Abstract
Two interrelated Omani families are described with eight children manifesting a genetic disorder with widespread brain calcifications. Brain imaging showed extensive scattered calcifications of basal ganglia and cortex, suggesting possible Aicardi-Goutiéres syndrome (AGS) or Coats’ Plus syndrome. However, the clinical features in the present families diverge substantially from these two conditions. Growth delay, mild developmental delay and poor school performance were present in all affected individuals, but progressive deterioration of neurological function was not apparent, nor were there significant cortical whitematter disease or retinopathy. Genome-wide linkage and fine-mapping analyses of the extended family members and affected individuals indicate a genetic locus for this disorder on Chromosome 2 with a LOD score of 6.17. The Chromosome 2 locus is novel and the clinical presentation displays features distinguishing the condition from either Coats’ or AGS, making this a new variant or possibly a new disorder of inherited brain calcification.
Keywords: brain calcifications, hydrocephalus, autosomal recessive inheritance, microcephaly, developmental delay, Aicardi-Goutiéres syndrome, Coats’ Plus syndrome
INTRODUCTION
More than 50 acquired and familial conditions that involve bilateral calcium accumulation in the brain parenchyma, sometimes referred to as brain calcinosis, have been reported [Baba et al., 2005]. In children, intracerebral calcifications are most often acquired in association with intrauterine infections, intrauterine or early postnatal hemorrhage, or neoplasia. Some features of early onset cerebral calcifications may be shared between inherited and acquired conditions, but the appearance of multiple affected individuals in a single family assures an underlying genetic cause.
Many hereditary forms of brain calcinosis involve the basal ganglia, but only rarely do they involve both deep nuclear structures and cortical grey matter. Several of them have overlapping phenotypes, and probably represent examples of the same entity. Several of the familial forms of brain calcifications may be recognized by their associated endocrinopathies, which are characterized by abnormalities of calcium, phosphorous and parathyroid hormone (PTH). These include hypoparathyroidism [Ahn et al., 1986] and pseudohypoparathyroidism (Albright hereditary osteodystrophy) [Patten et al., 1990; Juppner et al., 1998]. Other metabolic disorders associated with brain calcification include carbonic anhydrase II deficiency [Sly et al., 1985], Krabbe disease [Farley et al., 1992], dihydropteridine reductase deficiency [Woody et al., 1989], and mitochondriopathies including MELAS and MERRF [Hirano and Pavlakis, 1994].
Another group of brain calcinoses is associated with immune system disorders and systemic involvement. Examples include systemic lupus erythematosis (SLE) [Baba et al., 2005], IgG (lambda-M) proteinemia, and autoimmune polyglandular syndrome (AIRE) [Baumert et al., 1993]. A significant member of this class is Aicardi-Goutiéres syndrome (AGS), a genetically heterogeneous autosomal recessive encephalopathy associated with CSF lymphocytosis and elevated alpha-interferon [Aicardi and Goutieres 1984; Lebon et al., 1988; Tolmie et al., 1995].
A similar genetic syndrome distinguished by retinopathy and extensive cerebral calcification is cerebroretinal microangiopathy with calcifications and cysts (CRMCC) or “Coats’ plus” syndrome [Tolmie et al., 1988; Crow et al., 2004; Briggs et al., 2008]. Other rare causes of intracerebral calcification have been reported such as the pseudo-TORCH syndrome, a very rare autosomal recessive disorder that resembles intrauterine infections, consisting of severe postnatal microcephaly, seizures and profound mental retardation, fronto-parietal polymicrogyria, and a distinct pattern of frontal predominant, band-like intracranial calcifications [Burn et al., 1986; Reardon et al., 1994; Briggs et al., in press]. Brain calcinosis has also been reported with prominent extrapyramidal movement disorders, as in autosomal dominant dystonia or idiopathic basal ganglia calcification (Fahr Disease) [Geschwind et al., 1999]. In still other brain calcification syndromes, bone dysplasia may be prominentas in Raine syndrome or polycystic lipomembranous osteodysplasia [Kan and Kozlowski 1992; Al Mane et al., 1996].
Here we describe a novel autosomal recessive syndrome consisting of early developmental delay, small stature, poor school performance and extensive brain calcifications in 8 individuals from two consanguineous and interrelated sibships, whose clinical features differed from described brain calcinoses. Linkage analysis mapped the causative locus to 2q36.2, indicating a new gene associated with intracerebral calcification.
PATIENTS AND METHODS
Clinical studies
Two Omani families from the same tribal unit were studied. The two couples and eight affected children were examined by A.R. and M.E.R. and the brain imaging results were reviewed independently by M.E.R and W.B.D. Plain skull films were obtained on all affected children and calcifications were apparent. Population based growth curves have not been established for Oman. Experience indicates that head circumferences are equivalent to those published in the UK and US, but height and weight curves of Omani children between the ages of 2 and 20 years are shifted approximately −1.5 SD, so that the 50th centile for Oman corresponds to −1.5 SD on growth curves in the West. Therefore, percentiles here were based on the growth curves published by the Center for Disease Control (CDC) and adjustments for height and weight were also estimated for the Omani population. Brain computerized tomography (CT) was performed and reviewed on all affected individuals and parents and magnetic resonance imaging (MRI) was obtained on the eldest daughter in family #1 at age 18. Medical records were reviewed by A.R. and skeletal surveys were reviewed by M.E.R. with orthopaedic specialists at the Hospital for Special Surgery, New York.
