Abstract
We report the development of a new mosquito aspirator with the same aspiration capacity (airflow) of the CDC Backpack Aspirator (CDC-BP), but smaller and lighter (0.8 kg without battery), less expensive (US$45–70), easier to build, and compatible with the use of telescoping extension poles to access hard-to-reach locations. The performance of this new aspirator, named “Prokopack,” was compared with the CDC-BP in laboratory settings as well as in paired collections in combined sewer overflow (CSO) tunnels in Atlanta, GA, and indoor mosquito collections in Iquitos, Peru. The difference in suction power between both aspirators (average, 0.29–0.43 m/s) was negligible. However, 2.3 times more mosquitoes were collected using the Prokopack in the upper wall (> 1.5 m) and ceilings of CSO tunnels than with the CDC-BP in lower walls. Indoor collection in Iquitos yielded significantly more total mosquito numbers [including Culex pipiens complex, Culex (melanoconion) sp., and Mansonia sp.] and Aedes aegypti (L.) in the Prokopack than in the CDC-BP. Our results demonstrate the effectiveness of the Prokopack to collect different mosquito species in different epidemiological settings.
Keywords: sampling, mosquito abundance, mosquito aspirator
Knowledge of adult mosquito population density is paramount to our understanding of host–vector contacts, pathogen inoculation rates, and, ultimately, vector-borne disease transmission risk (Lehane 2005, Silver 2008). A myriad of mosquito collection methods exist; most of them have limitations in their sensitivity and bias toward preferentially collecting mosquitoes of certain stages or nutritional states, or they are limited operationally by their cost or convenience for massive and long-term deployment (Silver 2008).
Human landing-biting and pyrethroid knockdown collections, although relatively inexpensive, are time-and personnel-consuming, subject to interoperator and location heterogeneity, and impractical in many urban environments (Silver 2008). Active traps (e.g., gravid, CDC-light trap, BG-sentinel trap), although more effective at capturing large numbers of mosquitoes, are biased toward collections of female and target particular species and life stages or nutritional status (Silver 2008). In addition, the high cost (e.g., US$100–200 per trap) and the dependence on personnel for setup and recovery preclude their use for large-scale collections, particularly in resource-poor settings.
Battery-powered aspirators collect mosquitoes of both sexes and all physiological stages directly from their resting sites, allowing better estimations of richness (number of different mosquito species in a given area), abundance, sex ratio, age structure, and physiological condition of sampled populations (Silver 2008). The CDC-Backpack Aspirator (CDC-BP) is considered the most effective method for indoor collections of certain domestic mosquito species such as Aedes aegypti (L.) (Edman et al. 1992, Clark et al. 1994, Reiter and Gubler 1997, Scott et al. 2000, Scott and Morrison 2008). Created by improving the AFS sweeper (Meyer et al. 1983), the CDC-BP is designed to provide the needed suction and increased maneuverability with a backpack design (Clark et al. 1994). However, its heavy weight (12 kg, including battery) coupled with its nonextendable and primarily rigid suction hose makes it difficult to maneuver in confined spaces, dramatically limiting the ability to aspirate mosquitoes indoors. Furthermore, because its reach cannot be extended, it cannot be used to collect mosquitoes in elevated and out of reach locations such as upper walls, ceilings, and under beds. As a consequence, mosquito collections commonly yield low capture rates (Scott and Morrison 2008). Finally, commercially available CDC-BPs are very expensive (US$ 468–758 in the United States), limiting their potential for widespread mosquito surveillance.
Here, we report the development of a new mosquito aspirator with the same aspiration capacity of the CDC-BP but which is more maneuverable (smaller and lighter), less expensive, easy to build, and compatible with telescoping extension poles to access hard to reach locations. The performance of this new aspirator, named “Prokopack” (patent pending), was compared with the CDC-BP in the laboratory and in two epidemiological settings: collecting overwintering mosquitoes in urban tunnels in Atlanta, GA, and indoor mosquito collections in Iquitos, Peru.
