Abstract
Over the past five decades, those interested in markers of radiation effect have focused primarily on tumor response. More recently, however, the view has broadened to include irradiated normal tissues—markers that predict unusual risk of side-effects, prognosticate during the prodromal and therapeutic phases, diagnose a particular toxicity as radiation-related, and, in the case of bioterror, allow for tissue-specific biodosimetry. Currently, there are few clinically useful radiation-related biomarkers. Notably, levels of some hormones such as thyroid-stimulating hormone (TSH) have been used successfully as markers of dysfunction, indicative of the need for replacement therapy, and for prevention of cancers. The most promising macromolecular markers are cytokines: TGFβ, IL-1, IL-6, and TNFα being lead molecules in this class as both markers and targets for therapy. Genomics and proteomics are still in nascent stages and are actively being studied and developed.
Keywords: Biomarker, prostaglandin, metabolomics, cancer survivorship, medical countermeasures against radiation
1. Introduction: A Brief History of Radiation-related Biomarker Research
An early focus of research into cellular and molecular radiation targets involved examination of the DNA molecule and radiation-induced, unrepaired single- or double-strand breaks that express as chromosomal aberrations. Similar examinations of cell integrity following radiation insult led early investigators to conclude that radiation-induced damage to the cellular membrane often led to cell death [1, 2]. These investigations of the cellular target itself led to broader questions of signaling and inquiry into the systemic response to radiation injury. Researchers began to examine the molecular targets of radiation-induced signaling, the cytokines being recruited to the site of injury, such as TGFβ, IL-1, and TNF [3-5]. In 1995, Rubin and colleagues explored what they termed a radiation-induced “perpetual cascade of cytokines” [6], a breakthrough notion that cast aside the classical division of radiation-induced damage into 3 temporal categories (acute, subacute, and late), and which led to a new understanding of the pathogenesis of radiation injury as a continuum [7].
2. Challenges of Marker Identification
A holy-grail of radiation oncology translational research has been the identification of markers of both tumor and normal tissue responses [8-11]. Identification of patterns of molecular expression indicative of human disease is exceedingly important. This is particularly true for two types of expressed molecules: those that are predictive of future disease and those that cause disease. The former can be used to identify patients for preventive therapies and the latter as a target for drug development. Markers can be classified into several categories and are measured with a variety of techniques. The most useful markers are those that are easily collected and immediately available, inexpensive, diagnostic and prognostic, and specific for a given disease. As an example, a high blood glucose level alone can be diagnostic of diabetes and prognostic of impending immediate and long-term disease; and determination of the glucose level is inexpensive and immediately available. While some simple markers are still waiting to be discovered, most researchers in the field of normal tissue radiation biology expect that matrixes of molecular markers will likely be needed for both prediction and for identifying targets for therapy.
A unique marker profile associated with normal tissue damage after irradiation is unlikely to be found. More likely marker profiles will vary with tissue and time due to the complex nature and protracted course of radiation toxicity. Briefly, radiation injury is a continuum of biological processes sometimes simplified by the terms acute and late toxicity, which follow a sequential process beginning within hours and progressing over weeks, months, and years [6, 11, 12]. The earliest events are often followed by a recovery period, which can maintain for years, and subsequent late injury is often exacerbated by any additional trauma leading to late organ dysfunction or malignancy. Thus, at any given time, markers might be valuable for predicting the likelihood of subsequent events in the sequential process, and for identifying prophylactic therapies that might be utilized to prevent those events. Attempting to identify markers for each of these types of events and for detecting progression during the latency periods is challenging. Since there are not yet any fully satisfactory markers, in this chapter we will discuss the experimentally promising markers that can be measured in easily acquired samples.
Preceding exposure to irradiation, genetic predisposition for decreased DNA repair, diabetes and other metabolic diseases affecting small vessel health, previous trauma and medical history, and pre-existing autoimmune or collagen vascular disease predispose to increased toxicity. During the first few hours after irradiation, cells primed for apoptosis or those that readily undergo apoptosis are at risk (Figure 1). Here, damage to mucosa and cutaneous tissues can allow brief organ dysfunction that progresses over time. Over the subsequent weeks, proliferation of cells required to maintain organ function is altered leading to damage in some tissues. Healing thereafter may include apparent complete recovery but can result in very late fibrovascular complications, decreased wound healing after subsequent trauma, or may predispose to oncogenesis. The process of identifying markers for all these potential toxicities, many of which are organ specific and vary with time, is a major and complex task.
Figure 1.
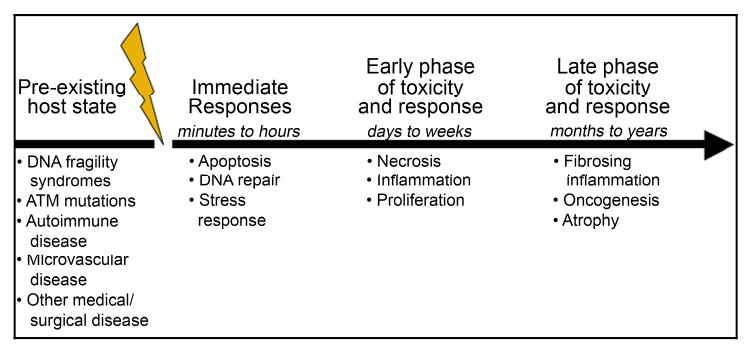
Different physiological processes, many of which are not specifically related to the direct instantaneous damage caused by radiation, progress over time to create normal tissue toxicity. Markers are needed at all stages of the process including markers for both the direct effects of the radiation and the indirect and thus potentially reversible radiation effects.
3. Definition of Markers
The term molecular marker is used broadly. It includes metabolites, proteins, RNA, DNA, and even complicated physiological states. The term is used to describe predictive assays, diagnostic tests, and targets for therapeutics. The terminology has not been developed to separate markers that can be used to follow disease progression and response to therapy as compared with markers that simply identify increased risk of development of disease after radiation exposure. A language has been coined to distinguish drugs that might be used to reduce the severity of impending radiation toxicity (protectors), treat subclinical radiation injury (mitigators), or treat radiation illness (therapeutics) [13]. A parallel language might be used to define at least these categories of markers for radiation effects on normal tissues (vis. predictive markers, prognostic markers, diagnostic markers, and dosimetric markers) (Table 1). Genotoxicity markers are a category that cross several of the others. Many genotoxicity markers are proven biodosimeters, most prominently dicentric chromosome aberrations which are essentially unique to irradiation. Genotoxicity markers including chromosome aberrations, micronuclei, and DNA complexes to phosphorylated histone H2AX may also be prognostic markers of oncogenesis [17-19].
