are > 0.50 on correct trials, and all but two ROC area values are > 0.50 on error trials indicating that, overall, the FEF neurons were selective for the target location despite the monkeys’ behavioral report.
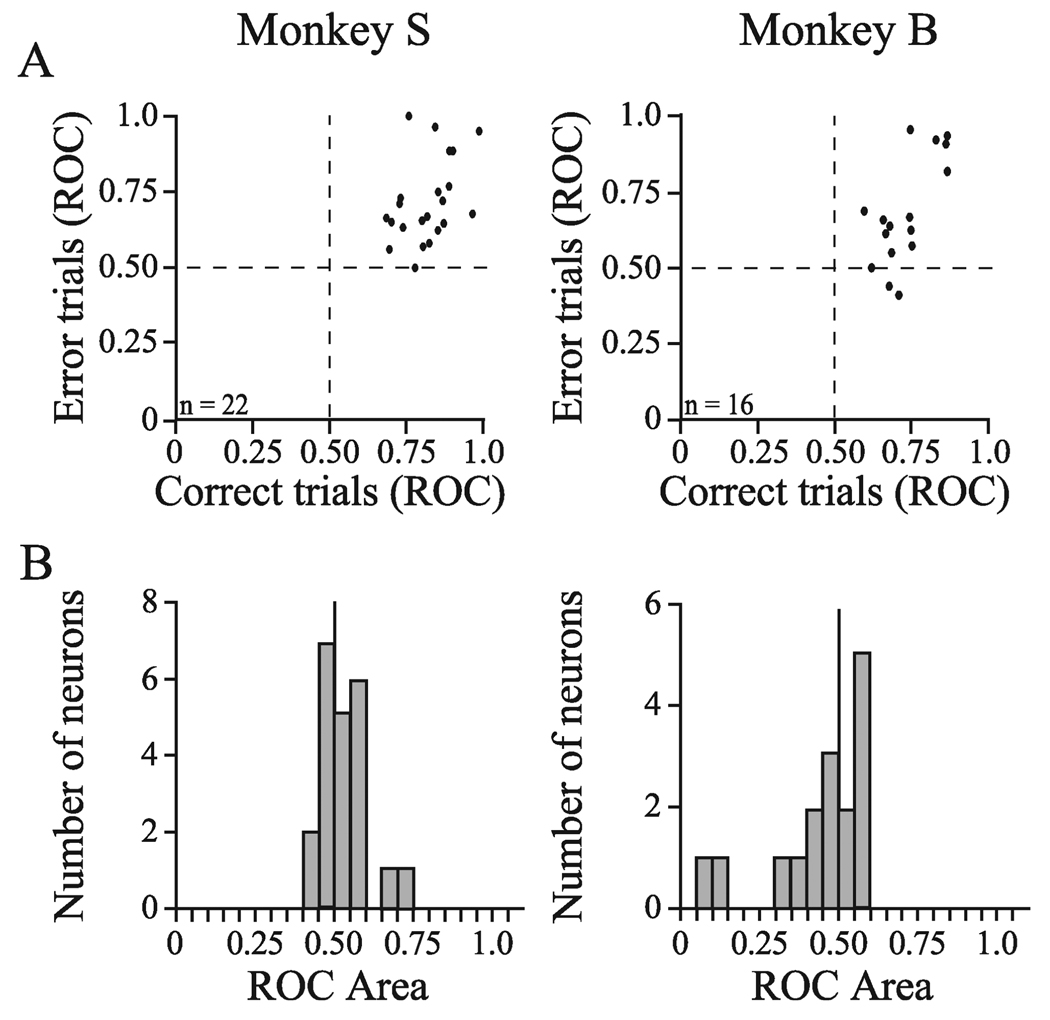
The magnitudes of selectivity of individual neurons on correct and error trials are compared in Fig. 5A. The average ROC areas measured from the activity on correct trials was slightly higher than the average ROC areas measured from the activity on error trials for both monkeys. For monkey S this difference was significant (paired t-test, t42 = 2.86, P = 0.006, Fig. 4A, top-left panel), but for monkey B the difference did not reach statistical significance (paired t-test, t30 = 1.04 P = 0.305, Fig. 4A, bottom-left panel). Even though the ROC values were slightly higher on correct trials, the most important result is that the ROC values from both correct and error trials were considerably higher than 0.50. This result demonstrates that FEF neurons reliably encode target location on correct and error trials and that one cannot always infer from the overt behavioral choice the presence or absence of an accurate perceptual representation in the brain.