Materials and Methods
Device Description
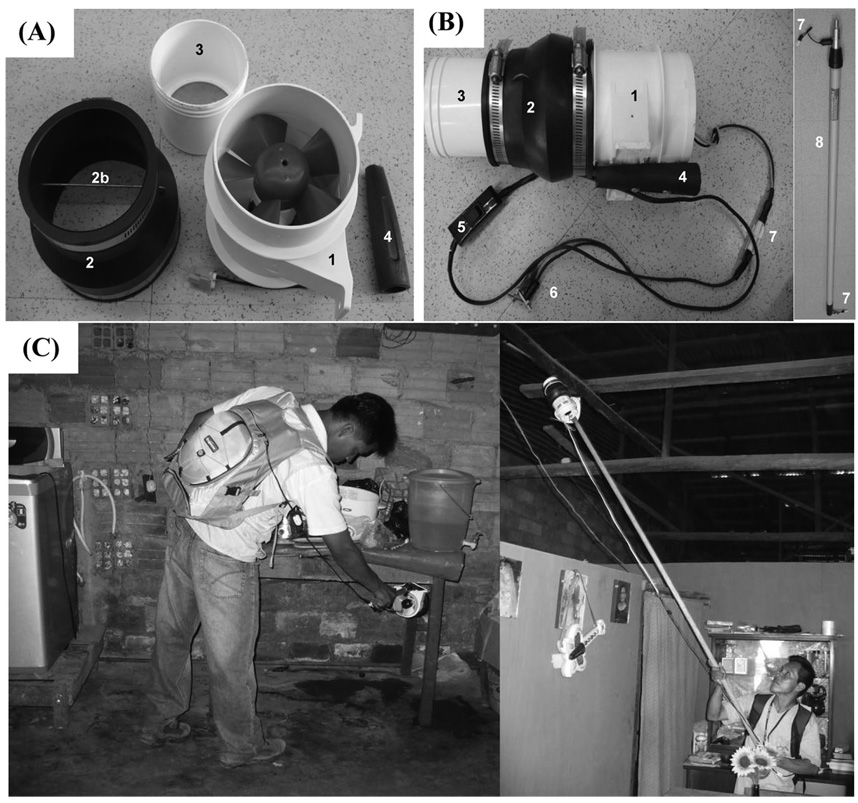
Unlike the CDC-BP, the Prokopack has its light-weight motor located close to the collection cups and its 12-V battery mounted on the operator’s back, reducing the overall weight and bulkiness of the unit (Fig. 1). The screened collection cups used with the CDC-BP also are used with the Prokopack. Several commercially available battery-powered motors were tested. A 10.2-cm (4-inch) inline blower designed for boat ventilation systems (Rule model 240, ITT Co., White Plains, NY) was chosen for use in the Prokopack due to its suction power, light-weight (0.5-kg) low rate of power consumption (4.3 amps), and water resistance. A 4:3-inch flexible rubber coupling (PlumbQuik model P1056-43, Fernco Inc., Davison, MI) commonly found in hardware stores was added to the aspirating end of the blower (Fig. 1). Between the coupler and the fan a thick wire and a piece of metal mesh were added to separate the cups that contain captured mosquitoes from the fan. Straight wire with an on/off switch was connected to a 12 VDC 17 ampHR sealed-electrolyte battery (Enersys, Reading, PA). A threaded handle from a paint roller was bolted to the side of the blower. A 2–4-m adjustable length extension painting pole was threaded with a coiled (telephone style) electric wire to allow for easy extension and collapse of the pole without excess wire. The in-line blower can be purchased online or in boating stores of >50 countries (www.rule-industries.com), whereas all other parts can be easily obtained in any hardware store. The total weight of the aspirator (without battery) is 0.88 kg (3.0 kg with battery), the cost for construction materials is ≈$45 for the aspirator and $25 for the telescoping pole extension (not including collection cups and batteries); assembling one unit takes ≈1 h.
Fig. 1.
Unassembled (A) and assembled (B) Prokopack aspirator and extension pole. (C) The Prokopack being used in indoor collections of mosquitoes from low (right) and high (left) locations in Iquitos, Peru. (1) In-line blower motor. (2) 4:3-inch rubber coupler. (2b) Rigid wire to prevent the cups to get close to the fan. (3) Collection cup. (4) Threaded paint handle. (5) In-line on/off switch. (6) Ring terminal. (7) Ballast disconnect. (8) Telescoping extension pole.