Table 1. Types of Molecular Markers.
| Class of Marker | Definition | Example |
|---|---|---|
| Predictive | Available before irradiation that predicts a subsequent increased risk for organ specific radiation toxicity. | ATM mutant homozygosity [14], circulating IL-1 and IL-6 may be in this class for pulmonary toxicity [15]. Chromosomal aberrations and micronuclei might be in this category for oncogenic risk |
| Prognostic | Available at any time after the exposure that predicts a subsequent increased risk for a more severe disease course. | TGFβ1 levels may be in this class for pulmonary toxicity [10, 16], FGF2 may also be in this class for fibrovascular complications [16]. |
| Diagnostic | Available at the time of symptom presentation and which implicate radiation as the specific cause. | None. |
| Dosimetric | Available at some point after exposure that can determine the dose delivered to one or more organs. | Lymphocyte chromosomal aberrations for bone marrow exposure. |
Biological markers are usually circulating but can be tissue-based. The latter are necessarily more difficult to measure since they require tissue specimens. Metabolites can be very useful markers of toxicity, but they are usually only available in the therapeutic mode: they can however be used to follow the effectiveness of therapy. An example of a diagnostic marker might include blood urea nitrogen and creatinine levels for radiation-induced renal failure. Protein markers can be very specific, as is the case with liver enzyme measurements for hepatic toxicity, and can be elevated in the absence of symptoms and thus can be used to follow the severity of illness and its rate of improvement (prognostic marker).
Measurement of nucleic acid markers, RNA or DNA, from tissue or in the circulation is usually performed non-specifically using array technologies [9, 20]. The power of these methods to examine thousands of specific sequences is extraordinary. Finding the true needle(s) in the haystack of measurements, however, can be very challenging. Array studies are rarely specific or fully diagnostic. None have been used to follow the severity of illness, in part due to the expense but mostly because the associations are rarely quantitative. In contrast, identification of particular gene mutations can be very specific and diagnostic. The prominent example of this is the ATM gene mutation, homozygosity for which creates severe radiation sensitivity [21]. Heterozygosity to mutations of ATM leads to intermediate sensitivity in cell culture; however, it is not a useful clinical marker for prediction of increased radiation sensitivity [21-23].
4. Metabolic Markers of Normal Tissue Response to Radiation in Select Organs and Tissues
All tissues are damaged by radiation. Tissues that undergo rapid renewal have earlier side effects that relate to depopulation. Slowly renewing, or non-proliferating tissues, have side effects that include inflammatory changes and devascularization. The toxicity process is sequential and progressive in most tissues, but the pace varies between tissues and differs from patient to patient. Treatment doses are as much determined by the tolerance of the tissues being irradiated as they are by the tumor being treated. For many tumors, for example pancreatic cancer, the tolerance dose dominates and curative doses are unsafe. Some critical organs and possible markers of toxicity are discussed in the next sections.
4.1.1. Brain
Biomarkers of radiation damage to the brain are not yet available clinically. The phenomenon of brain damage that progresses during and after a course of radiation has been extensively studied but remains confusing. There is a prominent fatigue syndrome associated with brain irradiation that appears to be accompanied by alterations in circulating levels of FGF2, VEGF, IL-1, and cortisol [24, 25]. Animal studies suggest that these cytokines might be a more long-lived response seen in the circulation to the local production of TNF [26, 27], TNF being a more locally active and short lived cytokine. In receptor knockout mice, the effect appears in part controlled by the relative expression levels of the two TNF receptors. The cellular transport of tryptophan and metabolism of serotonin also appear prominent in the early effects of radiation on the brain. The fatigue generally recovers completely after a month. During the years following irradiation, however, patients develop progressive dysfunction including decreased concentration and memory, processes that are attributed to a combination of progressive microvessel dysfunction, and to killing or premature maturation of brain stem cells [28, 29]. Thus there are some potential markers, but they involve collecting brain specimens and measuring BrdU labeling or Ki67 stains. The utility of apolipoprotein E genotype, a marker associated with premature dementia after traumatic brain injury, deserves study in survivors of cranial irradiation. Other markers of brain trauma that deserve consideration as a measure for radiation-related brain damage include 14-3-3beta from cerebral spinal fluid and neuro-specific eolase in the serum [30, 31].
4.1.2. Salivary glands and Pancreas
Organs containing digestive enzymes can have amplified reactions following radiation-induced cell killing. Salivary amylase levels constitute roughly 60% of total serum amylase levels in the body. Within 12 hours of exposure to just 2 Gy, salivary function decreases by as much as 50% [32]. Maintaining a total dose under tolerance is crucial to recovery. Pilocarpine stimulation or growth factors to prevent apoptosis also appears to reduce the severity of dry mouth [33-35]. The reduced salivary function occurs along with an increase in serum amylase levels [36]. Increases in amylase but not other digestive enzymes is also seen after irradiation of the pancreas, but radiation does not appear to cause symptomatic pancreatitis or much acute pancreatic exocrine dysfunction. While serum amylase increases acutely in the first hours after irradiation, the loss of salivary gland function over time leads to subsequent low circulating amylase. An association between acute increase or chronic decrease in circulating amylase and subsequent severity of xerostomia is logical but has not been established. Interventions for xerostomia include growth factors. Keratinocyte growth factor (KGF), also known as FGF7, has shown promise for prevention of both radiation- and chemotherapy-induced xerostomia [35, 37, 38]. The mechanism is believed to include inhibition of epithelial apoptosis, and improved proliferation and repair. There are not yet markers to identify which patients might benefit, however, nor are there markers for assessing response. Therefore, optimizing drug dose and schedule is not possible and benefits remain limited. Thus predictive and prognostic markers are needed.
4.1.3. Lung
Pulmonary surfactant is a polar molecule that allows the alveoli of the lung to re-expand after atelectasis, a process that would otherwise be nearly impossible due to surface tension. After radiation, surfactant levels in the blood quickly rise as do the levels of surfactant in the exposed lung tissue [11, 39, 40]. The mechanism of acute respiratory distress syndrome that can occur following lung radiation is thought to be a result of impaired metabolism of surfactant from high density to low density forms [41]. This can lead to failure of the lungs to expand properly. Gas exchange is not much affected shortly after irradiation, unless there is associated pneumonitis or atelectasis. At later times, gas exchange is reduced due to pulmonary fibrovascular changes and inflammation. The latter is usually compensated for by increased pulmonary rate. Respiratory acidosis or alkalosis, however, is uncommon. Additionally, certain circulating cytokines including TGFβ1, IL-1, and IL-6 are naturally increased in many patients who go on to develop pulmonary fibrosis [42, 43]. The utility of TGFβ1 has been the most extensively studied and is reviewed elsewhere [8, 10, 44]; a difficulty in use of this marker is the need to measure it sequentially over long periods of time in order to assess risk. Studies in which IL-1 and IL-6 were measured appear more useful in that measurements were applicable to both prediction and prognosis [42, 43]. The latter, however, were not sufficiently quantitative to allow assessment of the value of an intervention.
4.1.4. Liver
The liver is a hypoxic organ, having a blood supply that is chiefly venous [45]. The liver's role includes the detoxification of ingested toxins, and thus the low oxygen environment is likely a safety measure reducing oxidation damage. It is therefore confusing as to why the liver is among the most intrinsically radiosensitive organs. Massive cell death can be seen after only 30 Gy when the whole organ is exposed to fractionated treatment. The liver however has an enormous capacity for regeneration, a process that occurs with partial hepatectomy or high dose localized liver irradiation. The advent of extracranial radiosurgery for liver metastases would otherwise not be possible. There are some obvious candidate markers for examining the damage to the liver and its rate of regeneration. In the former category are liver enzymes, the most useful of which appears to be alkaline phosphatase. Bilirubin and other markers of obstruction are usually not involved. Regarding the healing process, many growth factors are likely involved though none have been proven. These include epidermal growth factor (EGF) and hepatocyte growth factor (HGF), and the angiogenesis factor, basic fibroblast growth factor (FGF2). It is worth commenting that liver regeneration is not significantly impaired in patients treated concurrently with anti-Her2 antibodies (trastuzumab).