Genotyping
Genomic DNA was purified from the two parental couples and their 15 children after obtaining informed consent in accordance with protocols approved by the Institutional Review Board of Weill Cornell Medical College, and the Office of Human Research Protection, Ministry of Health, Sultanate of Oman. DNA was extracted from peripheral blood samples using commercial kits (Qiagen). The Affymetrix 50K XbaI SNP Array was used for genotyping as per the standard protocol described elsewhere [Matsuzaki et al., 2004] by The University of Chicago Functional Genomics Facility. Fine mapping was performed using established microsatellite markers from the deCODE genetic map identified through the NCBI Human Map Viewer (Build 35). PCR reactions were performed in 10 μl volumes that contained 50 ng genomic DNA; 0.25 U Platinum Taq DNA polymerase (Invitrogen); 1X PCR buffer, minus Mg; 1.5 mM MgCl2; 250 μM primers; and 250 μM dNTPs. Amplification was performed using the following PCR conditions (95°C × 75 s, 10 cycles of 94°C × 45 s, 55°C × 45 s, 72°C × 75s, 30 cycles of 89°C × 45 s, 55°C × 55 s, 72°C × 45 s followed by a final extension of 72°C × 10 min). Amplified microsatellites were sequenced (ABI 3130 capillary DNA sequencer) and fragment analysis was performed using the ABI GeneMapper v4.0 analysis software.
Linkage analysis
Of the 58,690 total SNPs, 2,186 were discarded from analysis because they were not placed on the deCODE map or had a missing rate > 25%. Mendelian incompatibilities were identified using PedCheck [O’Connell and Weeks, 1998]. Any markers that were associated with Mendelian errors were dropped from subsequent analysis. Linkage analysis was initially performed at 1 cM intervals across each chromosome using Allegro 2.0 [Gudbjartsson et al., 2005]. Parametric LOD scores assuming a fully penetrant autosomal recessive mode of inheritance and a disease allele frequency estimated at 1/10,000 were calculated.
RESULTS
The parents in each family were first cousins and both girls and boys were affected, consistent with autosomal recessive inheritance (Fig 1A). The consanguinity, lack of cerebral calcifications in CT scans of parents, and frequency of affected children supported a fully penetrant autosomal recessive disorder, though an autosomal dominant mutation with incomplete penetrance could not be entirely ruled out.
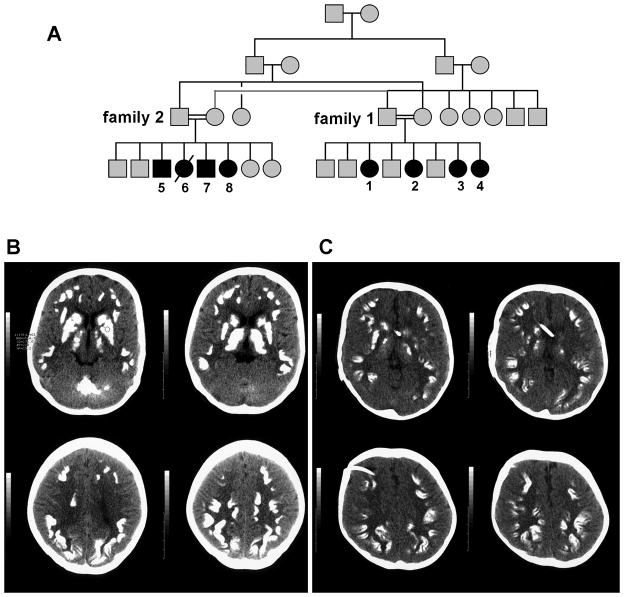
Figure 1.
(A) Pedigree of extended families 1 and 2. Brain CT images from Patient 1 (B) and Patient 4 (C) show extensive brain involvement with calcifications of deep cerebellar nuclei, caudate, putamen, thalamus, and cerebral cortex. Cerebral cortical calcifications tend to occur at the depths of sulci where U-fibers are located.
History and physical examination
Salient clinical features are summarized in Table I. Birth parameters are noted below when available, but none of the affected children were thought to be unusually small or underweight as neonates.
Table I.
Summary of clinical features of affected patients
| Patient |
||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Widespread Brain calcifications | + | + | + | + | + | + | + | + |
| Microcephaly (−2 to −3 SD) | + | + | + | + | + | + | + | + |
| Postnatal growth delay | + | + | + | + | + | + | + | + |
| Short stature | + | + | + | + | + | + | + | + |
| Thin build | + | + | + | + | + | + | + | + |
| Poor muscle mass | + | + | + | + | + | + | + | + |
| Learning difficulties | + | + | + | + | + | NA | + | − |
| Reduced exercise tolerance | + | + | + | + | + | + | + | + |
| Headaches | + | + | + | + | + | NA | + | − |
| Bone Fractures | − | − | − | + | − | − | − | − |
| Thin cortex bone | + | + | + | + | + | NA | + | + |
| Delayed bone age | + | + | + | + | + | NA | + | + |
| Anemia | + | + | + | + | + | NA | + | + |
Family 1
Patient 1, the eldest affected, was a female with family history of intracerebral calcifications in three other siblings. She was born at full term by normal vertex delivery with birth weight 2.8 kg (−1 SD for length), length 50 cm and occipito-frontal head circumference (OFC) 30 cm (−2.8 SD), and had no perinatal problems. Her language development was slow with a small vocabulary, and she did not walk until two years, later than her older brothers. She was noted to have short stature, thin build, and reduced vigour compared to school mates, but had no major illnesses during childhood. Her parents described school difficulties with poor academic progress, but normal behavior. She complained of periodic headaches from age 15 years, and at about that age lost the ability to walk fast or run. Brain calcifications were discovered at 17 years during family screening for brain calcifications diagnosed in a younger sister. She performed activities of daily living independently, but her fine motor coordination was poor. When examined at 18 years, her weight was 29 kg (− 5 SD on CDC charts, estimated − 3.5 SD in the Omani population; hereafter −5/− 3.5 SD), height 141 cm (− 5/− 3.5 SD) and OFC 50 cm (−3 SD). She had no dysmorphic features or skin changes, and her hair and nails were normal. Speech was fluent with a normal cranial nerve exam. She walked without ataxia or spasticity but was unable to run. Tendon reflexes were mildly brisk.