Laboratory and Field Tests
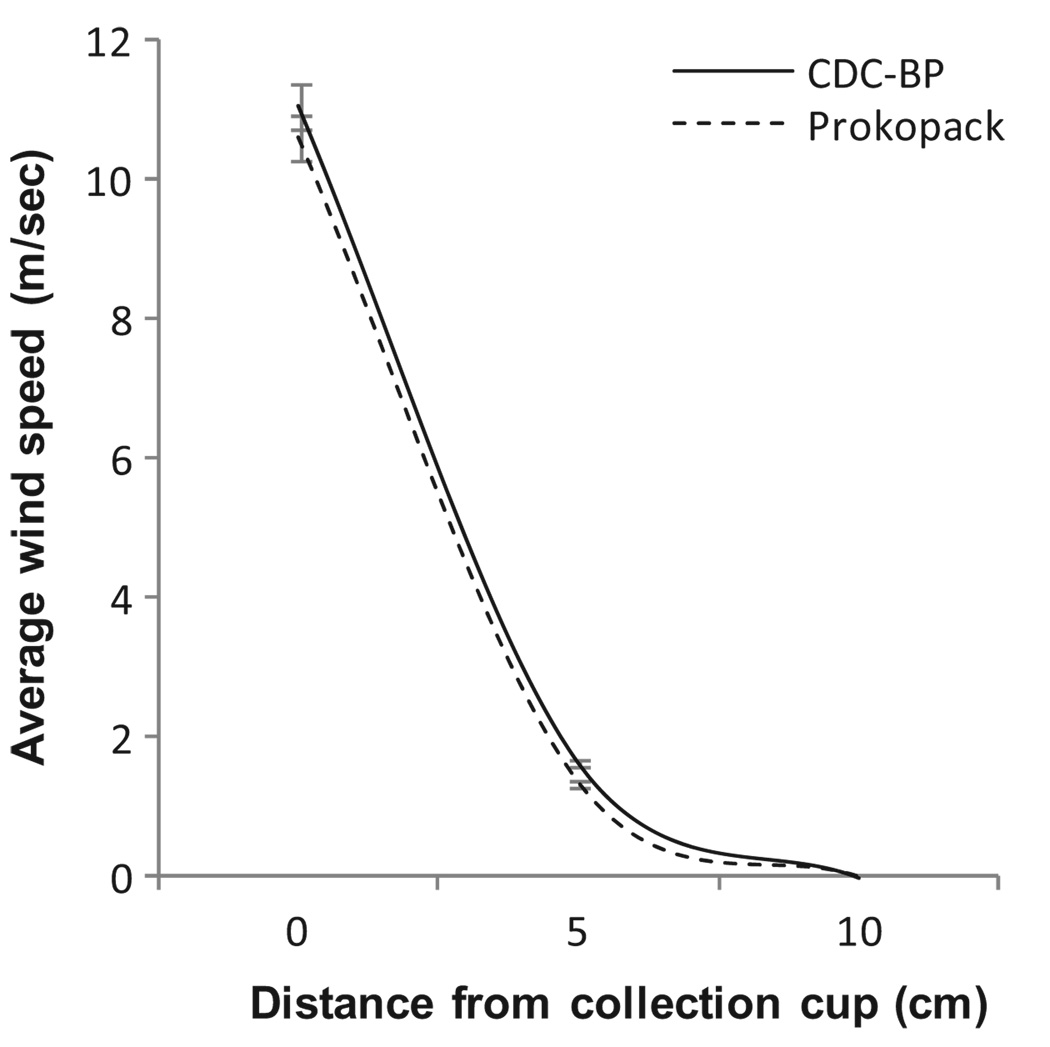
We aimed to have a motor with the same aspirating power of the CDC-BP because blowers that have more suction generally injure or kill mosquitoes (Clark et al. 1994). We measured the aspiration power of a brand-new CDC-BP (John W.Hook, Gainesville, FL) and of the Prokopack at 0, 5, and 10 cm from end of collection cup by using a hand-held digital wind gauge (Kestrel 4000; Kestrel Meters, Sylvan Lake, MI). For each aspiration device and distance, we recorded the average wind speed over a 1-min interval for a total of 10 repetitions.