FIG. 5.
(A) Scatterplots comparing average ROC area values obtained from individual neurons on correct and error trials between 125 and 225 ms following the search array for monkey S (left) and monkey B (right). The majority of values are in the upper-right sectors, indicating selectivity for the target on both correct and error trials. (B) Distributions of average ROC area values that compare distractor activity between 125 and 225 ms following the search array on correct and error trials for monkey S (left) and monkey B (right). ROC area values > 0.50 indicate greater distractor-related activity on error trials whereas ROC area values < 0.50 indicate greater distractor-related activity on correct trials. The distributions of ROC area values did not differ significantly from 0.50 for either monkey.
We examined whether the monkeys’ incorrect behavioral reports could have been attributed to an enhanced response for one of the distractors in the hemifield opposite the target. If this were the case, then distractor-related activity would be higher on error trials than on correct trials. To test this we compared the distractor-related activity on error trials to that on correct trials by calculating the average ROC areas between 125 and 225 ms following the onset of the search array (Fig. 5B). In this calculation, ROC areas > 0.5 indicated higher activation on error trials than on correct trials. The ROC values did not differ from 0.50 during the selective period for either monkey (Fig. 5B: monkey S, mean = 0.53, t-test, t21 = 1.739, P = 0.10; monkey B, mean = 0.44, t15 = −1.449, P = 0.17). Therefore, we conclude that FEF neurons responded similarly to the presence of the distractor in the RF on correct and error trials, indicating that the monkeys’ decision to turn the lever in a particular direction was not influenced by distractor-related activity.
Discussion
The main goal of this study was to determine whether variability in perceptual representations could fully account for variability in choice behavior in an easy perceptual task. The main finding is that well trained monkeys made errors in reporting target location when there was an accurate neural representation of target location in the FEF. This result is opposite to the results when monkeys made saccades to the target of the search array. When monkeys make saccades during visual search, the activity of FEF neurons identifies the location of the saccade goal even when the saccade is directed to a distractor stimulus on error trials (Thompson et al., 2005a). However, psychophysical studies have shown that subjects effortlessly identify the target in a pop-out search task such as the one used in this study (Nothdurft, 2002). Therefore, it could be inferred that somewhere in the brain an accurate neural representation of target location exists. Although this is often assumed it has never been experimentally confirmed. Our results provide direct evidence that the fidelity of the perceptual evidence is not always the limiting factor in initiating a behavioral report denoting the outcome of a perceptual decision. In contrast, our results support the view that under certain circumstances perceptual and motor processes can run somewhat independently, and variability introduced into the decision process subsequent to the formation of the perceptual representation of target location affects the behavior used to report the perceptual decision (Murthy et al., 2001; Holroyd et al., 2005; Camalier et al., 2007; Madelain et al., 2007).
A perceptual representation in FEF in the absence of eye movements
The FEF is one of the brain structures involved in the sensorimotor decision of identifying the visual target for a gaze shift and planning the saccade to that target (Schall & Thompson, 1999). Previous studies of FEF that required monkeys to make a saccade to a visual target describe a growth of activity in saccade-related neurons that precedes the eye movement and resembles an accumulation of evidence to a threshold (Hanes & Schall, 1996; Schall & Thompson, 1999; Camalier et al., 2007; Murthy et al., 2007). In tasks requiring an eye movement to indicate choice, both sensory and motor processes are ongoing within the FEF and the variability in FEF activity probably reflects the variability in both the perceptual and motor aspects of the task. In monkeys performing visual search tasks using eye movements as the behavioral choice, neurons in FEF and the superior colliculus exhibit a growth of activity preceding the saccade on both correct and error trials that predicts the behavioral choice, not the location of the singleton target (Thompson et al., 2005a; Kim & Basso, 2008). Here we show that, in the absence of saccades, FEF neurons exhibit accurate spatial selectivity for the target even when the monkey incorrectly reports the target’s location. The difference between the previous studies and this report is the monkeys’ behavioral report of target location.