4.1.5. Gastrointestinal Tract
Irradiation of the gastrointestinal tract causes a series of biological effects that begin with immediate nausea and vomiting, delayed malnutrition and diarrhea, and which can progress to late fibrosis and necrosis. The malnutrition-related changes arise from altered absorption [46] and alterations in bowel motility [47]. Many metabolic markers of these gastrointestinal syndromes are not specific and can be simulated by controlled diet in non-irradiated animals [46-48]. Likewise, replacing the diet of irradiated animals with a low protein or liquid elemental diet repairs some metabolic changes that occur after irradiation; therefore, metabolic markers specific for radiation exposure remain undetermined. Thus while radiation-related gastrointestinal dysfunction is a common phenomenon from both total body and local abdominal irradiation exposure, there may not be specific metabolic patterns for radiation syndromes.
4.2. Types of markers available in accessible specimens
Accessibility of sequential specimens is a high priority for marker studies. The mechanism of the interaction between the marker and a biological process is important if the marker is to be used rationally. Optimally, a marker can be easily followed before therapy and then during therapy to evaluate progression of toxicity or adequacy of preventive measures and therapy. Markers come in several classes in addition to the obvious macro-molecular protein, DNA and RNA markers. Importantly, some of the most easily measured markers and simplest markers are already in routine use. The next sections will discuss some important classes of markers and how they are being used clinically and experimentally.
4.2.1. Electrolytes and Cell Metabolic Products
Radiation, whether localized or systemic, can have a number of immediate and delayed physiological effects. Polydipsia due to altered plasma volume occurs in many animal species. It can occur within a few hours of exposure and maintain for a few days [49, 50]. It is rarely clinically evident and most patients correct the effect (asymptomatically) by oral fluid and electrolyte replacement. An electrolyte imbalance in animal models is a marker for diuresis. It begins within a few hours and continues for a few days after exposure. Depletions of calcium, phosphate, and sodium have been reported [51]. In animals, the diuresis can be prevented by hypophysectomy and adrenalectomy, implicating a corticoid hormonal basis [52]. The diuresis phase is followed by decreased appetite and this is associated with changes in electrolytes and blood glucose, and decreased protein absorption. Urine volume decreases and urine concentration increases. None of these changes, however, are specific.
The bowel also impacts the fluid and electrolyte balance [53, 54]. The small bowel mucosa is composed of the villous and crypt. The former is highly differentiated tissue that is naturally sloughed and replaced approximately weekly in humans. Its function is mostly absorptive of fluid and electrolytes. The crypts are less differentiated and in contrast are more secretory. After irradiation there is proliferation and regeneration that favors the crypt cells and thus a secretory diarrhea. Villous cells slough even more rapidly than normal. The electrolyte imbalance due to gastrointestinal dysfunction likely reaches a maximum at 3 to 5 days and recovers a few days later. As with the renal effects, none of these changes are specific for radiation and can be corrected by appropriate modification of diet.
Some tissue degradation products can be measured in the urine and plasma shortly after irradiation. These include polyamines, small cationic compounds derived from L-ornithine and L-arginie. Polyamines are increased in tissues undergoing proliferation and differentiation and are sometimes increased in cancers. Examples of these include putrescine, spermidine, and spermine. Interestingly, tissue levels of these compounds can decrease within hours after irradiation in the spleen and bowel, while increasing in the blood. Late injury from localized irradiation can result in high levels of these metabolites [55]. The association with these circulating tissue breakdown products with specific toxicity experienced by patients undergoing abdominal or head and neck radiation is unknown.
It is obvious that radiation dose is the most powerful predictor of radiation toxicity. The search for radiation-specific electrolyte or metabolic markers that vary with dose is an active area of biodosimetry research. Unfortunately most of this research is aimed at victims of total body radiation exposure and not local organ toxicity in the cancer patient. All the electrolyte markers from the blood or urine are non-specific for radiation, have non-monotonic responses to radiation, circadian and other temporal effects, or large inter-individual variations; thus none are satisfactory biodosimeters even for total body exposure. Similarly, use of the available markers as a measure of specific organ toxicity during or after a course of therapeutic radiation does not yet add to the management or diagnosis of radiation-related injury. Unfortunately, none of these metabolic markers are yet useful for prediction of impending radiation-related organ injury.
4.2.2. Hormones and prostaglandins
Hormone and prostaglandin levels are altered in the tissue and circulation by local and large field irradiation. As with cytokines, these molecules are known to affect the proliferation, repair, healing, and inflammation of tissues. These molecules could be more than just prognostic, since therapeutic interventions aimed at normalizing these factors have beneficial effects in animals. Cox-2 inhibitors, which affect prostaglandin synthesis, in particular show great promise [56, 57]. Hormone replacement therapy is offered for some very specific conditions such as hypothyroid in patients with elevated TSH after thyroid irradiation, as a mechanism for reducing the overstimulation of the thyroid and thus subsequent thyroid cancer [58]. It has also been shown that bone remodeling and the health of cartilage is affected by a combination of cytokines and parathyroid hormone. Hormones like cytokines are powerful mitogenic and differentiating agents for a number of stem and progenitor cells. Despite the promise of hormone and Cox-2 inhibition therapies as a prevention for radiation-related toxicity, lack of markers limits their use. In the case of hormone aberrations after pituitary, thyroid, or gonadal irradiation, many circulating markers have been identified [59]. Appropriate prostaglandin balance is unknown, which limits the use of Cox-2 inhibitors. More markers related to both hormone and prostaglandin balance are needed since therapy with these agents would be very simple.
4.2.3. Thiols and other antioxidants
In-vitro thiol levels produce substantial alterations in the shape of the radiation survival curve and, since they also inactivate many chemotherapy drugs, were among the first and most studied natural radiation protectors [60]. Other antioxidants including vitamins C and E have shown benefits when used as part of therapy for radiation-related side effects [61, 62]. Studies also show that high concentrations of these vitamins can be protective against whole body irradiation. There has however been very little study of the role that naturally high or low oxidative states in plasma (or tissues or circulating cells) might play in the progression of radiation toxicity. Oxidative states can be measured a number of ways. For example, tests include those that measure reduction capacity, reduction potential, and reactivity in soluble phase (cytoplasm) or lipid phase (membrane) [63, 64]. Cellular compartments that experience high free radical levels, such as the mitochondria, may be the key subcellular target. Tests for oxidative stress are all readily available, can be performed on leukocytes or plasma, and deserve further investigation.
