Abstract
Lithium has been and continues to be the mainstay of bipolar disorder (BD) pharmacotherapy for acute mood episodes, switch prevention, prophylactic treatment, and suicide prevention. Lithium is also the definitive proof-of-concept agent in BD, although it has recently been studied in other psychoses as well as diverse neurodegenerative disorders. Its neurotrophic effects can be viewed as a unifying model to explain several integrated aspects of the pathophysiology of mood disorders and putative therapeutics for those disorders. Enhancing neuroprotection (which directly involves neurotrophic effects) is a therapeutic strategy intended to slow or halt the progression of neuronal loss, thus producing long-term benefits by favorably influencing outcome and preventing either the onset of disease or clinical decline. The present article: (i) reviews what has been learned regarding lithium’s neurotrophic effects since Cade’s original studies with this compound; (ii) presents human data supporting the presence of cellular atrophy and death in BD as well as neurotrophic effects associated with lithium in human studies; (iii) describes key direct targets of lithium involved in these neurotrophic effects, including neurotrophins, glycogen synthase kinase 3 (GSK-3), and mitochondrial/endoplasmic reticulum key proteins; and (iv) discusses lithium’s neurotrophic effects in models of apoptosis and excitotoxicity as well as its potential neurotrophic effects in models of neurological disorders. Taken together, the evidence reviewed here suggests that lithium’s neurotrophic effects in BD are an example of an old molecule acting as a new proof-of-concept agent. Continued work to decipher lithium’s molecular actions will likely lead to the development of not only improved therapeutics for BD, but to neurotrophic enhancers that could prove useful in the treatment of many other illnesses.
Keywords: bipolar disorder, depression, GSK-3, lithium, mania, mitochondria, mood stabilizer, neurodegenerative, neurotrophic effects, oxidative stress, PKC, protein kinase C
Bipolar disorder (BD) is one of the most severe of the major mental illnesses, and recent estimates place its lifetime prevalence (including BD spectrum) at about 4.4% in the U.S. population (1). Several pathophysiological findings in BD associated with loss of neurotrophic effects and consequent cellular injury have been widely described in different targets regulated by a wide range of specific mechanisms [reviewed in (2, 3)].
The mood stabilizer lithium has been the standard pharmacological treatment for BD for over 60 years. Its introduction led to an immediate improvement in outcome for many patients suffering from this illness whose treatment options had, until that point, been quite bleak. Lithium was found be effective in treating acute manic and depressive episodes, as well as in reducing the recurrence of mood episodes and minimizing the risk of suicidal behaviors (4, 5).
Lithium is the lightest of all metals, with a density only half that of water. It induces multiple biochemical and molecular effects on neurotransmitter/receptor-mediated signaling, signal transduction cascades, hormonal and circadian regulation, ion transport, and gene expression (6). These effects have been widely associated with the activation of neurotrophic pathways involved in the pathophysiology of BD. Indeed, since John Cade’s preclinical studies using lithium to protect against urea toxicity (7), protection has been the most expected and replicated biological effect associated with lithium use in both human and preclinical studies. Briefly, enhancing neuroprotection (which directly involves neurotrophic effects) is a therapeutic strategy intended to slow or halt the progression of neuronal loss (8) or pharmacological interventions that produce long-term benefits by favorably influencing underlying etiology or pathogenesis, and thereby preventing onset of disease or clinical decline (9). In other words, a neurotrophic therapy is expected to act on the pathophysiological mechanisms underlying the clinical presentation of the disease, thus altering its natural course and outcome. It is also logical to assume that a neurotrophic therapy capable of concomitantly preventing and reversing dysfunctional cellular and molecular effects in diverse targets may represent the standard treatment for the illness. In this context, converging evidence has supported neurotrophic effects as a unifying hypothesis for lithium’s efficacy in treating BD. Lithium would act by limiting or reversing disease progression directly associated with the activation of neurotrophic effects; these have been widely described in studies evaluating targets such as neurotransmitters, second messengers, signaling pathways, hormones, neurotrophic factors, ion channels, organelles, genes, and others.
It is important to emphasize that lithium has already demonstrated diverse molecular effects reversing well-described pathophysiological changes such as increased oxidative stress, programmed cell death (apoptosis), inflammation, environmental stress, glial dysfunction, neurotrophic factor dysfunction, excitotoxicity, mitochondrial and endoplasmic reticulum (ER) dysfunction, and disruption in epigenetic mechanisms. All these changes strongly suggest molecular imbalances that trigger the cellular stress, atrophy, and death described over the course of BD.
In this article, we discuss lithium’s neurotrophic properties and review their potential clinical implications for the development of therapeutics that are more effective than existing ones. First, human studies showing evidence for neural cell atrophy and loss in BD are described, as well as lithium’s ability to reverse these pathological findings. Second, the potential roles of intracellular cascade systems in BD are described; these have been shown to directly regulate cell survival/death pathways and are directly targeted by lithium. Yucel et al. showed increased bilateral hippocampal volume after 2–4 years of lithium treatment in previously drug-naïve BD subjects (10). Also, a recent study using high-resolution volumetric MRI showed a direct therapeutic relevance of lithium neurotrophic effects in BD. It was observed that only lithium-responders showed increases in gray matter in the prefrontal areas (11). Third, the neurotrophic effects of lithium are described in preclinical models in vivo and in vitro and in clinical studies, demonstrating lithium’s clinical relevance not only in BD, but as a potential neurotrophic agent for use in several neurological disorders. We anticipate that understanding lithium’s relevant therapeutic molecular targets will eventually enable us to develop novel and improved drugs with lithium-mimetic properties.
Cellular stress, atrophy, and loss in BD: therapeutic targets for lithium
Cellular stress, atrophy, and/or loss in BD: data from human studies
Although BD is not a typical neurodegenerative disorder, several postmortem morphometric and brain imaging studies [structural imaging and magnetic resonance spectroscopy (MRS)] have demonstrated the presence of neuronal/glial stress, atrophy, and death associated with the illness [reviewed in (2)].
Postmortem studies evaluating BD subjects matched to healthy controls have shown a significant decrease in brain volume in areas directly involved in mood regulation, mostly characterized by reduced number, density, and/or size of neurons and glial cells, with consistent evidence for the prefrontal and anterior cingulate cortices and amygdala [reviewed in (12, 13)]. For instance, Öngur and colleagues (14) described a significant decrease (41.2%) in glial cell density in the subgenual prefrontal cortex in BD subjects with a positive family history of mood disorders.