We propose that the covert visual search task used in the present study isolated neuronal activity related to the perceptual stage of the decision process from the motor stage that generated the overt choice. The manual movements that turned the lever in this task are not represented in the FEF activity (Thompson et al., 2005b), and there was no behavioral or physiological evidence for saccade planning on correct or error trials. Here we show that there was no pre-motor buildup of activity nor was there an increase in spatial selectivity preceding the behavioral report in the FEF neurons. Therefore, we suggest that the FEF is not involved in the accumulation of sensory evidence towards motor initiation threshold in this task, a process that probably occurs in brain regions responsible for or closely linked to the generation of the hand and arm movements required to turn the lever (Romo et al., 2004; Cisek & Kalaska, 2005; Gold & Shadlen, 2007). Although the activity observed in the FEF is not consistent with an accumulation process towards a threshold, it is consistent with FEF’s role in generating a salience map identifying the location of behaviorally relevant targets (Thompson & Bichot, 2005) and as a part of a frontoparietal spatial attention network (Corbetta & Shulman, 2002; Kincade et al., 2005).
It could be argued that the representation of target location in FEF is not used by the motor systems responsible for generating the manual lever turn. By extension, it is conceivable that on error trials the activity in other brain regions is more variable, thus leading to the erroneous lever turns. Importantly, although a more variable representation may exist in the brain, our data demonstrate that the brain does possess an accurate representation of target location in the FEF. This indicates that the perceptual system itself is not the primary source of this variability. In addition, FEF is directly connected with the postarcuate premotor cortex, suggesting that the relevant motor systems do have access to its information (Stanton et al., 1993). An unanswered empirical question is whether there are separate perceptual representations of target location in the brain for the same easily identified pop-out target. We believe this is unlikely. However, the possibility of multiple representations of target location raises the interesting question of how the brain chooses the appropriate representation to accurately guide behavior.
It is noteworthy that the target selectivity in the FEF on error trials tended to be less than that on correct trials. We hypothesize that post-perceptual noise would have a greater influence on trials with weaker perceptual signals and make motor errors more likely. The decreased selectivity on error trials is consistent with this hypothesis. Therefore, we speculate that the most likely explanation for the incorrect behavior in this task is variability in processes related to motor response generation.
Relationship to previous studies
Many studies have investigated the relationship between the formation of a perceptual representation and its influence on choice behavior (for a review see Gold & Shadlen, 2007). Determining the causes of choice variability is often complicated by the fact that many brain regions display activity related to both the perceptual stage and the motor stage of the decision process. Additionally, specific aspects of experimental design can modulate the evolving perceptual representation, for example movement planning, attentional shifts, the arrangement of stimuli in the search array or a perturbation of the target itself (Bisley & Goldberg, 2003; Balan & Gottlieb, 2006; Oristaglio et al., 2006). These factors can often make it difficult to attribute choice variability to a particular processing stage. However, it is important to recognize that these modulations of the perceptual representation do not reflect a failure of the perceptual system to accurately encode information about the target stimulus. Rather, these modulatory influences reflect the impact of other systems on the overall formation of the decision. Our task design sufficiently reduces these modulatory influences, allowing for a clearer interpretation of the source of choice variability.
Numerous studies have identified a post-perceptual stage of response preparation, independent of perceptual processing, that contributes to the delay and variability in reaction time (Thompson et al., 1996; Hooge & Erkelens, 1998; Sato et al., 2001; Madelain et al., 2007). A study by Murthy et al. (2001) demonstrated that spatial selection in FEF does not obligate a corresponding movement. In that study monkeys were rewarded for making a saccade to a pop-out target in a visual search array that on infrequent random trials changed location before the saccade was made. In some of these trials the monkeys made a saccade to the initial target location even though the activity of FEF neurons accurately identified the new target location. This result demonstrates that an explicit perceptual representation of target location in FEF does not oblige a corresponding movement. The difference between our results and the study by Murthy et al. (2001) is that the sudden target change generated a competition between two different movement plans which led to incorrect behavioral responses (Camalier et al., 2007; Murthy et al., 2007). In contrast, there was not a task-generated motor response conflict in the covert visual search task. The monkeys made errors in reporting target location while performing a perceptually easy, well learned stimulus–response association even though there was an accurate perceptual representation in the brain.