Patient 2 was the product of a full term normal delivery, birth weight 2.9 kg (−1 SD for length), length 50 cm and OFC 32 cm (−2 SD). Like patient 1, her postnatal development was slow, though her learning difficulties were less severe than her affected sisters. At age 14 years her school evaluations indicated cognitive skills commensurate with a 10 year old. Examined at age 14 years, she was not dysmorphic except that low set ears were noted. Skin, hair and nails were normal with no lesions. She weighed 17.3 kg (−5/−3.5 SD), height measured 124 cm (− 5/− 3.5 SD), and OFC 47 cm (−3.3 SD).
Patient 3 was 11 years of age when examined. Slow growth and developmental delay was noted from infancy. Her weight was 13.9 kg (−4.5/−3 SD), height 113 cm (−5/−3.5 SD) and OFC 47 cm (−3 SD). She was said to suffer severe learning difficulties, and was unable to read; her speech was difficult to understand. She was pleasant and cooperative with thin build and short stature. There were no skin lesions or chilblains, but palmar erythema was noted. Fundoscopic exam was normal with no retinal exudates and normal retinal vessels. Cognitive difficulties were more pronounced than her sisters and considerable dysarthria was apparent. She had mild dyspraxia and her ability to imitate movements was limited. Gait was slightly broad based with reduced arm swing. She was unable to run or move fast. Deep tendon reflexes were brisk.
Patient 4 was seen at 7 years of age. She was the product of a full term normal delivery. Early infancy was uneventful, but an unusual increase in head size was noted by age 3 months. A transcranial ultrasound at age 6 months diagnosed right-sided hydrocephalus and brain calcifications, and she appeared lethargic and was not feeding well. Serological investigations for congenital infections were negative. She had a ventriculo-peritoneal (VP) shunt inserted at 8 months of age. Calcifications were evident on imaging at that time. Her activity and growth improved post shunt. Seizures were noted one year post VP shunt insertion and she was placed on anticonvulsants. Like her sisters, she was slow to sit up, walk and talk. Mild to moderate learning difficulties were noted by her parents. She assisted with dressing but was not independent. At the age of 5 years she underwent an operative revision of the shunt that was complicated by seizures. At age 6 years she sustained a fracture of the left arm. At age 7 her weight was 13.4 kg (−3.5/−2 SD), height 100 cm (−5/−3.5 SD), and OFC 47 cm (−2.3 SD). Cognitive development was delayed but social interactions were normal. Speech was intelligible without dysarthria, and she was able to follow commands and imitate simple motions. She had no gross motor abnormalities; tendon reflexes were normal with flexor plantar responses.
Family 2
Patient 5 was ascertained at age 28 years. He was unaware of brain calcifications until the age of 28 when CT showed diffuse calcifications in bilateral frontal, temporal and parietal lobes, basal ganglia and cerebellum, but no hydrocephalus or brain atrophy. He had no history of neonatal or perinatal complications, nor any major illness or hospitalisations during childhood. Postnatal development and growth were slower than his healthy siblings. Learning difficulties were noted in school and he was not able to complete intermediate school. He reported periodic headaches occurring mostly in the mornings that responded only partially to analgesics and were not associated with visual disturbance, nausea or vomiting. He had a history of hand tremors and unprovoked sweating episodes. Several medical opinions were sought for inability to gain weight despite heavy food intake but no cause was found. He worked as a driver. When examined at 29 years, his weight was 40 kg (−3.5/−2 SD) height 153cm (−3.5/−2 SD) and OFC 53cm (−2 SD), and he appeared emaciated with poor muscle mass. His exam also showed no refractive errors, normal vision, retina and retinal vessels, normal muscle tone, power and tendon reflexes, and no cerebellar signs. Fine hand tremors were noted at rest.
Patient 6 was the full term product of a normal pregnancy with birth weight 2.5 kg. At age 2 years she developed an acute febrile illness. Lumbar puncture for assessment of possible meningitis was complicated by seizure and decorticate rigidity, which may have been due to downward herniation at the time of lumbar puncture. Cerebrospinal fluid examination did not indicate meningitis. CT scan showed widespread brain calcifications that were attributed to congenital infection, though a TORCH screen at the age of 2 years was negative. Spastic quadriplegia was noted on recovery. From that time the patient suffered from severe spasticity and was bed-ridden for life. She died at age 20 years due to pneumonia.
Patient 7 had a history of slow growth and developmental delay with mild learning difficulties in school. He suffered headaches 2–3 times per week occurring at different times of the day and complained of difficulty gaining weight despite adequate caloric intake. Ascertained at the age of 18 years, he was a part time college student. Examination showed a thin-built young man with poor muscle mass, 38 kg (−4/−2.5 SD), height 155 cm (−3/−1.5 SD), and OFC 52cm (−2 SD). Brain calcifications were present on his CT scan.