4.2.4. Metabolomics
Mass spectroscopy and other modern techniques have made it possible to analyze thousands of metabolites in blood and urine. These techniques have revealed the existence of several short-lived and apparently unique metabolites in these fluids following irradiation [10]. Some appear to be oxidized lipid product involved in apoptosis signaling [65]. Several commercial ventures have grown with the premise that fingerprints of unique metabolites should be useful for following responses of cancer to treatment and of normal tissue to radiation exposure (e.g., Metabolon, North Carolina, USA; Sionex Corp., Massachusettes, USA). The practical value of these metabolites with regard to prevention, prognosis, or diagnosis remains unclear.
5. Physiological processes
Radiation treatments have multiple physiological effects. In clinical radiation therapy, the majority of tissue being irradiated (often to much lower dose) is actually non-tumor. Thus the majority of physiological changes are likely related to this exposure. Radiation causes cell death through a variety of mechanisms including apoptosis, necrosis, DNA damage, and reproductive inactivation. Radiation damages some cells without killing them, causing the stimulation of pathways involved in repopulation, maturation, angiogenesis, and inflammation. It damages vasculature leading to perfusion dysfunction and hypoxia. The role that cytokines might play in these physiological processes has been extensively studied in recent years. The following sections will briefly discuss some physiological markers of great promise.
5.1. Markers of Cell Death (Apoptosis, Necrosis, and Reproductive Inactivation) in Response to Radiation
Radiation-induced cell killing includes apoptosis, necrosis, and reproductive inactivation. Fortunately most solid organ tissues are resistant to apoptosis following irradiation. Such cells alternatively might, sometimes over a long period of time, undergo reproductive death, necrosis, or delayed apoptosis. These mechanisms of cell death occur at a rate too slow to be substantially detectable by circulating markers or with a biopsy. Also, unlike apoptosis, there are very few candidate markers for reproductive or necrotic cell loss. Thus death markers are not yet useful for predictive, prognostic, or diagnostic purposes and their development for use in dosimetry will be a challenge as well.
Regarding early side effects of radiation, apoptosis can be extremely important even if its impact on late toxicity is limited. Apoptosis is important for acute effects because it is a rapid process that can dramatically reduce cell number. Among the most studied tissues undergoing brisk apoptosis are the bone marrow progenitor cells, lymphocytes, and endothelial cells. They undergo apoptosis via both the intrinsic and extrinsic pathways, including p53-dependent and -independent pathways, and ceramide-dependent and -independent pathways. Apoptosis signaling can begin with oxidative products and the mitochondrial pathway. In apoptosis prone cells, the process is brisk, reaching a maximum a few hours after radiation exposure and usually decreasing to a low level within 24 hours [66-68]. Because the process is short lived and most of the signaling proteins are intracellular, to date there are few useful apoptosis-related proteins to consider as molecular markers of radiation effect.
One marker related to reproductive health of cells has been useful as a biodosimetric marker, namely chromosomal aberrations. Aberrations are visualized when hematopoietic cells are cultured and metaphase spreads are examined. Improperly repaired chromosomes following radiation have altered structure leading to abnormal and missing chromosomes. Chromosomal aberrations, at present specifically the dicentric assay, remain the gold standard for radiation biodosimetry [69, 70] (Figure 2). New approaches to biodosimetry are in development that also quantify chromosomal damage and include assays that examine for micronuclei in hematopoietic cells [71], reticulocytes [72], and cutaneous and pulmonary fibroblasts [73]. Flow cytometric assays are also in development for biodosimetry [71] as are techniques for measuring DNA repair complexes such as histone H2AX [17-19]. These markers of biodosimetry are actually measures of genotoxicity. Thus they may also be prognostic markers for oncogenic risk in exposed individuals and predictive markers for individuals considering work that might involve radiation exposure. Other biodosimetric techniques are physical dosimeters that measure unpaired electrons in crystalline biological materials such as dental enamel [74] and finger nails.
Figure 2.
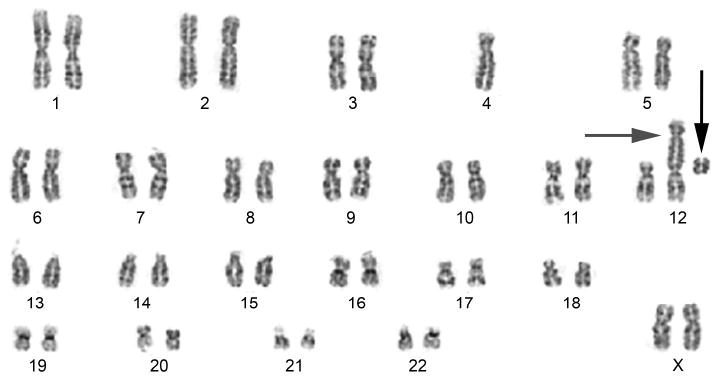
Cytogenetic preparation of irradiated lymphocytes (3 Gy) from a female subject. The radiation produced a dicentric chromosome (#12, grey horizontal arrow) by the translocation of chromosome #4 onto #12, also forming an acentric fragment (black vertical arrow).
5.2 Markers of Hypoxia in Response to Radiation
A common pathway of radiation toxicity is microvascular dysfunction. Many genes are upregulated in response to hypoxia including several angiogenic peptides that are easily detected in the circulation. High levels of FGF2 have been seen in long-term survivors of radiation with concurrent fibrovascular complications of radiation [26]. When these subjects were treated with pentoxifylline to improve microcirculation, most had a reduction of their circulating FGF2 protein. Other angiogenic proteins are likely expressed and deserve consideration. The anti-angiogenic effects of radiation on vasculature are likely a combination of decreased localized stem cells and local changes in the cytokine milieu reducing the recruitment of new stem cells from the circulation. Some of these factors might also be available for long-term evaluation as prognostic markers and as targets for therapy.
5.3 Cytokines and inflammatory mediators in Response to Radiation
Cytokines are a class of proteins and glycoproteins involved in intercellular signaling. Most act through autocrine and paracrine cellular communication but can be found in the circulation. They include growth factors, angiogenesis and angiostatic proteins, interleukins, adhesion molecules and chemokines. High, low, or prolonged expression of angiogenic or angiostatic cytokines have been associated with human diseases that pathologically simulate radiation toxicity [10, 39, 40, 75]. Chemokines are chemotactic proteins that recruit inflammatory cells to irradiated tissues and, like interleukins, can activate inflammatory cells. Prolonged or high expression of these proteins, as with the angiogenic peptides, has been associated with delayed radiation side effects that emulate autoimmune disease. Growth factors are double-edged swords that cause the proliferation and maturation of stem and progenitor cells. These typically reduce the early side effects of radiation but can be a cause of deleterious changes to organ architecture and premature differentiation of stem cells leading to later organ dysfunction. The cytokines and their effects are particularly exciting in that they themselves are therapeutic targets, making cytokine-mediated disease reversible. Thus cytokine levels can serve as predictive, prognostic, and diagnostic markers for radiation toxicity; they are not, however, specific for the radiation effect.