Changes in brain volume and structure, as well as altered energy parameters in specific brain areas related to mood regulation, have been described in neuroimaging studies evaluating individuals with BD. It has consistently been shown that there is decreased gray matter volume in diverse neural areas that regulate cognitive and emotional processing in BD, such as ventral/orbital/medial prefrontal cortex and amygdala [reviewed in (15, 16)]. In a seminal study, Drevets and colleagues (17) observed an approximate 40% reduction in subgenual prefrontal cortex volumes in familial mood disorder subjects. In addition, MRS studies showed regional abnormalities of N-acetyl-aspartate (NAA), choline, and myoinositol in BD, especially in the prefrontal and anterior cingulate cortices, hippocampus, and basal ganglia [reviewed in (18)]. These markers are related to the regulation of neurotrophic pathways (described elsewhere) and responded to lithium treatment (see next section), making them potential state-dependent markers. For instance, BD subjects showed abnormally high myoinositol levels in diverse brain areas during manic and depressive episodes; such levels were not observed during euthymia, in healthy controls, or after lithium treatment in BD subjects [reviewed in (19)].
Notably, while BD is not considered a classic neurodegenerative disorder based on the absence of gliosis (a marker of neurdegeneration), it has specific patterns of glial loss possibly associated with the decreased NAA and elevated choline levels described above in BD subjects (18, 20, 21). Furthermore, NAA synthesis occurs in the mitochondria, which are implicated in the altered cell energy regulation (e.g., decreased pH and increased lactate levels) described in the pathophysiology of BD (22, 23) (see “Mitochondrial and ER regulation of oxidative stress and apoptosis: a role for Bcl-2, IP3, and calcium”, p. 99); this has been hypothesized to be critical to the altered oxidative stress parameters, apoptosis, and disruption in gene expression that lead to loss of neurotrophic effects.
Lithium’s neurotrophic effects in BD: data from human studies
The most replicated finding from structural neuroimaging studies is an association between lithium treatment and increased gray matter volume in brain areas implicated in emotional processing and cognitive control, such as the anterior cingulate gyrus, amygdala, and hippocampus, which suggests that lithium has considerable neurotrophic effects (24, 25). One study found that patients with BD who were not treated with lithium had significantly reduced left anterior cingulate volumes compared to healthy volunteers and lithium-treated patients (26). Additional magnetic resonance imaging (MRI) studies examined the gray and white matter volumes of 12 untreated and 17 lithium-treated patients with BD and 46 healthy controls and found that total gray matter volumes were significantly increased in lithium-treated relative to untreated patients and healthy controls (27). Another study found that gray matter density was significantly greater in patients with BD relative to healthy controls in diffuse cortical regions (28). In an MRI study of the hippocampus conducted in 33 patients with BD (21 treated with lithium and 12 unmedicated) and 62 matched healthy controls, investigators found that total hippocampal volume was significantly greater in lithium-treated patients with BD compared with healthy controls (by 10%) and unmedicated patients (by 14%) (29).
As noted above, MRS studies have evaluated the potential effects of lithium treatment in BD subjects on abnormal concentrations of markers of neuronal integrity, including NAA and myoinositol (see also “Mitochondrial and ER regulation of oxidative stress and apoptosis: a role for Bcl-2, IP3, and calcium”, p. 99). Increased NAA levels were observed after four weeks of lithium treatment in different brain areas (30, 31), and decreased choline and myoinositol were reported after chronic lithium treatment in individuals with BD [(32), reviewed in (19)]. Despite some limitations, including the potential for a Type I or II error and negative findings in specific brain areas, human postmortem and imaging studies in BD suggest that neurotrophic effects play a critical role in lithium’s therapeutic effects.
Direct targets of lithium involving neuroprotection: potential relevance in the pathophysiology and treatment of BD
Glycogen synthase kinase 3 (GSK-3)
General aspects of GSK-3 and integration with other neurotrophic pathways
GSK-3 is a serine/threonine kinase that regulates diverse cellular processes and directly regulates cell apoptosis. Interest in GSK-3 as a target for BD drug development arose from findings that it is key to a number of central functions, such as glycogen synthesis, gene transcription, synaptic plasticity, apoptosis (cell death), cellular structure and resilience, and the circadian cycle (33); notably, all of these functions are significantly implicated in the pathophysiology of severe recurrent mood disorders. Indeed, Benedetti and colleagues (34) showed that a polymorphism in the GSK-3 gene was associated with earlier onset of BD.
Activation of GSK-3β functionally inhibits cyclic AMP response element binding protein (CREB), β-catenin, and other survival-promoting transcription factors. GSK-3 is also directly regulated by signals originating from a number of different signaling pathways including the Wnt pathway, the PI-3kinase (PI3K) pathway, protein kinase A (PKA), and protein kinase C (PKC) (see Fig. 1). For instance, certain PKC isoforms have been shown to phosphorylate and inactivate GSK-3β in vitro (35). Modulation of GSK-3 by these and other signaling pathways fine-tunes the activity of this critical enzyme. The downstream targets of GSK-3 are many, and the resulting functional consequences of GSK-3 activation or inhibition often depend on the signaling pathway targeted. For example, GSK inhibition by the Wnt pathway activates the transcription factor β -catenin (an important component of memory consolidation). Other targets of GSK-3 include transcription factors like c-Jun, proteins bound to microtubules [Tau, microtubule-associated protein (MAP)-1B, kinesin light chain], cell cycle mediators (cyclin D), and regulators of metabolism (glycogen synthase, pyruvate dehydrogenase) (36). GSK-3 also directly regulates different neurotransmitter systems involved in the pathophysiology of BD, such as the dopaminergic, glutamatergic, and serotonergic systems [reviewed in (37, 38)].
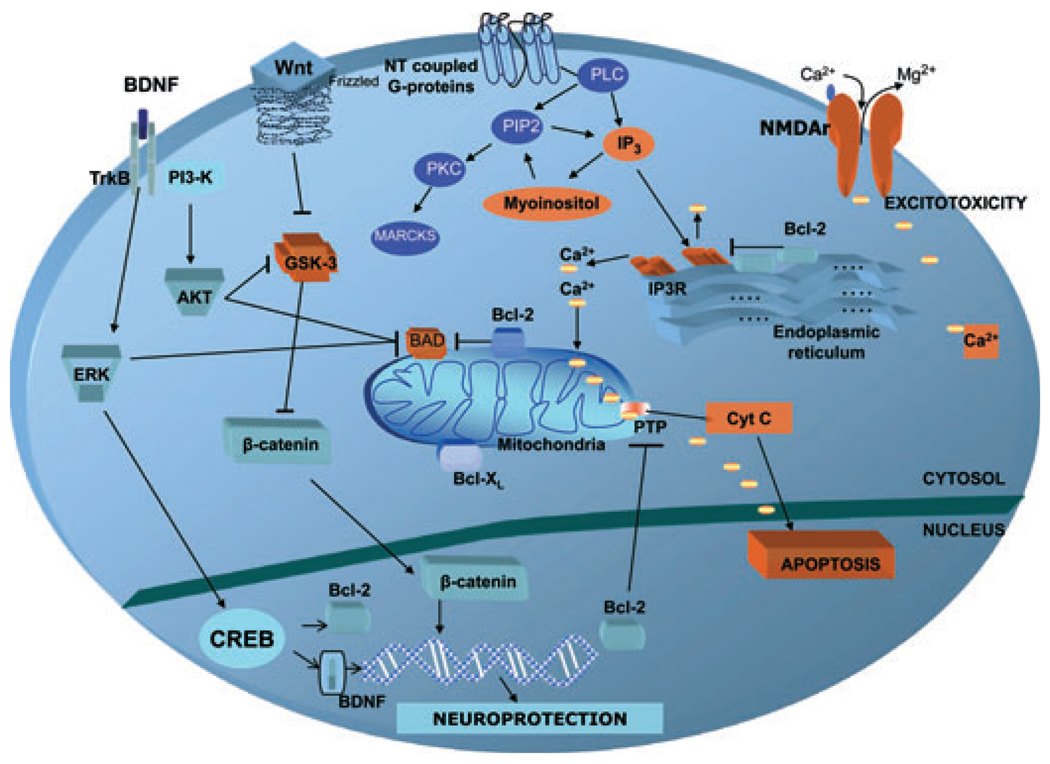
Fig. 1.