Patient 8 was born at full term by normal vertex delivery with no neonatal complications. She had no history of headaches, no visual deficit, no history of fractures, no anaemia. Examined at age 15 years, she was small in stature, of slight build and poor muscle mass with no skin lesions, normal hair and nails. Her school performance was said to be satisfactory. She weighed 30 kg (−4/−2.5 SD), height measured 145 cm (−3/− 1.5 SD) and OFC 51 cm (−2 SD), and she had normal pubertal development. Neurological examination showed normal vision, retina and retinal vessels. Like her other affected siblings, she had reduced fine motor dexterity and slow rapid alternating movements. A brain CT showed widespread parenchymal calcifications and mild dilatation of lateral ventricles.
Laboratory Investigations
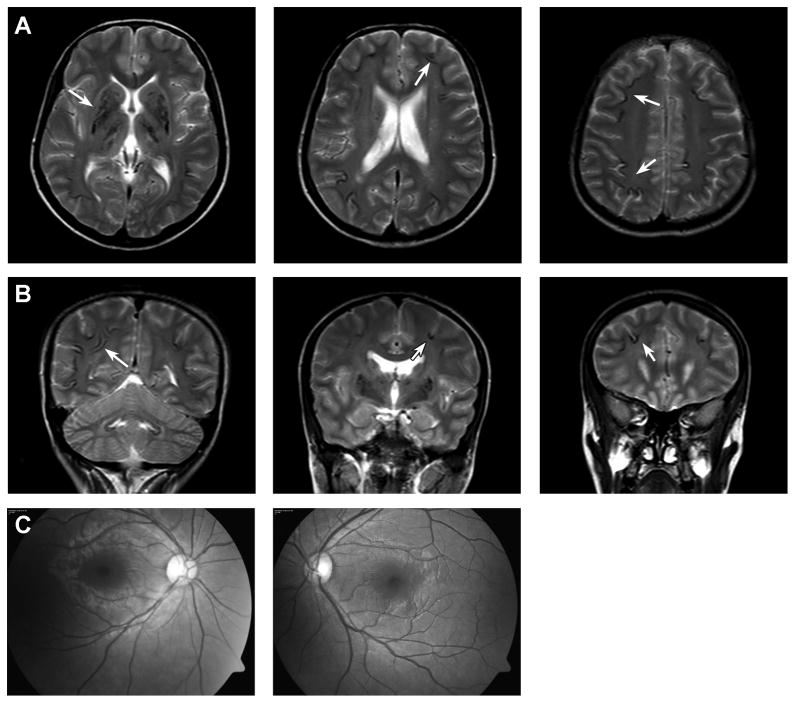
Brain imaging of all 8 children in families 1 and 2 were reviewed and were remarkably similar. Brain CT scans obtained on the parents in both families showed no calcifications. CT scans from patients 1 and 4 are shown (Figure 1). A brain CT of the eldest affected daughter in Family 1 (patient 1, Figure 1B) showed diffuse serpiginous or globular calcifications involving frontal, temporal, parietal and occipital cortex, basal ganglia, thalamus and deep cerebellar nuclei. No significant brain atrophy was seen, and ventricular size appeared normal. A brain CT obtained on her youngest sister (Patient 4) at age 5 years (Fig 1C) showed widespread curvilinear calcifications in both cerebral hemispheres and small ventricles with shunt catheter in place. Like her older siblings, the cortical calcifications occurred predominantly at the base of sulci at the grey-white matter junction. An MRI obtained on Patient 1 (Figure 2) showed signal voids corresponding to the calcifications. The white matter appeared intact with essentially normal signal intensity. Detailed retinal exam in this girl showed no vasculopathy or angiomas (Figure 2C).
Figure 2.
Brain MRI and retinal photographs from patient 1. (A, B) MR images show hypodense areas of calcification (e.g. arrows) that occur at the base of a sulcus or in deep structures including caudate, putamen, thalamus and dentate nuclei of the cerebellum. There is little or no white matter dystrophic change. (C) retinal photographs show a normal vascular pattern and absence of exudates.
The youngest girl in Family 1 (Patient 4), who received a VP shunt, had CSF examined on three occasions and was found to have a normal protein of 40 mg/dl and 2 cells per ml. In addition, Patient 6 (Family 2) had a lumbar puncture at age 2 during a febrile illness, and the CSF protein and cell count were normal.
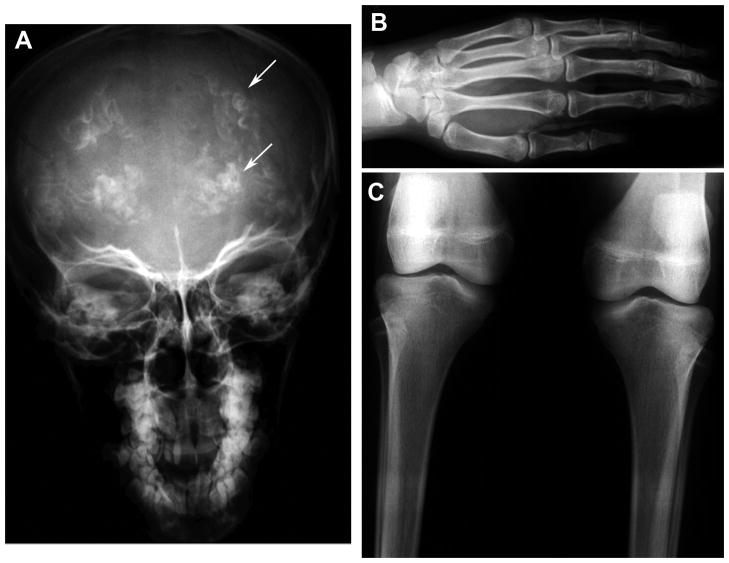
Skeletal X-rays on all four affected girls in Family 1 showed intracranial calcification on skull films (Figure 3). X-Rays of hands and long bones showed signs of thin cortical bone and osteopenia, with the trabecular bone too readily visible (Figure 3). Abdominal ultrasound showed normal liver, spleen and kidneys, and no calcifications outside of brain were found in any of the affected individuals.