The role that cytokines play in radiation toxicity was first discovered and described by Rubin and colleagues [39]. Shortly thereafter, transforming growth factor β1 (TGFβ1) was identified in the circulation of breast cancer patients who developed pulmonary and hepatic complications from chemotherapy for bone marrow transplantation [75]. Subsequently, the impact of TGFβ1 on pulmonary radiation effects has now been studied extensively in humans. The bulk of current evidence suggests that if TGFβ1 is elevated for a long period of time before, during, or after irradiation, the risk of pulmonary toxicity increases [3, 44, 75-77]. An example of the powerful role TGFβ1 appears to play in radiation-related fibrosis is shown in Figure 3. More importantly, however, extensive work on cytokine levels and their relation to radiation exposure and toxicity has indicated an exquisitely complex picture; cascades of cytokines—messengers, recruiters, and modifiers—are involved. Chen and colleagues found that subjects undergoing thoracic radiation who had elevated IL-1 or IL-6 before or during radiation all developed some degree of radiation changes (clinical or radiographic). Angiogenic factors have also been associated with late pulmonary toxicity. For example, we found that FGF2 was markedly elevated in the circulation of most subjects with severe late radiation fibrovascular toxicity of the extremities [26].
Figure 3.
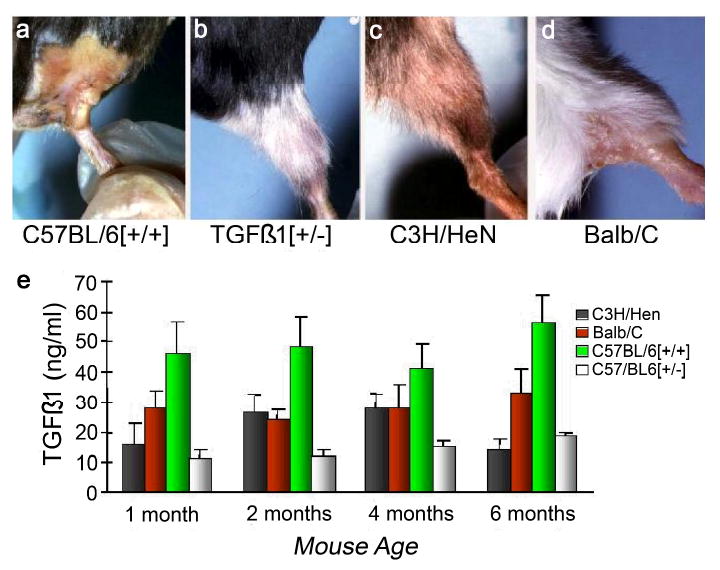
TGFβ1 is among the most studied circulating biomarkers of radiation fibrosis, and there is a strong association between circulating levels of this cytokine and fibrosis in irradiated mice and in human disease. Panels a-d show mice irradiated to the hind limb with 40 Gy at 4 weeks post-exposure. Note the depilation of the C57BL/6[+/+] and Balb/C mice compared to the relatively healthy appearance of the C3H/HeN and heterozygous knockout TGFB1[+/-] mice. The C57Bl/6 mouse is known to have a high propensity for radiation fibrosis and the C3H/HeN a low, while the Balb/C is intermediately fibrosing following irradiation. Heterozygous knockout TGFβ1 mice are phenotypically normal, but have a life-long low level of circulating TGFβ1 and a similarly low level of depilation, muscle wasting, and scarring following irradiation. The histogram (panel e) indicates the naturally occurring plasma levels of TGFβ1 (no radiation given) for these 4 mouse strains as a function of age.
There is a “cytokine storm” that occurs shortly after a tissue is irradiated. It can be very prominent in patients undergoing whole body radiation for bone marrow transplant. This short-lived, multi-cytokine expression has led some to speculate that the intensity of that expression might identify at-risk patients for later toxicity. Most investigators, however, judge this storm to be a general stress response and not a predictive assay. The prediction therefore lies in the slower progressive cascade of cytokines previously described. It remains unclear whether measurements of either the storm or the cascade will be specific and sensitive enough to be useful as biomarkers for anything other than acute biodosimetry.
5.4 Other protein markers in Response to Radiation
Marchetti and colleagues [78] performed an admirable and exhaustive review of all protein radiation biomarkers in the literature between 1973 and 2006. The review included studies using modern mass spectroscopy techniques. The potential of protein markers for clinical usefulness was ranked on a 9 point scale, with six criteria: the highest scores emphasizing long lived proteins seen in vivo, studies with better scientific justifications and studies in humans. Of the 322 proteins in their list, only 29 were documented in vivo in humans. Among their top ranked proteins were the best studied cytokine families but not necessarily the family stars. For example, many cytokines in the TGFβ1 family were acknowledged, but TGFβ1 itself was poorly ranked. Likewise, the receptors in the TNF family were highly ranked, but the very short lived and hard to measure TNFα was ranked poorly. IL-1 and IL-6, two of the most researched interleukins, were outranked by many other inflammatory cytokines. Pulmonary surfactant did not make the list, and plasma amylase was ranked poorly. In general, proteins involved in maintenance of a stable redox state and of mitochondrial function were toward the top of the list, as well as many proteins involved in DNA repair and inflammation. While the ranking system necessarily has significant bias, it demonstrates the absence of any fully adequate markers and the difficulty inherent in choosing a set of best protein markers.
6. Gene expression and amplification in Response to Radiation, and the status of microarray analysis
While mRNA expression profiles for late effects of radiation or other cancer treatments are still few in number [9, 79-81], this area of research is of great interest and is expected to continue to grow rapidly. Most clinical studies to date feature cultured cells and are thus limited to lymphocytes and fibroblasts. As an added artifact, the cells are necessarily grown in medium and thus have a greatly altered environment compared with their native in vivo state. Patients with purely radiation-related side effects are hard to group, making appropriate comparison difficult. Thus specimens are necessarily stored, and different categories of side effects are often mixed. Microdissection of cells within tissues to confirm the type of cells responsible for the gene expression is also difficult to perform.
Among the studies, Quarmby et al examined 268 transcripts using the Atlas Clontech Array system [81]. All subjects had breast cancer treated with 40 Gy in 15 fractions. Those who developed fibrosis (3 subjects) were compared with those without fibrosis (3 subjects). The cells were cultured fibroblasts from biopsies far from the irradiated field. They found 9 transcripts that were significantly elevated in the 3 fibrosis sensitive patients. These included mRNA transcripts relevant to TNFα, PDGF, and nerve growth factor. In another study by Rieger and colleagues using more advanced arrays with far more transcripts, 14 patients with unusual skin reactions were compared with 43 others with more normal responses [80]. A variety of advanced statistical techniques were used including analyses that grouped genes with those that are functionally or structurally related (the GO system). They tested radiation response using UV light on lymphocytes and ultimately found 24 genes that predicted toxicity in 9 of 14 patients; however, the genes represented by their transcripts differed from those identified by Quarmby and colleagues.
Among normal tissue radiation studies published to date, there are no mRNA arrays from cell types other than lymphoblastoid cells [82]. Though all studies to date employed lymphoblastoid cells, the prominent transcripts in each report were different with little overlap. The selected transcripts rarely represented genes with protein products known to be important in radiation response, making their results suspect. While this summary is disappointing, the science of gene arrays is in its nascent phase and more promising studies are expected.