Targets for lithium’s neuroprotective effects. Targets shown in orange are mainly pro-apoptotic proteins/receptors; lithium either significantly downregulates these proteins or decreases their expression. Anti-apoptotic proteins are showed in blue. Lithium increases their expression and/or levels, thus inducing neuroprotective and neurotrophic effects. Activation of brain neurotransmitter-coupled G-proteins induces PLC hydrolysis of PIP2 to IP3 and DAG (not shown), which activates PKC. IP3 binds to the IP3R, thus inducing the release of ER calcium stores. Increased intracellular calcium levels have been described in bipolar disorder and may increase the risk of apoptosis. The neuroprotective protein Bcl-2 downregulates ER calcium release through an IP3R-dependent mechanism. The same effect is induced by lithium treatment, which also increases Bcl-2 levels. IP3 is recycled by IMPase, another of lithium’s targets. Cellular signaling through Wnt glycoproteins and frizzled receptors result in GSK-3β inhibition, a critical cellular target and effector for diverse proteins. Inhibition of GSK-3β prevents β-catenin phosphorylation and stimulates its translocation to the nucleus, thus targeting transcription of specific genes activating neurotrophic effects and synaptogenesis. Activation of the BDNF receptor (Trk-B) activates the ERK/MAPK pathway, which inhibits GSK-3β and BAD. Activation of the ERK/MAPK pathway by BDNF increases the expression of nuclear CREB, which facilitates the expression of neurotrophic/neuroprotective proteins such as Bcl-2 and BDNF. BDNF also activates the PI3K pathway, which indirectly inhibits GSK-3β and BAD. Mitochondrial Bcl-2 and Bcl-xl also inhibit pro-apoptotic activation of BAD, as well as consequent mitochondrial increase of calcium influx and cytochrome C release. Bcl-2 = B-cell lymphoma-2; BDNF = brain-derived neurotrophic factor; Ca2+ = calcium; CREB = cAMP response element binding protein; Cyt C = cytochrome C; DAG = diacylglycerol; ER = endoplasmic reticulum; ERK = extracellular regulated kinase; GSK = glycogen-synthase kinase; IMPase = inositol monophosphatase; IP3 = inositol 1,4,5-triphosphate 3; IP3R = inositol 1,4,5-triphosphate 3 receptor; Mg2+ = magnesium; MAPK = mitogen activated protein kinase; NMDA = N-methyl D-aspartate; NT = neurotransmitter; PI3K = phosphatidylinositol-3 kinase; PIP2 = phosphoinositide 4,5-biphosphate; PKC = protein kinase C; PLC = phospholipase C; PTP = permeability membrane pore; TrkB = tyrosine receptor kinase B.
Lithium’s neurotrophic effects targeting at GSK-3 pathways
GSK-3 inhibition is commonly associated with the neurotrophic effects of different survival factors. GSK-3 inhibition directly influences gene transcription, leading to anti-apoptotic effects and improved cell structural stability (39). Notably, GSK-3 was shown to be downregulated by lithium in diverse studies, inducing direct neuronal protection against different injuries (40) and providing new insights into lithium’s neurotrophic effects [reviewed in (41)]. Indeed, GSK-3 is potently inhibited by chronic administration of lithium (42–44); specifically, lithium inhibits GSK-3 with an enzyme inhibition constant (Ki) of 1–2 mM (serum therapeutic range 0.6–1.2 mM) (44–46). In rats, lithium increases cytosolic protein levels of β-catenin, a transcription factor regulated directly by GSK-3 (42), and in mouse brain it activates β-catenin-dependent transcription (47), and decreases the quantity of amyloid-β peptide (an animal model of Alzheimer’s disease) (48) (see also “Lithium’s neurotrophic effects: potential therapeutic relevance in other psychiatric and neurological disorders”, p. 100). More recently, lithium was found to modulate GSK-3 transcription in vitro and in vivo (49). Akt activation and indirect inhibition of GSK-3 by lithium have also been demonstrated after acute or chronic treatment with therapeutically relevant levels of lithium [reviewed in (50)]. In addition, GSK-3 modulation is either the direct or downstream target of action for other mood stabilizers (36).
In behavioral models, O’Brien and collaborators (47) demonstrated that knocking out a single copy of the GSK-3β gene in mice resulted in antidepressant-like effects that were analogous to lithium administration. Also, peripheral administration of a GSK-3 inhibitor decreased amphetamine-induced hyperactivity in rats (51). More recently, transgenic mice overexpressing β-catenin demonstrated changes comparable to those observed following the administration of lithium, including decreased immobility time in the forced swim test and the inhibition of d-amphetamine-induced hyperlocomotion. These findings are consistent with the hypothesis that the behavioral effects of lithium are mediated through its direct inhibition of GSK-3 and the consequent increase in β-catenin (52).
In summary, these findings suggest that GSK-3 inhibition is key to lithium’s antimanic and antidepressant effects, and offers a promising target in the pursuit of drug development for BD. Although there are currently no available GSK-3 inhibitors for clinical testing in BD, a number of inhibitors of this enzyme are currently in various stages of development for other illnesses, including diabetes, Alzheimer’s disease, other neurodegenerative disorders, and stroke (48, 53–60); presumably, GSK-3 is also involved in their pathophysiology. Currently, there is a growing interest in the regulatory effects on GSK-3 on cell survival/death because several conditions that cause apoptosis in association with GSK-3 activation are also counteracted by lithium; furthermore, newly developed GSK-3 inhibitors have a similar neurotrophic profile to lithium (61). However, because isozymes of GSK-3 are a target in several organ systems (more than 40 proteins are phosphorylated by GSK-3, including over a dozen transcription factors) (33), caution is needed when developing such compounds due to the possibility of congenital defects (36, 62).