A recent study by Messinger et al. (2005) showed that neurons in the inferior temporal cortex could identify learned stimulus pairs even when the monkey did not. The results of our study are similar, but they were produced under very different experimental conditions. In Messinger et al. (2005), the monkeys learned a new stimulus–response pairing each day and the monkeys performed at near chance levels even at the end of the experimental sessions. Therefore, the errors could be related to the monkeys’ partial learning of the appropriate stimulus–response association. In our study, however, the monkeys performed the same stimulus–response pairings for several weeks and exhibited stable behavior. One monkey performed the task at 82% correct and the other at 94% correct. Therefore, we believe that the monkeys knew the sensorimotor association in the task and this was not the reason for the errors in this study. Our study, therefore, strengthens and extends the argument made by Messinger et al. (2005) that behavioral reports of perceptual decisions are influenced by post-perceptual processes, and that these post-perceptual influences occur in perceptually easy well-learned tasks.
Even in well-learned tasks, performance accuracy often varies widely across individuals such as the two monkeys in this study (see also Cisek & Kalaska, 2005; Oristaglio et al., 2006). Under the right conditions, the performance on perceptually easy trials can approach near perfect levels (e.g. Roitman & Shadlen, 2002). Therefore, variability in response selection may be controlled by individuals or may be manipulated by task demands (Madelain et al., 2007). Despite their less-than-perfect performance, the accuracy of the monkeys in this study performing the lever search task is in line with other easy color search tasks using eye movements (Bichot & Schall, 2002; Thompson et al., 2005a; Shen & Pare, 2006; Kim & Basso, 2008) and manual reaches (Bolster & Pribram, 1993; Cisek & Kalaska, 2005). Variability in response selection in spite of an easily discriminated stimulus may be adaptive, producing exploratory behaviors and promote the learning of arbitrary stimulus–response associations (Neuringer, 2002).
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, National Eye Institute. We thank E. Yomtovian for help with the experiments and J. D. Schall, P. Pouget, G. Woodman and Asaf Keller for helpful discussions and valuable comments.
Abbreviations
- FEF
frontal eye field
- LIP
lateral intraparietal cortex
- PA
performance accuracy
- RF
receptive field
- ROC
receiver operating characteristic
- SC
superior colliculus.
References
- Allport A. Selection for action: some behavioral and neurophysiological considerations of attention and action. In: Heuer H, Sanders AF, editors. Perspectives on Perception and Action. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. pp. 395–419. [Google Scholar]
- Balan PF, Gottlieb J. Integration of exogenous input into a dynamic salience map revealed by perturbing attention. J. Neurosci. 2006;26:9239–9249. doi: 10.1523/JNEUROSCI.1898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J. Neurosci. 2002;22:4675–4685. doi: 10.1523/JNEUROSCI.22-11-04675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bolster RB, Pribram KH. Cortical involvement in visual scan in the monkey. Percept. Psychophys. 1993;53:505–518. doi: 10.3758/bf03205199. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields: I. Single neurons discharging before saccades. J. Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Camalier CR, Gotler A, Murthy A, Thompson KG, Logan GD, Palmeri TJ, Schall JD. Dynamics of saccade target selection: race model analysis of double step and search step saccade production in human and macaque. Vision Res. 2007;47:2187–2211. doi: 10.1016/j.visres.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature. 2000;404:390–394. doi: 10.1038/35006062. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J. Neurosci. 2003;23:632–651. doi: 10.1523/JNEUROSCI.23-02-00632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu. Rev. Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York, NY: Wiley; 1966. [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J. Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, Coles MG, Cohen JD. A mechanism for error detection in speeded response time tasks. J. Exp. Psychol. Gen. 2005;134:163–191. doi: 10.1037/0096-3445.134.2.163. [DOI] [PubMed] [Google Scholar]