It is too early to determine whether the mRNA array approach to the identification of markers will bear fruit. There are many issues that need to be studied [83]. The culturing of cells is likely to introduce artifacts, as is the choice of cells to culture and evaluate. The statistical analysis of arrays is likewise a difficult task that naturally introduces bias [83]. Ultimately, some reproducible system that avoids cell culture and examines a panel of cell types will likely be needed. Lymphocytes might prove a poor substitute for epithelial tissues, hence normal tissue samples might best be collected at the time of surgery. Gene processing is likely to play an important role; this includes methylation and change in copy number. The latter phenomenon also relates very critically to the rarely studied mitochondrial genome, which like the somatic chromosomes are damaged by radiation but must also reproduce at all phases of the cell's life cycle. Finally, arrays with a limited number of transcripts with high information content should be employed to allow emphasis on critical but minimally expressed markers and conversely prevent false positives from irrelevant but highly expressed markers.
7. Future of research for molecular markers of normal tissue tolerance
We do not yet have adequate markers for the vast majority of clinical needs, and their discovery remains a very high priority in radiation research. The need for these markers has intensified due to the growing number of cancer survivors at risk for developing toxicity from radiation, chemotherapy, surgery, and combinations of all three. Currently the number of cancer-treatment survivors is numbered at over 10 million in the United States and is growing rapidly. This number dwarfs the number of new cancers detected each year. The National Cancer Institute has identified survivorship research as a high priority over the next decade. Cooperative clinical trial groups are beginning to design studies aimed at determining the best management of treatment related side effects (rather than just how to manage cancers). Concurrent with the growing number of cancer-treatment survivors is the real risk for radiological exposure of otherwise healthy victims in an accident, an act of terror, or war-related scenarios. Taken together, the need for better markers is growing rapidly and is of high importance considering the paucity of currently available markers. As the cooperative group clinical trials grow, and the funding for research in the area increases, progress in identifying markers for prediction, prognostication, mitigation, and therapy is expected to be rapid in the next decade.
8. Conclusions
Markers of radiation-related side effects include those that can be used to identify subjects at risk of greater than normal toxicity, before exposure, in addition to markers useful for diagnosis, prognosis, biodosimetry, and therapy. The available markers are often organ-specific, and some are in routine use; however, there are currently very few markers and many organs-at-risk have no satisfactory markers. The most useful markers are those that are most specific and quantitatively predictive of side-effect severity. The available markers include metabolites, physiological markers, and protein products of tissue damage or signaling processes. Among these markers, cytokines appear to play the role of both causative agent and marker. Thus interventions that target cytokines might be effective. Gene expression profiles might ultimately play a role in better understanding normal tissue tolerances to radiation. More work on better models of radiation exposure is likely to be needed. The future of research on markers of radiation tolerance is increasingly important due to the growing number of cancer treatment survivors. Advances in the field of radiation markers of normal tissue toxicity are expected in the next decade.
Acknowledgments
This research was in support of the Centers for Medical Countermeasures against Radiation Program, U19AI067733, National Institute of Allergy and Infectious Diseases (USA).
References
- 1.Radford IR. Evidence for a general relationship between the induced level of DNA double-strand breakage and cell-killing after X-irradiation of mammalian cells. Int J Radiat Biol. 1986;49(4):611–620. doi: 10.1080/09553008514552861. [DOI] [PubMed] [Google Scholar]
- 2.Chapman JD, Allalunis-Turner MJ. Cellular and molecular targets in normal tissue radiation injury. In: Gutin PH, Liebel SA, Sheline GE, editors. Radiation injury to the nervous system. New York: Raven Press; 1991. pp. 1–16. [Google Scholar]
- 3.Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor-beta 1 expression in irradiated liver. Radiat Res. 1990;122(1):77–85. [PubMed] [Google Scholar]
- 4.Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28(3):621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 5.Piguet PF. Is “tumor necrosis factor” the major effector of pulmonary fibrosis? Eur Cytokine Netw. 1990;1(4):257–258. [PubMed] [Google Scholar]
- 6.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33(1):99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 7.McBride WH. Cytokine cascades in late normal tissue radiation responses. Int J Radiat Oncol Biol Phys. 1995;33(1):233–234. doi: 10.1016/0360-3016(95)02019-8. [DOI] [PubMed] [Google Scholar]
- 8.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4(9):529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 9.Kruse JJ, Stewart FA. Gene expression arrays as a tool to unravel mechanisms of normal tissue radiation injury and prediction of response. World J Gastroenterol. 2007;13(19):2669–2674. doi: 10.3748/wjg.v13.i19.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17(2):89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hartsell WF, Scott CB, Dundas GS, Mohiuddin M, Meredith RF, Rubin P, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Analysis of RTOG 91-03. Am J Clin Oncol. 2007;30(4):368–376. doi: 10.1097/01.coc.0000260950.44761.74. [DOI] [PubMed] [Google Scholar]
- 12.Brush J, Lipnick SL, Phillips T, Sitko J, McDonald JT, McBride WH. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol. 2007;17(2):121–130. doi: 10.1016/j.semradonc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res. 2004;162(6):711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 14.Williams JR, Zhang Y, Russell J, Koch C, Little JB. Human tumor cells segregate into radiosensitivity groups that associate with ATM and TP53 status. Acta Oncol. 2007;46(5):628–638. doi: 10.1080/02841860601080407. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. 2002;12(1 Suppl 1):26–33. doi: 10.1053/srao.2002.31360. [DOI] [PubMed] [Google Scholar]
- 16.Andreassen CN, Alsner J, Overgaard M, Sorensen FB, Overgaard J. Risk of radiation-induced subcutaneous fibrosis in relation to single nucleotide polymorphisms in TGFB1, SOD2, XRCC1, XRCC3, APEX and ATM--a study based on DNA from formalin fixed paraffin embedded tissue samples. Int J Radiat Biol. 2006;82(8):577–586. doi: 10.1080/09553000600876637. [DOI] [PubMed] [Google Scholar]
- 17.Al Rashid ST, Dellaire G, Cuddihy A, Jalali F, Vaid M, Coackley C, et al. Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo. Cancer Res. 2005;65(23):10810–10821. doi: 10.1158/0008-5472.CAN-05-0729. [DOI] [PubMed] [Google Scholar]