The phosphoinositol (PI) cycle, PKC, and myristoylated alanine-rich C-kinase substrate (MARCKS)
General aspects of the PI cycle, PKC, and MARCKS: relevance for BD pathophysiology
Phospholipids are precursors for many signaling molecules. Inositol phospholipids play a key role in receptor-mediated signal-transduction pathways, and are implicated in a variety of responses such as cell division, secretion, neuronal excitability, and responsiveness. The PI pathway is initiated by the activation of G-protein-coupled receptors, which couple neurotransmitter receptors to multiple types of intracellular effector proteins. M1, M2, M3, α1, and serotonin receptors coupled to G α q/11 induce phospholipase C (PLC), which hydrolyzes phosphatidylinositol- 4,5-bisphosphate (PIP2) to yield two second messengers: inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG), which is an endogenous activator of PKC. IP3 binds to the IP3 receptor, facilitating the release of calcium from intracellular stores, in particular the ER (63, 64) (see also “Mitochondrial and ER regulation of oxidative stress and apoptosis: a role for Bcl-2, IP3, and calcium”, p. 99).
PKC comprises a family of 12 structurally related isozymes subspecies with a heterogeneous distribution throughout the body (65, 66). PKC isoforms differ in structure, subcellular localization, tissue specificity, mode of activation, and substrate specificity (67). These are classically divided into conventional (α, βI, βII, γ), novel (δ, ε, η, θ, μ), and atypical (ζ, λ/ι) isoforms based on their molecular structure enzyme characterization and activation requirements. PKC is a primary target of DAG, and as such has been the object of intense research. PKC is a ubiquitous enzyme, highly enriched in the brain, where it plays a significant role in regulating both pre- and postsynaptic aspects of neurotransmission (68). In addition, PKC is active in many other cellular processes, including transmembrane glucose transport, secretion, exocytosis, long-term alterations in gene expression and plasticity, modulation of ion conductance, cell proliferation, and desensitization of extracellular receptors (68). The ratios of platelet membrane bound to cytosolic PKC activities were found to be elevated in subjects experiencing a manic episode (69). In addition, serotonin-elicited platelet PKC translocation, as well as PKC isozyme levels, activity, and translocation, were found to be enhanced in postmortem brain tissue of patients with BD (70). Certain PKC isoforms have been also shown to phosphorylate and inactivate GSK-3 in vitro (35).
The protein MARCKS, a downstream target for PI/PKC signaling, is a fundamental substrate of PKC, and has been implicated in signaling and neuroplastic events associated with cytoskeletal architecture (71, 72). Indeed, activation of PKC results in the post-translational phosphorylation of various proteins, but MARCKS is its major substrate in the brain (73).
Lithium’s neurotrophic effects targeting at the PI cycle, PKC, and MARCKS: general aspects
In the PI cycle, lithium blocks inositol monophosphatase (IMPase), preventing conversion of inositol-1-phosphate (IP1) to myoinositol. This leads to accumulation of inositol monophosphates and depletion of myoinositol, which reduces PIP2 and its cleavage products—IP3 and DAG. Dampening the PI cycle with lithium treatment downregulates PKC isozymes. Among PKC substrates, MARCKS is particularly relevant (Fig. 1). MARCKS is an actin-binding protein expressed in neurites and implicated in cytoskeletal restructuring; its expression is downregulated by lithium, thereby stabilizing the neuronal membrane and representing an important target for its neurotrophic effects.
Lithium’s neurotrophic effects on the PI cycle
It has been proposed that lithium induces its therapeutic effects in BD by reducing cellular levels of myoinositol and PIP2 concentrations (see Fig. 1), but data are not homogeneous, and depend largely on the model/cell under investigation and the sample size (74–79). IMPase is the final, common step for conversion of monophosphorylated inositols into myoinositol, and thus inhibition of IMPase can decrease myoinositol levels. Indeed, lithium’s inhibition of these enzymes led to the inositol depletion hypothesis of lithium’s action, which suggests that lithium, via inhibition of IMPase, decreases the availability of myoinositol, and thus the amount of PIP2 available for G-protein-mediated signaling events that rely upon this pathway (80); this has not been well established using in vivo studies.
A significant decrease in myoinositol levels in the right frontal lobe induced by lithium in BD subjects prior to clinical response was described using MRS (32). Inhibition of IMPase and the consequent reduction of myoinositol levels have been observed after lithium treatment. Also, lithium-induced reductions of myoinositol levels were found in children and adults with BD in brain regions previously implicated in this disorder (32, 81, 82). Another recent study using lithium-7 and proton magnetic resonance spectroscopy (1H-MRS) evaluated the effects of brain lithium on NAA and myoinositol levels in older adults with BD treated with lithium. In this cross-sectional study, lithium treatment was associated with higher NAA levels and higher myoinositol levels. Mitochondrial NAA is a key regulator of neuronal viability; thus, the investigators suggested that the elevated NAA levels after lithium treatment supports lithium’s mitochondrial-enhancing properties (see also “General aspects of mitochondrial and ER regulation of oxidative stress and apoptosis: relevance for BD pathophysiology”, p. 99), in addition to its neurotrophic effects (83).
Hypothetically, the brain would be particularly sensitive to lithium because of inositol’s relatively poor penetration across the blood-brain barrier (84), or because of the reduced ability of specific neuronal populations to transport inositol across their cell membranes (74). Notably, lithium was also found to reduce free inositol levels in brain sections and in the brains of rodents (85). However, at least one recent study challenges this hypothesis; investigators found that the reduced intracellular inositol associated with brain sodium–myoinositol transporter (SMIT1) knockout in mice had no effect on PI levels (86).
Lithium’s neurotrophic effects at the PKC/MARCKS pathways
Lithium has been shown to alter signaling pathways that also directly regulate neurotrophic effects (3, 69, 87–90). In particular, chronic treatment with lithium in rats significantly decreased PKC stimulation-induced release with phorbol esters in cortex, hypothalamus, and hippocampus. In addition, chronic lithium administration resulted in reduced isoforms of PKC α and ε (91). Interestingly, addition of inositol reverses the lithium-induced downregulation of MARCKS, suggesting that MARCKS downregulation was regulated by the PI pathway (72). Further support for the involvement of the PKC signaling pathway in the pathophysiology of BD comes from two independent genome-wide association studies that identified diacylglycerol kinase eta (DGKH) as a risk gene for BD (92). DGKH is a major regulator of DAG, which activates all known conventional and novel isoforms of PKC, but may also involve non-PKC targets.