From 24 November 2008 to 11 March 2009, two combined sewer overflow (CSO) tunnels (Greensferry and Tanyard Creek) in Atlanta, GA, were visited to collect overwintering mosquitoes by using one Prokopack in the upper walls (above 1.5 m) and ceiling and one CDC-BP in the lower walls (<1.5 m). Seven 10-m sections of the tunnels (three in Greensferry and four in Tanyard) were carefully aspirated by three field technicians with the aid of flashlights to spot overwintering mosquitoes. Collection effort was fixed (≈20 min per tunnel section) for each aspirator. We aimed to assess how our collections could be improved by aspirating on the upper wall and ceiling. The tunnel concrete surface walls were uneven and required maneuvering around pipes and drains, the ceilings were high (up to 5 m), and some surfaces were partially wet. Collected mosquitoes were kept alive in glass breeding chambers (30×30×30 cm) containing a 10% sucrose solution and then identified by species and individually stored at −80°C for further virus testing.
During 7–22 May 2009, a paired trial between the Prokopack and the CDC-BP was performed in 71 houses in Iquitos, Peru. Randomly selected houses were visited by two field technicians who tested the performance of each mosquito aspirator in indoor collections. At each house, a collection sequence alternating the use of the CDC-BP and the Prokopack in the lower (<1.5 m) walls and furniture was followed. After using one of the aspirators (e.g., Prokopack), the same technician was in charge of repeating the collection with the alternative aspirator (e.g., CDC-BP), making sure to cover a similar area as in the initial collection. Concurrently with the lower wall collections, a Prokopack with an extension pole was used to collect the mosquitoes resting on the higher (>1.5 m) walls and the ceiling. Collection effort in each house was fixed (≈10 min) for each aspirator. Aspiration was performed in all rooms and hallways of each house as described by Scott et al. (2000) and collected mosquitoes were processed as described above. In a first assessment, we found several damaged mosquitoes, because the collection cups were too close to the aspirator fan. We fixed this problem by adding a rigid wire transversally at 2.5 cm from the end of the rubber coupler (see 2b in Fig. 1A).
Results and Discussion
In the laboratory tests, the average suction power of both aspirators decreased exponentially from an average of 11.0-10.6 m/s at 0 cm to 0.0 m/s at 10 cm from the collection cup (Fig. 2). The difference in suction power between both aspirators (average, 0.43 m/s at 0 cm and 0.29 m/s at 5 cm) was negligible.
Fig. 2.
Average aspiration power (measured in meters per second) for the CDC-Backpack aspirator (CDC-BP) and the Prokopack at increasing distances from the collection cup. Gray vertical lines show the standard error of measurements.
In total, 132 mosquitoes (120 females and 12 males) were collected in CSO tunnels in Atlanta, 40 in the lower wall by using the CDC-BP and 24 and 68 in the upper wall and the ceiling, respectively, with the Prokopack. Although not statistically significant, the percentage of tunnel sections infested by overwintering mosquitoes on the upper wall and ceiling (9/42; 21.4%) was much higher (threefold) than on the lower wall (3/42; 7.1%) (Fisher exact test, χ2 = 3.5, P = 0.058). From the 90 identified mosquitoes, 96.7% were Culex pipiens complex, 2.2% Culex territans (Walker), and 1.1% Culex erraticus (Dyar & Knab). No signs of damage were observed in the collected live mosquitoes, and they survived at room temperature for an average of 3.4 d (SD, 2.4 d) after being collected.
Table 1 summarizes the results of the paired collections for all mosquito species and for Ae. aegypti in Iquitos. Although the Prokopack detected mosquitoes in more houses than the CDC-BP (Table 1), the difference was not statistically significant either for all mosquitoes (McNemar test for paired samples χ2 < 1.8, P > 0.18) or for Ae. aegypti (χ2 < 2.5, P > 0.11). When comparing the total number of mosquitoes collected per house, the Prokopack collected significantly more mosquitoes when used before (Wilcoxon signed rank test for paired samples, Z = −2.98, P = 0.003) but not after (Z = −0.474, P = 0.635) the CDC-BP. The same analysis performed for Ae. aegypti collections showed that the Prokopack outperformed the CDC-BP both when used before (Z = −2.14, P = 0.032) and after (Z = −2.30; P = 0.02) the CDC-BP. Unlike the CSO tunnels, a small proportion of mosquitoes (13–17%) and Ae. aegypti (14–17%) was found resting in the upper walls and ceilings of the houses (Table 1). We attribute the low Ae. aegypti yields by either collection method to the peak of low vector abundance prevailing in Iquitos during May (Morrison et al. 2004) coupled to the random inclusion of houses located in the periphery of the city, where this species is less abundant (Morrison et al. 2004).
Table 1.