Figure 3.
Skeletal X-rays from Patient 1. (A) skull film shows prominent intracranial calcifications (Arrows). (B) Hand and (C) knee films show signs of thinned cortical bone and prominent trabecular pattern consistent with osteopenia.
Additional normal laboratory values obtained on all affected children included blood and platelet counts, arterial blood gases (on Patients 1, 3 and 4), serum electrolytes, liver function tests (SGPT and SGOT), alkaline phosphatase, calcium, parathormone, thyroid function tests (TSH, T4), renal function tests (blood urea nitrogen, creatinine) and glucose. In addition, HPLC studies excluded sickle cell anaemia and beta-thalassemia.
Recombination frequency mapping
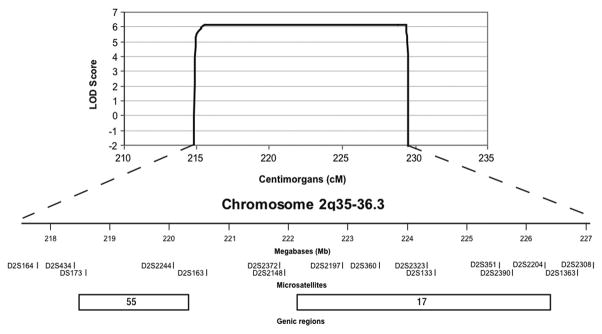
An affected only genome-wide linkage analysis was performed on Family 1. The most significant scores were observed in two regions, the first spanning 15 cM on chromosome 2 (214–229 cM) with a LOD of 3.01 and the second spanning 11 cM on chromosome 12 (86–97 cM) with a LOD of 2.00. Fine-mapping using polymorphic microsatellite markers between rs2373041 and rs10498202 on 2q35-q36.3 was performed in Families 1 and 2. The resulting peak LOD score increased to 6.17 at D2S351 and D2S2390 on 2q36.2 (Figure 4). Fine-mapping on 12q15-q21.31 excluded this locus (data not shown).
Figure 4.
Close-up of the chromosome 2 locus. Multipoint parametric LOD scores obtained from the fine-mapping (black line) analysis are shown. Physical positions (UCSC Human Genome Browser May 2004), microsatellite markers and two genic regions harbouring 72 RefSeq genes within the region of interest are depicted.
DISCUSSION
All affected members of this extended, inbred family manifested a syndrome of microcephaly and cerebral calcifications associated with developmental delay, small stature and osteopenia. The calcifications were striking, and involved the cerebral cortex, particularly the gray-white border in the depths of sulci where they appeared to trace the U-fibers, as well as the basal ganglia and cerebellum. While the ‘railroad track’ appearance suggested calcified vessels, no vascular abnormalities were seen on magnetic resonance angiogram in the studies obtained on Patient 1. All patients displayed poor weight gain from infancy and all were less vigorous than their normal siblings. All affected individuals were small with height and weight proportional to OFCs of −2 to −3 SD below the mean. Although delayed, neurological milestones once acquired were maintained and psychosocial development was normal. School performance was suboptimal in all, but to variable degrees, and fine dexterity was poor in most patients. One 18-year-old displayed dyspraxia, brisk deep tendon reflexes and lost her ability to move fast and to run in her teens; another had fine hand tremors at rest at the age of 28 years. Thin cortical bone with prominent trabecular pattern and osteopenia on skeletal X-ray was noted in all patients. However, only one child had a history of traumatic fracture. Another child developed hydrocephalus, perhaps due to the placement of calcifications, and seizures most likely related to her VP shunt.
Among the inherited developmental disorders associated with intracranial calcifications, perhaps four – AGS, CRMCC, Fried syndrome and carbonic anhydrase II deficiency – most closely approximate the pattern of calcifications seen here, though our patients’ syndrome differs significantly from each of them. AGS is a severe autosomal recessive encephalopathy characterized by stereotyped subacute onset of irritability, inconsolable crying, loss of skills and frequently intermittent sterile fevers usually after the first few days but within the first year of life, and subsequently severe mental retardation, spasticity and dystonia with no further progression, and recurrent chilblain lesions [Crow et al., 2006a; Crow et al., 2006b; Rice et al., 2007]. A few AGS patients are affected at birth with neonatal seizures, jitteriness, poor feeding, thrombocytopenia, hepatosplenomegaly and elevated transaminases. Brain imaging shows progressive brain atrophy, leukoencephalopathy, intracerebral calcifications that typically spare the cortex (Table II). Chronic CSF lymphocytosis, increased CSF alpha-interferon, and negative serologic investigations for common prenatal infections are characteristic. The protean clinical manifestations of AGS have led to several different names for this syndrome, including familial infantile encephalopathy with intracranial calcification and chronic cerebrospinal fluid lymphocytosis, Cree encephalitis, and (incorrectly) pseudo-TORCH syndrome. These are considered to be the same entity, except that Briggs et al., [in press] have recently delineated a distinct syndrome with polymicrogyria and intracerebral calcifications that establish pseudo-TORCH as a separate entity.
Table II.