With regard to MARCKS regulation, studies have shown that despite being structurally dissimilar, both lithium and valproate (VPA) have very similar effects on MARCKS (88, 93). Lithium-induced decrease of MARCKS protein was followed by downregulation of MARCKS messenger ribonucleic acid (mRNA) (70). Also, synthesis of nascent RNA for MARCKS and its promoter Macs were significantly reduced in chronic, but not acute, lithium-treated immortalized hippocampal cells (70), suggesting a neurotrophic effect through this pathway only after chronic lithium treatment.
Neurotrophic signaling cascades
General aspects of neurotrophic signaling cascades: relevance for BD pathophysiology
Neurotrophic factors not only increase cell survival by providing necessary trophic support, but also play an important role in inhibiting apoptosis cascades and activating cell survival pathways. The cascade reactions of the two major cell survival pathways—the extracellular regulated kinase (ERK)/mitogen activated protein kinase (MAPK) signaling pathway and the PI3K–Akt pathway—are known to critically regulate cell survival and growth of neurons during development and the survival and function of adult neurons, as well as modulate synaptic transmission and plasticity. p90 ribosomal S6 kinase (RSK) also activates transcription factors that positively influence survival, and its upstream target, ERK, is activated by Raf and MAPK (Fig. 1).
Members of the neurotrophin family include brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin (NT)-3, NT-4, NT-5, and NT-6. BDNF and other neurotrophic factors are essential for neuronal survival and functioning; thus, it is possible that a continuous diminution of these factors could affect neuronal viability [reviewed in (94, 95)]. BDNF also has acute effects on synaptic plasticity and on the facilitation of glutamate, γ-aminobutyric acid (GABA), dopamine, and serotonin release. In vitro and in vivo studies have shown that CREB and BDNF act together to mediate the neurotrophic and therapeutic effects of lithium (discussed in the next section).
One key issue is that dysregulation at any target in the BDNF-ERK-CREB elements (such as by unrelenting cellular stress) could possibly explain the consequent atrophic processes in a selective subpopulation of vulnerable neurons and/or distal neurons. It is therefore possible that the exact kinetics of ERK and CREB activation will ultimately determine whether the activated kinases participate in a cell survival- or death-promoting pathway. Chronic stress (i.e., foot shock, an animal model of depression) in rats resulted in ERK1/2 hyperphosphorylation in dendrites of the higher prefrontal cortical layers of rat brains, whereas phospho-CREB was reduced in several cortical regions including the frontal cortex (96). Because CREB is phosphorylated and activated by phospho-ERK1/2 directly, this phospho-CREB decrease indicates that chronic stress could downregulate CREB phosphorylation indirectly, and subsequently decrease the transcription of neurotrophic genes such as BDNF. Indeed, a number of human studies reported decreased BDNF levels in manic and depressive episodes, also considered a potential biomarker of neuronal dysfunction and chronic stress [reviewed in (97)].
Lithium’s neurotrophic effects targeting neurotrophic signaling cascades
In the ERK/MAPK signaling cascade, ERK activation results in enhanced transcription of neurogenesis and cell survival factors such as the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) (described in the next section) and BDNF. Lithium activated the ERK/MAPK cascade in human neuroblastoma SH-SY5Y cells (98). In vivo, chronic lithium increased activated ERK levels in the frontal cortex and hippocampus (99). Lithium also activated ERK1/2 after ischemia, and significantly increased cell proliferation in the hippocampal dentate gyrus (100).
Lithium’s effects on CREB phosphorylation and activity have been investigated in several preclinical studies, with mixed results (99, 101, 102). Postmortem studies in BD subjects have noted reduced CREB phosphorylation in lithium-treated BD subjects (103, 104), but the instability of phosphorylated proteins postmortem is well known and of concern in such reports. Also, chronic lithium treatment prevented stress-induced decreases in dendritic length, as well as stress-induced increases in CREB phosphorylation in the hippocampus (105). CREB is also modulated by the MAPK signaling pathway (Fig. 1). Thus, it is difficult to distinguish whether lithium’s actions result from either CREB or MAPK signaling pathways. Regarding other transcription factors, lithium increased the transactivation of AP-1, and also enhanced DNA binding (106).
Regarding BDNF regulation by lithium, its chronic administration increased BDNF expression in the rodent brain (99, 107), particularly in the hippocampus (108) and frontal cortex (109). Specifically, a recent study found that chronic treatment of cultured rat cortical neurons with therapeutic concentrations of lithium selectively increased the levels of exon IV-containing BDNF mRNA, and the activity of BDNF promoter IV (110). Also, a recent microarray gene expression study of the molecular pharmacology of lithium on mouse brain mRNA supports the importance of BDNF to lithium’s molecular effects (111). However, another study found that BDNF was not upregulated by chronic lithium treatment in therapeutically relevant doses (112). It is important to note that recent evidence suggests that the neurotrophic effect of lithium in cortical neurons requires BDNF expression (113).
Finally, human studies have also described decreased BDNF levels in lymphoblasts from patients with BD who responded to lithium compared with matched healthy controls and their unaffected relatives (114). Another study examining lithium response and BDNF gene polymorphism found that the Val/Met genotype of Val66Met polymorphism may be associated with degree of prophylactic response to lithium; there was a trend for a higher incidence of the Met allele in positive responders to lithium than in nonresponders (115).
Similar to BDNF, treatment with lithium for six weeks altered brain concentrations of NGF and glial cell line-derived neurotrophic factor in a rat model of depression (116). In another study, chronic (14 days) but not acute (one day) administration of lithium in adult rats at various doses (levels of 0.72 mM) significantly increased NGF concentrations in the frontal cortex (+23%), limbic forebrain (+47%), hippocampus (+72%), and amygdala (+74%) (117). Correspondingly, lithium increased serum and hippocampal NT-3 levels in an animal model of mania (118). Recent studies have also pointed to the potential role of vascular endothelial growth factor (VEGF) in lithium’s neurotrophic effects. VEGF, which is considered to be a neurotrophic factor, has been implicated in neuronal survival, neurotrophic effects, regeneration, growth, and differentiation. A recent study found that lithium upregulated VEGF in brain endothelial cells and astrocytes (119); it also significantly attenuated the decreased expression of VEGF in the hippocampus in stressed animals (120).
Mitochondrial and ER regulation of oxidative stress and apoptosis: a role for Bcl-2, IP3, and calcium
General aspects of mitochondrial and ER regulation of oxidative stress and apoptosis: relevance for BD pathophysiology
The widely described presence of apoptosis in neural cells from individuals with BD occurs through diverse mechanisms. Two important pathophysiological effects in BD are increased oxidative stress parameters directly regulated by mitochondrial Bcl-2 and NAA (see “Cellular stress, atrophy, and loss in BD: therapeutic targets for lithium”, p. 93); these are associated with altered intracellular calcium levels through specific ER calcium regulation that occurs via the IP3 receptors (IP3R) and Bcl-2. Indeed, the primary function of IP3 is the release of calcium from the ER. IP3, along with DAG, is formed by members of the PI-PLC family through cleavage of PIP2 (Fig. 1) [described in “The phosphoinositol (PI) cycle, PKC, and myristoylated alanine-rich C-kinase substrate (MARCKS)”, p. 96]. The release of calcium from the ER is an important signaling event in all cells (63, 64). In this context, it is important to note that altered calcium dynamics has been the most reproducible biological measure in the pathophysiology of BD [for an excellent review, see (121)].