- 18.Yu T, MacPhail SH, Banath JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA Repair (Amst) 2006;5(8):935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 19.Klokov D, MacPhail SM, Banath JP, Byrne JP, Olive PL. Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother Oncol. 2006;80(2):223–229. doi: 10.1016/j.radonc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 20.West CM, Elliott RM, Burnet NG. The genomics revolution and radiotherapy. Clin Oncol (R Coll Radiol) 2007;19(6):470–480. doi: 10.1016/j.clon.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Fernet M, Hall J. Genetic biomarkers of therapeutic radiation sensitivity. DNA Repair (Amst) 2004;3(89):1237–1243. doi: 10.1016/j.dnarep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Meyer A, John E, Dork T, Sohn C, Karstens JH, Bremer M. Breast cancer in female carriers of ATM gene alterations: outcome of adjuvant radiotherapy. Radiother Oncol. 2004;72(3):319–323. doi: 10.1016/j.radonc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Andreassen CN. Can risk of radiotherapy-induced normal tissue complications be predicted from genetic profiles? Acta Oncol. 2005;44(8):801–815. doi: 10.1080/02841860500374513. [DOI] [PubMed] [Google Scholar]
- 24.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol. 2002;5(4):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 25.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12 1:22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 26.Okunieff P, Augustine E, Hicks JE, Cornelison TL, Altemus RM, Naydich BG, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22(11):2207–2213. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 27.Daigle JL, Hong JH, Chiang CS, McBride WH. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61(24):8859–8865. [PubMed] [Google Scholar]
- 28.Noble M, Dietrich J. Intersections between neurobiology and oncology: tumor origin, treatment and repair of treatment-associated damage. Trends Neurosci. 2002;25(2):103–107. doi: 10.1016/s0166-2236(02)02060-x. [DOI] [PubMed] [Google Scholar]
- 29.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18(1):115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Siman R, Zhang C, Roberts VL, Pitts-Kiefer A, Neumar RW. Novel surrogate markers for acute brain damage: cerebrospinal fluid levels corrrelate with severity of ischemic neurodegeneration in the rat. J Cereb Blood Flow Metab. 2005;25(11):1433–1444. doi: 10.1038/sj.jcbfm.9600138. [DOI] [PubMed] [Google Scholar]
- 31.Guan W, Yang YL, Xia WM, Li L, Gong DS. Significance of serum neuron-specific enolase in patients with acute traumatic brain injury. Chin J Traumatol. 2003;6(4):218–221. [PubMed] [Google Scholar]
- 32.McDonald S, Meyerowitz C, Smudzin T, Rubin P. Preliminary results of a pilot study using WR-2721 before fractionated irradiation of the head and neck to reduce salivary gland dysfunction. Int J Radiat Oncol Biol Phys. 1994;29(4):747–754. doi: 10.1016/0360-3016(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 33.Burlage FR, Roesink JM, Kampinga HH, Coppes RP, Terhaard C, Langendijk JA, et al. Protection of Salivary Function by Concomitant Pilocarpine During Radiotherapy: A Double-Blind, Randomized, Placebo-Controlled Study. Int J Radiat Oncol Biol Phys. 2007 Sep 14; doi: 10.1016/j.ijrobp.2007.06.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Thula TT, Schultz G, Tran-Son-Tay R, Batich C. Effects of EGF and bFGF on irradiated parotid glands. Ann Biomed Eng. 2005;33(5):685–695. doi: 10.1007/s10956-005-1853-z. [DOI] [PubMed] [Google Scholar]
- 35.Beaven AW, Shea TC. Recombinant human keratinocyte growth factor palifermin reduces oral mucositis and improves patient outcomes after stem cell transplant. Drugs Today (Barc) 2007;43(7):461–473. doi: 10.1358/dot.2007.43.7.1119723. [DOI] [PubMed] [Google Scholar]
- 36.Dubray B, Girinski T, Thames HD, Becciolini A, Porciani S, Hennequin C, et al. Post-irradiation hyperamylasemia as a biological dosimeter. Radiother Oncol. 1992;24(1):21–26. doi: 10.1016/0167-8140(92)90349-y. [DOI] [PubMed] [Google Scholar]
- 37.Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58(5):933–939. [PubMed] [Google Scholar]
- 38.Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, et al. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif. 2002;35 1:78–85. doi: 10.1046/j.1365-2184.35.s1.8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin P, Finkelstein JN, Siemann DW, Shapiro DL, Van Houtte P, Penney DP. Predictive biochemical assays for late radiation effects. Int J Radiat Oncol Biol Phys. 1986;12(4):469–476. doi: 10.1016/0360-3016(86)90054-4. [DOI] [PubMed] [Google Scholar]
- 40.Rubin P, McDonald S, Maasilta P, Finkelstein JN, Shapiro DL, Penney D, et al. Serum markers for prediction of pulmonary radiation syndromes. Part I: Surfactant apoprotein. Int J Radiat Oncol Biol Phys. 1989;17(3):553–558. doi: 10.1016/0360-3016(89)90106-5. [DOI] [PubMed] [Google Scholar]
- 41.Gross NJ. Surfactant subtypes in experimental lung damage: radiation pneumonitis. Am J Physiol. 1991;260(4 Pt 1):L302–L310. doi: 10.1152/ajplung.1991.260.4.L302. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Rubin P, Williams J, Hernady E, Smudzin T, Okunieff P. Circulating IL-6 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2001;49(3):641–648. doi: 10.1016/s0360-3016(00)01445-0. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Hyrien O, Williams J, Okunieff P, Smudzin T, Rubin P. Interleukin (IL)-1A and IL-6: applications to the predictive diagnostic testing of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2005;62(1):260–266. doi: 10.1016/j.ijrobp.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 44.Evans ES, Kocak Z, Zhou SM, Kahn DA, Huang H, Hollis DR, et al. Does transforming growth factor-beta1 predict for radiation-induced pneumonitis in patients treated for lung cancer? Cytokine. 2006;35(34):186–192. doi: 10.1016/j.cyto.2006.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 46.Classen J, Belka C, Paulsen F, Budach W, Hoffmann W, Bamberg M. Radiation-induced gastrointestinal toxicity. Pathophysiology, approaches to treatment and prophylaxis. Strahlenther Onkol. 1998;174 3:82–84. [PubMed] [Google Scholar]
- 47.Cionini L, Becciolini A, Dalla Palma L, De Giuli G. Intestinal absorption of radioiodine labeled human serum albumin, mono-iodotyrosine and di-iodotyrosine following abdominal radiation therapy. Acta Radiol Ther Phys Biol. 1971;10(3):341–352. doi: 10.3109/02841867109130798. [DOI] [PubMed] [Google Scholar]
- 48.Walden TL, Farzaneh NK. Biochemical response of normal tissues to ionizing radiation. In: Gutin PH, Liebel SA, Sheline GE, editors. Radiation injury to the nervous system. New York: Raven Press; 1991. pp. 17–36. [Google Scholar]
- 49.Prosser CL. Clinical sequence of physiological effects of ionizing radiation in animals. Radiology. 1947;49:299–312. doi: 10.1148/49.3.299. [DOI] [PubMed] [Google Scholar]
- 50.Osborn GK, Jones DC, Kimeldorf DJ. The effect of age at exposure on radiation-induced polydipsia in the rat. Radiat Res. 23:119–127. [PubMed] [Google Scholar]
- 51.Pento JT, Kenny AD. The influence of whole-body irradiation on calcium and phosphate homeostasis in the rat. Radiat Res. 1975;63(3):468–473. [PubMed] [Google Scholar]
- 52.Edelmann A, Eversole WJ. Changes in antidiuretic activity in rat serum after X-irradiation. Am Physiol Soc. 1950;163:709. [Google Scholar]