- Hooge IT, Erkelens CJ. Adjustment of fixation duration in visual search. Vision Res. 1998;38:1295–1302. doi: 10.1016/s0042-6989(97)00287-3. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc. Natl Acad. Sci. U.S.A. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J. Neurosci. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J. Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelain L, Champrenaut L, Chauvin A. Control of sensorimotor variability by consequences. J. Neurophysiol. 2007;98:2255–2265. doi: 10.1152/jn.01286.2006. [DOI] [PubMed] [Google Scholar]
- Messinger A, Squire LR, Zola SM, Albright TD. Neural correlates of knowledge: stable representation of stimulus associations across variations in behavioral performance. Neuron. 2005;48:359–371. doi: 10.1016/j.neuron.2005.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Osman AM, Irwin DE, Yantis S. Modern mental chronometry. Biol. Psychol. 1988;26:3–67. doi: 10.1016/0301-0511(88)90013-0. [DOI] [PubMed] [Google Scholar]
- Murthy A, Thompson KG, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. J. Neurophysiol. 2001;86:2634–2637. doi: 10.1152/jn.2001.86.5.2634. [DOI] [PubMed] [Google Scholar]
- Murthy A, Ray S, Shorter SM, Priddy EG, Schall JD, Thompson KG. Frontal eye field contributions to rapid corrective saccades. J. Neurophysiol. 2007;97:1457–1469. doi: 10.1152/jn.00433.2006. [DOI] [PubMed] [Google Scholar]
- Neuringer A. Operant variability: evidence, functions, and theory. Psychon. Bull. Rev. 2002;9:672–705. doi: 10.3758/bf03196324. [DOI] [PubMed] [Google Scholar]
- Norman DA. Categorization of action slips. Psych. Rev. 1981;88:1–15. [Google Scholar]
- Nothdurft HC. Attention shifts to salient targets. Vision Res. 2002;42:1287–1306. doi: 10.1016/s0042-6989(02)00016-0. [DOI] [PubMed] [Google Scholar]
- Oristaglio J, Schneider DM, Balan PF, Gottlieb J. Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area. J. Neurosci. 2006;26:8310–8319. doi: 10.1523/JNEUROSCI.1779-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Shifting visual attention and selecting motor responses: distinct attentional mechanisms. J. Exp. Psychol. Hum. Percept. Perform. 1991;17:1023–1040. doi: 10.1037//0096-1523.17.4.1023. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychol. Sci. 1998;9:347–356. [Google Scholar]
- Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J. Neurophysiol. 2003;90:1392–1407. doi: 10.1152/jn.01049.2002. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith P, Segraves MA. A dual diffusion model for single cell recording data from the superior colliculus in a brightness discrimination task. J. Neurophysiol. 2007;97:1756–1774. doi: 10.1152/jn.00393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron. 2001;30:583–591. doi: 10.1016/s0896-6273(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Ann. Rev. Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Shen K, Pare M. Guidance of eye movements during visual conjunction search: local and global contextual effects on target discriminability. J. Neurophysiol. 2006;95:2845–2855. doi: 10.1152/jn.00898.2005. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to the frontal lobe from the macaque frontal eye fields. J. Comp. Neurol. 1993;330:286–301. doi: 10.1002/cne.903300209. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: extensions of Donder’s method. In: Koster WG, editor. Attention and Performance II. North Holland, Amsterdam: 1969. pp. 276–315. [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog. Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J. Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J. Neurophysiol. 2005a;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J. Neurosci. 2005b;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Yomtovian E, Thompson KG. The Role of Post-Perceptual Noise in a Sensorimotor Decision. Society for Neuroscience Annual Meeting; Atlanta, GA. 2006. p. 439.4. [Google Scholar]
- Zhou HH, Thompson KG. Cognitively directed spatial selection in the frontal eye field in anticipation of visual stimuli to be discriminated. Vision Res. 2008 doi: 10.1016/j.visres.2008.03.024. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]