A large movement of calcium into the mitochondria will exceed mitochondrial capacity to export protons, potentially interrupting adenosine triphosphate (ATP) synthesis and the activation of the permeability transition pore with release of cytochrome c, thus initiating cellular apoptosis. In addition, excessive production of reactive oxygen species (ROS, or free radicals) triggered by mitochondrial dysfunction (whether or not this is related to lower antioxidant capacity) may lead to oxidative stress. Glutathione is the main antioxidant substrate in all tissues, and its production is rate-limited by its precursor, cysteine; notably, glutathione alterations have been reported in BD (122, 123). Furthermore, a recent study evaluated oxidative stress parameters in unmedicated and treated BD subjects during an initial manic episode; the investigators found that the end products of lipid peroxidation, thiobarbituric acid reactive substances (TBARS) and antioxidant enzyme activity [superoxide dismutase (SOD) and catalase (CAT)] were increased in unmedicated manic patients compared to healthy controls (124).
With regard to Bcl-2, it has been shown to attenuate cell death in the ER and mitochondria by limiting calcium overload and cytochrome c release, sequestering proforms of death-inducing caspase enzymes, and controlling mitochondrial calcium release. Severe stress has been shown to aggravate stroke outcome by suppressing Bcl-2 expression (125); mice in a situation of aggressive social stress showed a significantly lower (approximately 70%) expression of Bcl-2 mRNA compared to unstressed mice following ischemia. In addition, stress significantly worsened the size of the infarct area in control mice, but not in transgenic mice that constitutively express increased Bcl-2, which appears to be a neuronal neurotrophic factor.
Lithium’s neurotrophic effects targeting the mitochondrial and ER regulation of oxidative stress and apoptosis
Lithium’s regulatory effects on apoptosis-controlling proteins appear to occur in both the mitochondria and ER (126–128). Specifically, lithium’s neurotrophic effects against thapsigargin-induced intracellular calcium increase has been described, with elevated expression of the molecular chaperones GRP78, Bcl-2, and AP-1 (129). In another study, lithium also enhanced the expression of ER chaperones proteins GRP78, GRP94, and calreticulin, thus protecting against the deleterious effects of malfolded proteins (130). Interestingly, depletion of neuronal IP3 (located in the ER) represents a direct effect by lithium and other mood stabilizers to increase neuronal growth-cone area, and was reversed by inositol (131).
Increasing evidence exists that mood disorders are associated with oxidative stress and DNA damage, making lithium’s purported antioxidant properties particularly important. Chronic treatment with lithium also inhibited reactive oxygen metabolite H2O2-induced cell death and increased glutathione levels in primary cultured rat cerebral cortical cells (132). Chronic lithium treatment directly inhibited oxidative damage to lipids and proteins (133). It also increased mRNA and protein levels of the cytosolic glutathione s-transferase (GST) isoenyzmes and inhibited H2O2-induced cell death and DNA fragmentation (134). In bipolar mania, acute treatment with lithium significantly increased CAT levels and reduced TBARS levels, demonstrating antioxidant effects. The authors concluded that initial manic episodes are associated with both increased oxidative stress parameters and activated antioxidant defenses, which may be related to energy metabolism and neuroplasticity pathway dysfunction (124).
Several biochemical changes have been hypothesized for lithium’s neurotrophic properties against oxidative stress and apoptosis, including its ability to regulate Bcl-2. Bcl-2 levels were robustly increased in frontal cortex after lithium treatment, particularly in layers II and III (135). Also, long-term, but not acute, treatment with lithium of cultured cerebellar granule cells induced concentration-dependent decreases in mRNA of p53 and protein levels of Bax (both of which are pro-apoptotic); conversely, Bcl-2 mRNA and protein levels increased considerably. It was estimated that the Bcl-2/Bax protein-level ratio increased approximately fivefold after lithium treatment for five to seven days (136). In addition, chronic treatment with lithium in drinking water prevented the aluminum-induced translocation of cytochrome c, upregulating Bcl-2 and Bcl-X(L), and thus reducing DNA damage.
Lithium’s neurotrophic effects: potential therapeutic relevance in other psychiatric and neurological disorders
Preclinical models of apoptosis and excitotoxicity
Because of its neuroprotective effects against a variety of insults, lithium has garnered considerable interest as a neuroprotective drug for a broad range of central nervous system disorders. Indeed, a number of in vitro models have been used to evaluate lithium’s neuroprotective properties against excitotoxicity or apoptosis, including glutamate-induced excitotoxicity, C2-ceramide, oxygen and glucose deprivation, aluminum, low potassium medium, ouabain, β-bungarotoxin, and pilocarpine-induced mossy-fiber sprouting.
Over a decade ago, Nonaka and colleagues (137) reported that chronic, but not acute, lithium treatment at therapeutic doses robustly protected neurons against glutamate excitotoxicity by inhibiting N-methyl-D-aspartate (NMDA) receptor-mediated calcium influx. Lithium also markedly reduced the level of NR2B phosphorylation at Tyr1472, which inactivated NMDA receptors (NMDAR) and contributed to neuroprotective effects against excitotoxicity (138). Similarly, inhibition of NR2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by postsynaptic density (PSD)-95 may contribute to the lithium-induced neuroprotective effects against NMDAR excitotoxicity (139).
Regulation of c-Jun N-terminal kinase, p38 kinase and activator protein-1 (AP-1) DNA binding also appear to be relevant to lithium’s neuroprotective effects against glutamate excitotoxicity. Pretreatment with lithium blocked glutamate-induced effects and apoptosis in rat cerebellar granule cells by preventing the rapid activation of c-Jun N-terminal kinase and p38 kinase, and by increasing AP-1 binding. These neuroprotective effects were also mediated in part by suppressing NMDAR-mediated activation of the MAPK pathway (140). Additional studies have demonstrated that chronic treatment with lithium at therapeutically relevant concentrations attenuated NMDA-mediated cytoplasmic vacuolization in primary rat hippocampal neurons (141). Similarly, lithium in combination with a GSK inhibitor and VPA blocked against glutamate-induced cell excitotoxicity in aging cerebellar granule cells (127).