Location of intracerebral calcifications in five syndromes
| Calcification | Syndrome |
||||
|---|---|---|---|---|---|
| Fried | AGS | CRMCC | CAII | This report | |
| Cortex-superficial WM (U-fibers) | + | + | + | ||
| Deep and periventricular WM | + | + | |||
| Basal ganglia and thalamus | + | + | + | + | + |
| Cerebellar dentate nuclei | + | + | + | (+) | + |
+ typical location; (+) occasional location; CAII, carbonic anhydrase II deficiency; Fried, Fried syndrome; WM, white matter changes
At least four genes have been associated with AGS1-4, located on chromosomes 3p21.3 [Crow et al., 2000; Crow et al., 2006a], 13q14 [Ali et al., 2005], 11q13.2 [Crow et al., 2006b] and 19p13.13 [Crow et al., 2006b], as summarized in [Rice et al., 2007]. The causative genes are TREX1 (AGS1), encoding a 3′-5′ exonuclease, and RNASEH2A (AGS2), RNASEH2B (AGS3) and RNASEH2C (AGS4), all of which are involved in the degradation of DNA and RNA molecules [Crow et al., 2006a; Crow et al., 2006b]. It has been speculated that these genes are involved in nucleic acid removal during apoptosis and that failure of this process leads to activation of the innate immune system, accounting for the features that resemble infection or autoimmune lupus-like disease [Rice et al., 2007].
At least one AGS gene remains to be identified [Rice et al., 2007], leaving open the possibility that the disorder reported here represents a new AGS locus on chromosome 2. However, the disorder seen in this Omani family is distinct from any form of AGS. The clinical course is significantly milder than those AGS families described, although AGS2 patients have displayed slow progression and milder symptoms [Crow et al., 2006b]. The pattern of calcifications differs (Table II) and no evidence of leukoencephalopathy was present on MR imaging of Patient 1, which would have been evident in AGS by that age. No hematological or biochemical abnormalities and no signs of organomegaly or cutaneous lesions similar to chilblains were detected among affected individuals. The critical interval identified by linkage analysis is large (15 cM), but there are no known nucleotide exonucleases in the region. Thus, if this extended family were affected by a disorder in the AGS spectrum, the phenotype would expand the mild end of the spectrum.
Fried syndrome is a rare X-linked syndrome characterized by mental retardation, calcifications of the basal ganglia and deep cerebellar nuclei, and facial dysmorphism in boys. Mutations of the adapter protein gene, AP1S2, on Xp22 were recently identified [Saillour et al., 2007]. This locus was excluded in the family reported here, and the phenotype differs due to the lack of growth retardation, microcephaly, cortical calcifications and osteopenia. Another syndrome with brain calcification is carbonic anhydrase II deficiency (CAII), which is characterized by osteopetrosis, renal tubular acidosis, intracranial calcification and mental retardation [Cumming and Ohlsson, 1985). Although they share some features of brain calcification (Table II), the CAII syndrome has increased bone density and renal acidosis, which are not present in patients of this extended Omani family.
Perhaps a better fit for the Omani family is CRMCC or “Coats’ plus” syndrome [Crow et al., 2004]. This condition prominently includes a retinal pathology known as Coats’ disease characterized by the slow, insidious onset and progression of abnormal retinal vascular permeability and telangectasia [Tolmie et al., 1988]. It is recognized as raised patches of yellow-white exudates beneath normal vessels progressing to retinal detachment, cataract, glaucoma, phthisis bulbi and blindness. Other features of CRMCC include less severe neurological impairments than AGS, normal life expectancy, and osteopenia with frequent fractures. Brain imaging demonstrates a progressive leukoencephalopathy and extensive calcifications (Table II), and a brain biopsy in one patient showed local astrocytosis and calcification of small vessels [Crow et al., 2004). CRMCC was recently merged with “leukoencephalopathy with calcifications” or Labrune syndrome, in which brain pathology demonstrates a small vessel microangiopathy, but in which retinal pathology may be lacking [Briggs et al., 2008).
The phenotype in the Omani family reported here resembles CRMCC in the mild to moderate cognitive impairment range, mild motor impairment, growth deficiency and osteopenia, and distribution of intracerebral calcifications (Table II), but differs in many regards. None had visual impairment and detailed retinal exam in an 18-year-old affected girl and an affected man aged 28 years showed no evidence of vasculopathy. Brain CT scans in 8 patients demonstrated no cerebral cysts, and the single brain MRI showed no leukoencephalopathy or cerebral cysts. If the Omani family disorder were in the CRMCC spectrum, the phenotype would expand the mild end of the spectrum.
The critical interval encompassing the disease locus in this extended family is large at 15 cM, due to consanguinity and the relatively few informative markers available for this family. It is difficult to speculate regarding candidate genes in this interval as few genes have a demonstrated connection to intracerebral calcification or obvious mechanistic explanation. There is no gene in the region with known homology to RNAses or exonucleases, the protein class associated with AGS, nor are there genes with known roles in calcium homeostasis. Further refinement of the locus will require identification of additional families with this phenotype.