- 53.Goodman RD, Lewis AE, Schuck EA. Effects of x-irradiation on gastrointestinal transit and absorption availability. Am J Physiol. 1952;169(1):242–247. doi: 10.1152/ajplegacy.1952.169.1.242. [DOI] [PubMed] [Google Scholar]
- 54.Dublineau I, Ksas B, Joubert C, Aigueperse J, Gourmelon P, Griffiths NM. Alterations in water and electrolyte absorption in the rat colon following neutron irradiation: influence of neutron component and irradiation dose. Int J Radiat Biol. 2002;78(12):1127–1138. doi: 10.1080/0955300021000019221. [DOI] [PubMed] [Google Scholar]
- 55.Shideler CE, Johns ME, Cantrell RW, Shipe JR, Wills MR, Savory J. Erythrocyte polyamine determinations in patients with head and neck cancer. Arch Otolaryngol. 1981;107(12):752–754. doi: 10.1001/archotol.1981.00790480028007. [DOI] [PubMed] [Google Scholar]
- 56.Dicker AP. COX-2 inhibitors and cancer therapeutics: potential roles for inhibitors of COX-2 in combination with cytotoxic therapy: reports from a symposium held in conjunction with the Radiation Therapy Oncology Group June 2001 Meeting. Am J Clin Oncol. 2003;26(4):S46–S47. doi: 10.1097/01.COC.0000074180.16144.B3. [DOI] [PubMed] [Google Scholar]
- 57.Liang L, Hu D, Liu W, Williams JP, Okunieff P, Ding I. Celecoxib reduces skin damage after radiation: selective reduction of chemokine and receptor mRNA expression in irradiated skin but not in irradiated mammary tumor. Am J Clin Oncol. 2003;26(4):S114–S121. doi: 10.1097/01.COC.0000074149.95710.40. [DOI] [PubMed] [Google Scholar]
- 58.Hancock SL, McDougall IR, Constine LS. Thyroid abnormalities after therapeutic external radiation. Int J Radiat Oncol Biol Phys. 1995;31(5):1165–1170. doi: 10.1016/0360-3016(95)00019-U. [DOI] [PubMed] [Google Scholar]
- 59.Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell JB, Biaglow JE, Russo A. Role of glutathione and other endogenous thiols in radiation protection. Pharmacol Ther. 1988;39(13):269–274. doi: 10.1016/0163-7258(88)90072-1. [DOI] [PubMed] [Google Scholar]
- 61.Okunieff P, Suit HD. Toxicity, radiation sensitivity modification, and combined drug effects of ascorbic acid with misonidazole in vivo on FSaII murine fibrosarcomas. J Natl Cancer Inst. 1987;79(2):377–381. [PubMed] [Google Scholar]
- 62.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23(34):8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 63.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, et al. Radioprotective gene therapy. Curr Gene Ther. 2003;3(3):183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 64.Greenberger JS, Epperly MW. Review. Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization. In Vivo. 2007;21(2):141–146. [PubMed] [Google Scholar]
- 65.Belikova NA, Jiang J, Tyurina YY, Zhao Q, Epperly MW, Greenberger J, et al. Cardiolipin-specific peroxidase reactions of cytochrome C in mitochondria during irradiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2007;69(1):176–186. doi: 10.1016/j.ijrobp.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 66.Okunieff P, Li M, Liu W, Sun J, Fenton B, Zhang L, et al. Keratinocyte growth factors radioprotect bowel and bone marrow but not KHT sarcoma. Am J Clin Oncol. 2001;24(5):491–495. doi: 10.1097/00000421-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 67.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 68.Okunieff P, Mester M, Wang J, Maddox T, Gong X, Tang D, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiat Res. 1998;150(2):204–211. [PubMed] [Google Scholar]
- 69.Miller SM, Ferrarotto CL, Vlahovich S, Wilkins RC, Boreham DR, Dolling JA. Canadian Cytogenetic Emergency network (CEN) for biological dosimetry following radiological/nuclear accidents. Int J Radiat Biol. 2007;83(7):471–477. doi: 10.1080/09553000701370860. [DOI] [PubMed] [Google Scholar]
- 70.Blakely WF, Salter CA, Prasanna PG. Early-response biological dosimetry--recommended countermeasure enhancements for mass-casualty radiological incidents and terrorism. Health Phys. 2005;89(5):494–504. doi: 10.1097/01.hp.0000175913.36594.a4. [DOI] [PubMed] [Google Scholar]
- 71.Thierens H, De Ruyck K, Vral A, de Gelder V, Whitehouse CA, Tawn EJ, et al. Cytogenetic biodosimetry of an accidental exposure of a radiological worker using multiple assays. Radiat Prot Dosimetry. 2005;113(4):408–414. doi: 10.1093/rpd/nch483. [DOI] [PubMed] [Google Scholar]
- 72.Dertinger SD, Miller RK, Brewer K, Smudzin T, Torous DK, Roberts DJ, et al. Automated human blood micronucleated reticulocyte measurements for rapid assessment of chromosomal damage. Mutat Res. 2007;626(12):111–119. doi: 10.1016/j.mrgentox.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moiseenko VV, Battista JJ, Hill RP, Travis EL, Van Dyk J. In-field and out-of-field effects in partial volume lung irradiation in rodents: possible correlation between early DNA damage and functional endpoints. Int J Radiat Oncol Biol Phys. 2000;48(5):1539–1548. doi: 10.1016/s0360-3016(00)00802-6. [DOI] [PubMed] [Google Scholar]
- 74.Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikhov I, Khan N, et al. In vivo EPR dosimetry to quantify exposures to clinically significant doses of ionising radiation. Radiat Prot Dosimetry. 2006;120(14):163–170. doi: 10.1093/rpd/nci554. [DOI] [PubMed] [Google Scholar]
- 75.Anscher MS, Peters WP, Reisenbichler H, Petros WP, Jirtle RL. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med. 1993;328(22):1592–1598. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 76.Zhao L, Sheldon K, Chen M, Yin MS, Hayman JA, Kalemkerian GP, et al. The predictive role of plasma TGF-beta1 during radiation therapy for radiation-induced lung toxicity deserves further study in patients with non-small cell lung cancer. Lung Cancer. 2007 Sep 28; doi: 10.1016/j.lungcan.2007.08.010. 2007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 77.Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst. 1996;88(13):918–922. doi: 10.1093/jnci/88.13.918. [DOI] [PubMed] [Google Scholar]
- 78.Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82(9):605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- 79.Chaudhry MA. Bystander effect: biological endpoints and microarray analysis. Mutat Res. 2006;597(12):98–112. doi: 10.1016/j.mrfmmm.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Rieger KE, Hong WJ, Tusher VG, Tang J, Tibshirani R, Chu G. Toxicity from radiation therapy associated with abnormal transcriptional responses to DNA damage. Proc Natl Acad Sci U S A. 2004;101(17):6635–6640. doi: 10.1073/pnas.0307761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quarmby S, West C, Magee B, Stewart A, Hunter R, Kumar S. Differential expression of cytokine genes in fibroblasts derived from skin biopsies of patients who developed minimal or severe normal tissue damage after radiotherapy. Radiat Res. 2002;157(3):243–248. doi: 10.1667/0033-7587(2002)157[0243:deocgi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 82.Ho AY, Atencio DP, Peters S, Stock RG, Formenti SC, Cesaretti JA, et al. Genetic predictors of adverse radiotherapy effects: the Gene-PARE project. Int J Radiat Oncol Biol Phys. 2006;65(3):646–655. doi: 10.1016/j.ijrobp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Mayo MS, Gajewski BJ, Morris JS. Some statistical issues in microarray gene expression data. Radiat Res. 2006;165(6):745–748. doi: 10.1667/RR3576.1. [DOI] [PubMed] [Google Scholar]