High doses of lithium (5 mM) had neuroprotective effects against C2-ceramide (N-acetyl-sphingosine)-induced apoptosis in primary cultures of cerebellar granule neurons (142), presumably by blocking the dephosphorylation of both protein kinase B (PKB) and GSK (143); however, the doses used in this study would be toxic for humans. In cultures exposed to oxygen and glucose deprivation in a model of ischemia, lithium treatment (0.2–1.2 mM) had neuroprotective effects in hippocampal slices at different doses (144) (see next section). In another model of excitotoxicity using ouabain (a potent inhibitor of sodium-potassium adenosine triphosphatase), lithium pretreatment in human neuroblastoma SH-SY5Y cells at 1 mM diminished ouabain-induced lactate dehydrogenase (LDH, a measure of cellular damage) (145).
Lithium also prevented colchicine-induced apoptosis in rat cerebellar granule neurons (146). Finally, in another cell culture model of neurotoxicity, therapeutically relevant concentrations of lithium (1.2 mM) protected against β-bungarotoxin in primary cultured granular neurons. The investigators hypothesized that the neuroprotective effects of lithium were due to its ability to attenuate intracellular calcium overload (147). Indeed, one study suggested that lithium selectively prevents apoptosis in cortical neurons by increasing calcium through activation of PI3-K and PLC (148). Treatment with therapeutic doses of lithium was also neuroprotective against ATP-induced cell death in hippocampal slices of adult rats (149).
As is clear from the evidence reviewed above, lithium’s neuroprotective properties occur via diverse mechanisms. Furthermore, some of the mechanisms involved in lithium’s neuroprotective effects are not shared by other mood stabilizers. For instance, lithium and VPA were found to have dissimilar effects on the PI3-K/PKB pathways; in addition, only lithium (and not VPA) protected against C2-ceramide-induced apoptosis, presumably due to the inhibition of PKB (150). Similarly, lithium (but not VPA) reduced ouabain-induced LDH release (145).
Lithium’s neuroprotective effects: modeling other psychiatric and neurological disorders
In this section, we review the neuroprotective effects of lithium in a series of models of brain ischemia, injury (especially spinal cord and optic nerve injury), infection, irradiation, neurodegeneration, and neuroinflammation [e.g., cerebral ischemia, Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis (ALS), human immunodeficiency virus (HIV)-associated cognitive impairments, spinocerebellar ataxia, and cranial irradiation].
Nonaka and Chuang (151) examined the effects of chronic lithium pretreatment (16 days) on neurological deficits and brain infarcts induced by left middle cerebral artery occlusion (MCAO, a model of brain ischemia) in rats. Lithium significantly improved neurological deficits, including abnormal posture and hemiplegia and reduced the size of ischemic infarct by nearly 60%, measured 24 h after MCAO. Another study (152) similarly showed that neurological deficits resulting from cerebral ischemia induced by MCAO were decreased in rats treated with lithium 25 and 48 h after ischemia; reduced infarct volume, ischemia-induced caspase-3 immunoreactivity, and AP-1 protein expression were also observed after lithium administration. Lithium also attenuated brain damage and facilitated neurological recovery in rats with cerebral ischemia following MCAO, suggesting that lithium’s neuroprotective effects were due to upregulation of cytoprotective heat shock protein 70 (HSP-70) (59). Chronic lithium treatment also protected against hypoxia in frontal cortex, caudate putamen, hippocampus, and cerebellum; expression of BDNF and phospho-CREB were higher in the frontal cortex (153). Lithium also reduced ischemia-induced hippocampal CA1 damage and behavioral deficits (memory impairment) in gerbils. These behavioral benefits were associated with an increase in viable cells associated with downregulation of pro-apoptotic p53 in the CA1, and upregulation of anti-apoptotic Bcl-2 and HSP-70 in the ischemic brain (154).
With regard to Alzheimer’s disease, diverse studies have suggested that lithium’s neuroprotective effects may have a potential role in the therapeutics of this disease. Lithium protected against the deposition of β-amyloid peptide, the hyperphosphorylation of tau protein, and the death of neurons in certain brain regions, all of which are characteristic features of Alzheimer’s disease. Specifically, lithium protected cultured neurons against β-amyloid-induced cell death (i.e., neurodegeneration) (155). Moreover, lithium treatment abolished GSK-3-mediated β-amyloid peptide increase and reduced the plaque burden in the brains of GSK-3 transgenic mice (57). Lithium-mediated reductions in GSK-3β and cyclin-dependent kinase 5 (cdk5) activity, tau phosphorylation, apoptotic activity, and cell death were observed, and provide a strong rationale for the use of lithium as a potential treatment in neurodegenerative diseases (156). Chronic lithium treatment was also effective in a transgenic model (amyloid precursor protein transgenic mice) of Alzheimer’s disease, as indicated by improved performance in the water maze paradigm, preservation of the dendritic structure in the frontal cortex and hippocampus, and decreased tau phosphophylation (157). In humans, ongoing clinical trials are evaluating lithium’s abilities to lower tau and β-amyloid levels in the cerebrospinal fluid of Alzheimer’s patients (158). Exposure of cultured cortical neurons to lithium decreased tau protein levels, a decrease associated with reduced tau mRNA levels (159).
While lithium has obvious neuroprotective properties, it is not clear whether it also reduces or prevents the risk of dementia. One recent study found that chronic lithium attenuated dementia risk (160). More recently, a large, observational study evaluating subjects with dementia in Denmark between 1995 and 2005 found that, while individuals who had used lithium at least once had an increased rate of dementia compared to those not exposed to lithium, individuals who used lithium continuously had similar rates of dementia to the general population (161). More formal longitudinal trials are currently underway to provide further information on the relevance of lithium’s neuroprotective effects in dementia disorders.
Lithium has been also tested in animal models of Huntington’s disease. Chronic lithium significantly reduced the size of quinolinic acid-induced striatal lesions, potentially associated with increased Bcl-2 protein levels (162). Correspondingly, Senatorov and colleagues (163) proposed that the neuroprotective properties of lithium are not only due to its ability to inhibit apoptosis but also its ability to stimulate neuronal and astroglial progenitor proliferation in the quinolinic acid-injected striatum. Also, lithium reduced striatal neurodegeneration induced in rats by 3-nitropropionic acid (3-NP) by inhibiting calpain and cdk5 activation, and consequent cellular death (164). Lithium treatment also reduced the intracellular increase in calcium induced by 3-NP (164). In another model of Huntington’s disease in which a mutation results in progressively deteriorating motor function, chronic treatment with lithium significantly improved motor performance when treatment was started post- but not presymptomatically (165). Such improvement suggests that lithium may improve motor symptoms in some patients with Huntington’s disease.
In a model of Parkinson’s disease, four weeks of lithium treatment prevented N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. MPTP depletes striatal dopamine and its metabolites in mice. Because lithium treatment prevented reduction of striatal Bcl-2 in MPTP-treated mice, but the opposite effect was seen with Bax, this effect was hypothesized to be mediated by Bcl-2 and Bax (166).