Acknowledgments
Supported by NIH funded RO1 NS058721 to WBD and P01 NS048120 to MER
References
- Ahn TG, Antonarakis SE, Kronenberg HM, Igarashi T, Levine MA. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore) 1986;65:73–81. [PubMed] [Google Scholar]
- Aicardi J, Goutieres F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. doi: 10.1002/ana.410150109. [DOI] [PubMed] [Google Scholar]
- Al Mane KA, Coates RK, McDonald P. Intracranial calcification in Raine syndrome. Pediatr Radiol. 1996;26:55–58. doi: 10.1007/BF01403707. [DOI] [PubMed] [Google Scholar]
- Ali M, Highet LJ, Lacombe D, Goizet C, King MD, Tacke U, van der Knaap MS, Lagae L, Rittey C, Brunner HG, von Bokhoven H, Hamel B, Oade YA, Sanchis A, Desguerre I, Cau D, Mathieu N, Moutard ML, Lebon P, Kumar D, Jackson AP, Crow YJ. A second locus for Aicardi-Goutieres syndrome at chromosome 13q14-21. J Med Genet. 2005 doi: 10.1136/jmg.2005.031880. Epub 2005 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Broderick DF, Uitti RJ, Hutton ML, Wszolek ZK. Heredofamilial brain calcinosis syndrome. Mayo Clin Proc. 2005;80:641–651. doi: 10.4065/80.5.641. [DOI] [PubMed] [Google Scholar]
- Baumert T, Kleber G, Schwarz J, Stabler A, Lamerz R, Mann K. Reversible hyperkinesia in a patient with autoimmune polyglandular syndrome type I. Clin Investig. 1993;71:924–927. doi: 10.1007/BF00185605. [DOI] [PubMed] [Google Scholar]
- Briggs TA, Abdel-Salam GM, Balicki M, Baxter P, Bertini E, Bishop N, Browne BH, Chitayat D, Chong WK, Eid MM, Halliday W, Hughes I, Klusmann-Koy A, Kurian M, Nischal KK, Rice GI, Stephenson JB, Surtees R, Talbot JF, Tehrani NN, Tolmie JL, Toomes C, van der Knaap MS, Crow YJ. Cerebroretinal microangiopathy with calcifications and cysts (CRMCC) Am J Med Genet A. 2008;146:182–190. doi: 10.1002/ajmg.a.32080. [DOI] [PubMed] [Google Scholar]
- Briggs TA, Wolf NI, D’arrigo S, Ebinger F, Harting I, Dobyns WB, Livingston JH, Rice GI, Crooks D, Rowland-Hill CA, Squier W, Stoodley N, Pilz DT, Crow YJ. Band-like intracranial calcification with simplified gyration and polymicrogyria; another recognizable ‘pseudo-torch’ phenotype. Am J Med Genet Part A. 2008;146A doi: 10.1002/ajmg.a.32614. in press. [DOI] [PubMed] [Google Scholar]
- Burn J, Wickramasinghe HT, Harding B, Baraitser M. A syndrome with intracranial calcification and microcephaly in two sibs, resembling intrauterine infection. Clin Genet. 1986;30:112–116. doi: 10.1111/j.1399-0004.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Crow YJ, Black DN, Ali M, Bond J, Jackson AP, Lefson M, Michaud J, Roberts E, Stephenson JB, Woods CG, Lebon P. Cree encephalitis is allelic with Aicardi-Goutieres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet. 2003;40:183–187. doi: 10.1136/jmg.40.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet. 2006a;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- Crow YJ, Jackson AP, Roberts E, van Beusekom E, Barth P, Corry P, Ferrie CD, Hamel BC, Jayatunga R, Karbani G, Kálmánchey R, Kelemen A, King M, Kumar R, Livingstone J, Massey R, McWilliam R, Meager A, Rittey C, Stephenson JB, Tolmie JL, Verrips A, Voit T, van Bokhoven H, Brunner HG, Woods CG. Aicardi-Goutieres syndrome displays genetic heterogeneity with one locus (AGS1) on chromosome 3p21. Am J Hum Genet. 2000;67:213–221. doi: 10.1086/302955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, Ali M, Semple C, Aicardi J, Babul-Hirji R, Baumann C, Baxter P, Bertini E, Chandler KE, Chitayat D, Cau D, Déry C, Fazzi E, Goizet C, King MD, Klepper J, Lacombe D, Lanzi G, Lyall H, Martínez-Frías ML, Mathieu M, McKeown C, Monier A, Oade Y, Quarrell OW, Rittey CD, Rogers RC, Sanchis A, Stephenson JB, Tacke U, Till M, Tolmie JL, Tomlin P, Voit T, Weschke B, Woods CG, Lebon P, Bonthron DT, Ponting CP, Jackson AP. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat Genet. 2006b;38:910–916. doi: 10.1038/ng1842. [DOI] [PubMed] [Google Scholar]
- Crow YJ, McMenamin J, Haenggeli CA, Hadley DM, Tirupathi S, Treacy EP, Zuberi SM, Browne BH, Tolmie JL, Stephenson JB. Coats’ plus: a progressive familial syndrome of bilateral Coats’ disease, characteristic cerebral calcification, leukoencephalopathy, slow pre- and post-natal linear growth and defects of bone marrow and integument. Neuropediatrics. 2004;35:10–19. doi: 10.1055/s-2003-43552. [DOI] [PubMed] [Google Scholar]
- Cumming WA, Ohlsson A. Intracranial calcification in children with osteopetrosis caused by carbonic anhydrase II deficiency. Radiology. 1985;157:325–327. doi: 10.1148/radiology.157.2.2413500. [DOI] [PubMed] [Google Scholar]
- Farley TJ, Ketonen LM, Bodensteiner JB, Wang DD. Serial MRI and CT findings in infantile Krabbe disease. Pediatr Neurol. 1992;8:455–458. doi: 10.1016/0887-8994(92)90009-n. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Loginov M, Stern JM. Identification of a locus on chromosome 14q for idiopathic basal ganglia calcification (Fahr disease) Am J Hum Genet. 1999;65:764–772. doi: 10.1086/302558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Thorvaldsson T, Kong A, Gunnarsson G, Ingolfsdottir A. Allegro version 2. Nat Genet. 2005;37:1015–1016. doi: 10.1038/ng1005-1015. [DOI] [PubMed] [Google Scholar]