Paired CDC-backpack aspirator and Prokopack mosquito collections performed in houses of the city of Iquitos, Peru
| All speciesa |
Ae. aegypti |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Collection sequenceb |
No. houses |
% houses positive only BP |
% houses positive only PKP |
No. collected |
% houses positive only BP |
% houses positive only PKP |
No. collected |
||||
| BP | PKP low |
PKP high |
BP | PKP low |
PKP high |
||||||
| BPc→PKPd | 36 | 16.7 | 16.7 | 359 | 504 | 183 | 5.6 | 22.2 | 8 | 29 | 6 |
| PKP→BP | 35 | 5.7 | 20.0 | 201 | 452 | 97 | 5.7 | 20.0 | 3 | 17 | 4 |
Includes Cx. pipiens complex, Ae. aegypti, Mansonia sp., and Cx. (Melanoconion) sp.
Represents the collection sequence each aspirator was used at each house (first → second) to collect mosquitoes from the lower (<1.5 m) walls and furniture. In all houses, the lower wall collections were paired with upper (>1.5 m) wall and ceiling collections by using a Prokopack with an extension pole.
BP, CDC-backpack aspirator.
PKP, Prokopack.
In indoor collections in Iquitos, the Prokopack collected 4.5 times more Mansonia sp. (9/2), 4.2 times more Ae. aegypti (53/11), 2.3 times more Culex pipiens complex (1,079/475), and 1.3 times less Culex (melanoconion) sp. (26/33) mosquitoes than the CDC-BP. Furthermore, most (86.7%) blood-feed Ae. aegypti females were collected with the Prokopack, suggesting a better ability of the latter to reach mosquito resting places. This large improvement in collecting blood-fed mosquitoes with the Prokopack (more than 6 times the proportion collected by the CDC-BP) is of particular importance in epidemiological studies of disease vectors.
Our results are conclusive about the effectiveness of the Prokopack to collect different mosquito species in different epidemiological settings. There is a need for effective and affordable mosquito sampling methods. Aspiration devices such as the Prokopack not only will increase the coverage (one team of two technicians can cover up to 20–30 houses a day) but also the quality of the entomologic data obtained, particularly for blood-fed mosquitoes. We also believe that the lower cost of our device (US$45–70) will make it affordable for countries where limited resources make impractical the purchase of the CDC-BP or other battery-powered trapping devices for mosquito surveillance.
Acknowledgments
We thank Jim McNelly, Abdurrahman Bouzid, Alexandra Vannostrand, and Donal Bisanzio for helping with the Atlanta CSO tunnel collections, and Thomas W. Scott and Amy Morrison for support of the testing of the Prokopack in Iquitos. Helvio Astete helped coordinate the collections in Iquitos. This project was supported by funds from Emory University and the National Institutes of Health National Institute of Allergy and Infectious Diseases award R01 AI069341-01.
References Cited
- Clark GC, Seda H, Gubler DJ. Use of the “CDC Backpack Aspirator” for Surveillance of Aedes aegypti in San Juan, Puerto Rico. J. Am. Mosq. Control Assoc. 1994;10:119–124. [PubMed] [Google Scholar]
- Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti in Thailand rarely feed on sugar. J. Med. Entomol. 1992;29:1035–1038. doi: 10.1093/jmedent/29.6.1035. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. The biology of blood-sucking in insects. 2nd. Cambridge, United Kingdom: Cambridge University Press; 2005. [Google Scholar]
- Meyer RP, Reisen WK, Hill BR, Martinez VM. The “AFS Sweeper,” a battery-powered backpack mechanical aspirator for collecting adult mosquitoes. Mosq. News. 1983;43:346–350. [Google Scholar]
- Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, Watts D, Stancil JD, Olson JG, Blair P, Scott TW. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J. Med. Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- Reiter P, Gubler DJ. Surveillance and control of urban dengue vectors. In: Gubler DJ, Kuno G, editors. Dengue and dengue hemorrhagic fever. New York: CAB International; 1997. pp. x–x. [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J. Med. Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Scott TW, Morrison AC. Vector-borne diseases: understanding the environmental, human health, and ecological connections. Washington, DC: The National Academies Press; 2008. Longitudinal field studies will guide a paradigm shift in dengue prevention; pp. 132–149. [PubMed] [Google Scholar]
- Silver JB. Mosquito ecology: field sampling methods. 3rd. New York: Springer; 2008. [Google Scholar]