- Hirano M, Pavlakis SG. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): current concepts. J Child Neurol. 1994;9:4–13. doi: 10.1177/088307389400900102. [DOI] [PubMed] [Google Scholar]
- Juppner H, Schipani E, Bastepe M, Cole DE, Lawson ML, Mannstadt M, Hendy GN, Plotkin H, Koshiyama H, Koh T, Crawford JD, Olsen BR, Vikkula M. The gene responsible for pseudohypoparathyroidism type Ib is paternally imprinted and maps in four unrelated kindreds to chromosome 20q13.3. Proc Natl Acad Sci U S A. 1998;95:11798–11803. doi: 10.1073/pnas.95.20.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan AE, Kozlowski K. New distinct lethal osteosclerotic bone dysplasia (Raine syndrome) Am J Med Genet. 1992;43:860–864. doi: 10.1002/ajmg.1320430522. [DOI] [PubMed] [Google Scholar]
- Lebon P, Badoual J, Ponsot G, Goutieres F, Hemeury-Cukier F, Aicardi J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci. 1988;84:201–208. doi: 10.1016/0022-510x(88)90125-6. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Dong S, Loi H, Di X, Liu G, Hubbell E, Law J, Berntsen T, Chadha M, Hui H, Yang G, Kennedy GC, Webster TA, Cawley S, Walsh PS, Jones KW, Fodor SP, Mei R. Genotyping over 100,000 SNPs on a pair of oligonucleotide arrays. Nat Methods. 2004;1:109–111. doi: 10.1038/nmeth718. [DOI] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten JL, Johns DR, Valle D, Eil C, Gruppuso PA, Steele G, Smallwood PM, Levine MA. Mutation in the gene encoding the stimulatory G protein of adenylate cyclase in Albright’s hereditary osteodystrophy. N Engl J Med. 1990;322:1412–1419. doi: 10.1056/NEJM199005173222002. [DOI] [PubMed] [Google Scholar]
- Reardon W, Hockey A, Silberstein P, Kendall B, Farag TI, Swash M, Stevenson R, Baraitser M. Autosomal recessive congenital intrauterine infection-like syndrome of microcephaly, intracranial calcification, and CNS disease. Am J Med Genet. 1994;52:58–65. doi: 10.1002/ajmg.1320520112. [DOI] [PubMed] [Google Scholar]
- Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, Artuch R, Montalto SA, Bacino CA, Barroso B, Baxter P, Benko WS, Bergmann C, Bertini E, Biancheri R, Blair EM, Blau N, Bonthron DT, Briggs T, Brueton LA, Brunner HG, Burke CJ, Carr IM, Carvalho DR, Chandler KE, Christen HJ, Corry PC, Cowan FM, Cox H, D’Arrigo S, Dean J, De Laet C, De Praeter C, Dery C, Ferrie CD, Flintoff K, Frints SG, Garcia-Cazorla A, Gener B, Goizet C, Goutieres F, Green AJ, Guet A, Hamel BC, Hayward BE, Heiberg A, Hennekam RC, Husson M, Jackson AP, Jayatunga R, Jiang YH, Kant SG, Kao A, King MD, Kingston HM, Klepper J, van der Knaap MS, Kornberg AJ, Kotzot D, Kratzer W, Lacombe D, Lagae L, Landrieu PG, Lanzi G, Leitch A, Lim MJ, Livingston JH, Lourenco CM, Lyall EG, Lynch SA, Lyons MJ, Marom D, McClure JP, McWilliam R, Melancon SB, Mewasingh LD, Moutard ML, Nischal KK, Ostergaard JR, Prendiville J, Rasmussen M, Rogers RC, Roland D, Rosser EM, Rostasy K, Roubertie A, Sanchis A, Schiffmann R, Scholl-Burgi S, Seal S, Shalev SA, Corcoles CS, Sinha GP, Soler D, Spiegel R, Stephenson JB, Tacke U, Tan TY, Till M, Tolmie JL, Tomlin P, Vagnarelli F, Valente EM, Van Coster RN, Van der Aa N, Vanderver A, Vles JS, Voit T, Wassmer E, Weschke B, Whiteford ML, Willemsen MA, Zankl A, Zuberi SM, Orcesi S, Fazzi E, Lebon P, Crow YJ. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;81:713–725. doi: 10.1086/521373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillour Y, Zanni G, Des Portes V, Heron D, Guibaud L, Iba-Zizen MT, Pedespan JL, Poirier K, Castelnau L, Julien C, Franconnet C, Bonthron D, Porteous ME, Chelly J, Bienvenu T. Mutations in the AP1S2 gene encoding the sigma 2 subunit of the adaptor protein 1 complex are associated with syndromic X-linked mental retardation with hydrocephalus and calcifications in basal ganglia. J Med Genet. 2007;44:739–744. doi: 10.1136/jmg.2007.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M, et al. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985;313:139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- Tolmie JL, Browne BH, McGettrick PM, Stephenson JB. A familial syndrome with coats’ reaction retinal angiomas, hair and nail defects and intracranial calcification. Eye. 1988;2 (Pt 3):297–303. doi: 10.1038/eye.1988.56. [DOI] [PubMed] [Google Scholar]
- Tolmie JL, Shillito P, Hughes-Benzie R, Stephenson JB. The Aicardi-Goutieres syndrome (familial, early onset encephalopathy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis) J Med Genet. 1995;32:881–884. doi: 10.1136/jmg.32.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody RC, Brewster MA, Glasier C. Progressive intracranial calcification in dihydropteridine reductase deficiency prior to folinic acid therapy. Neurology. 1989;39:673–675. doi: 10.1212/wnl.39.5.673. [DOI] [PubMed] [Google Scholar]