Recently, Feng and colleagues (167) investigated the effects of lithium and VPA on symptom onset, survival time, and neurological deficits in copper zinc superoxide dismutase (SOD1) G93A mutant mice, a model of ALS; they observed delayed onset and decreased neurological deficit after treatment. In a parallel study of G93A mice, lithium also delayed disease onset and duration and augmented life span (168). Notably, there is also clinical evidence to support the neuroprotective properties of lithium in ALS. A 15-month, parallel-group, randomized study of adults with ALS found that none of the patients with ALS treated with lithium (n = 16) died; in contrast, 29% of ALS patients who received riluzole died. Furthermore, disease progression was markedly attenuated in the lithium-treated group (168).
A study investigating the neuroprotective effects of lithium pretreatment against HIV-gp120-mediated toxicity in SY5Y neuronal cells found that lithium pretreatment significantly reduced gp120-associated neurotoxicity (169). However, post-treatment with lithium had minimal neuroprotective effects against gp120, both in vivo and in vitro. The investigators raised the possibility that prophylactic treatment with lithium may prevent the onset/progression of HIV-associated cognitive impairments. The presumed mechanism for this neuroprotective effect was hypothesized to be lithium’s ability to protect neurons from neurotoxic secretions of HIV-1-infected monocyte-derived macrophages (MDMs). This neuroprotective effect is believed to be mediated, in part, through the PI3K/Akt and GSK-3 pathways. In another study, lithium restored HIV-1 encephalitis (HIVE)-associated loss of MAP-2-positive neurites and synaptic density while reducing levels or activity of phospho-Tau Ser202, phospho-β-catenin, and GSK-3β (170). These encouraging preclinical findings were extended to humans. In an open-label study, 12 weeks of low-dose oral lithium (600–1200 mg/day) was associated with improved HIV-associated neurocognitive impairment (171).
Spinocerebellar ataxia type 1 (SCA1) is a dominantly inherited neurodegenerative disorder characterized by progressive motor and cognitive dysfunction. In a SCA1 mouse model, chronic administration of lithium had a positive effect on multiple behavioral measures and hippocampal neuropathology (172). Another type of brain injury, cranial irradiation, results in lifelong neurocognitive deficiency in cancer survivors, in part due to radiation-induced apoptosis and decreased neurogenesis in the subgranular zone of the hippocampus. A recent investigation examined whether lithium treatment protects irradiated hippocampal neurons from apoptosis and improves the cognitive performance of irradiated mice. As noted above, lithium treatment of cultured mouse hippocampal neurons activated Akt, inhibited GSK-3β, and increased Bcl-2 protein expression. These effects were sustained when cells were treated with lithium combined with ionizing radiation. Lithium treatment before cranial irradiation also improved performance in the Morris water maze paradigm, suggesting that lithium attenuates the cognitive deficits that result from cranial irradiation (173).
Treatment with GSK-3 inhibitors, including lithium to rats with thoracic spinal cord lesion or contusion injuries, induced significant sprouting in the caudal spinal cord and promoted locomotor functional recovery (174). Similarly, in a rat model of optic nerve injury, chronic treatment with lithium protected retinal ganglion cell survival and axon regeneration; an increase in Bcl-2 immunoreactivity was seen with lithium treatment in retinal ganglion cells (175, 176). Finally, a recent study reported that chronic treatment with lithium upregulated brain arachidonic acid (AA) metabolism in an animal model of neuroinflammation; thus, lithium could possibly be considered in the treatment of human brain diseases associated with neuroinflammation [for a good review, see (177)].
Conclusions and perspectives
BD is a disorder that entails mood episodes as well as considerable structural impairment, potentially secondary to changes in cellular plasticity and resilience. Indeed, a recent meta-analysis and meta-regression of 98 structural studies in BD showed a robust change in brain structure in BD, as well as evidence that lithium increases gray matter volume (178). Overall, lithium seems to be an exogenous support that activates adaptive mechanisms in the brain. Future effective treatments for BD possessing neurotrophic properties are expected to be (at least partially) lithium-mimetic agents.
Neuroprotection is the most consistent biological outcome associated with lithium treatment in both preclinical and clinical models of BD. Paradoxically, lithium is a simple metal with a complex mechanism of action. It is a monovalent cation that is also a very light, reactive, wide-spread, and unstable alkali metal, and thus it has outstanding and unique penetration in most tissues and cells. Its ability to be potentially omnipresent explains its high sensitivity and low specificity for biological effects targeting at neurotrophic pathways, as well as its potentially wide range of clinical indications and undesirable side effects profile.
When evaluating lithium’s neurotrophic effects and their potential relevance in BD in translational models, it is particularly important to consider only data obtained from studies evaluating chronic lithium treatment at therapeutically relevant doses. Studies showing that lithium has neurotrophic effects at doses that are extremely toxic (even lethal) for humans are not uncommon, but these cannot be reliably translated to human diseases, especially BD. Although not addressed by the present review, the study of lithium-responsive gene networks related to neurotrophic effects are promising and may improve our understanding of critical nuclear downstream targets expressing key proteins and peptides. Also, given that lithium has a narrow therapeutic margin and well-known side effects, and that good response rates are observed in only about half of patients, the search for biological predictors of better lithium response has begun [see (179)], although such work needs further replication.
Overall, lithium has been associated with significant neurotrophic properties not only in BD but also in a wide range of models for other brain and neurological disorders. Lithium’s neurotrophic effects in BD are an example of an old molecule acting as a new proof-of-concept agent. We are optimistic that recent novel insights into the mechanisms of action of lithium will ultimately lead to a better understanding of clinically relevant pathophysiological targets, and the consequent development of improved treatments for those who suffer from BD and other devastating disorders.
Acknowledgements
Funding for this work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH) and a NARSAD Award (CAZ). Ioline Henter provided outstanding editorial assistance.
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- 3.Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been over-looked? Biol Psychiatry. 1999;46:929–940. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290:1467–1473. doi: 10.1001/jama.290.11.1467. [DOI] [PubMed] [Google Scholar]
- 5.Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8:625–639. doi: 10.1111/j.1399-5618.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 6.Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- 7.Cade JFJ. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 8.Shoulson I. Neuroprotective clinical strategies for Parkinson’s disease. Ann Neurol. 1992;32:S143–S145. doi: 10.1002/ana.410320725. [DOI] [PubMed] [Google Scholar]
- 9.Shoulson I, Rosenberg RN. Neurotherapeutics, evidence-based neurology, and clinical equipoise. Arch Neurol. 1999;56:524. doi: 10.1001/archneur.56.5.524. [DOI] [PubMed] [Google Scholar]
- 10.Yucel K, McKinnon MC, Taylor VH, et al. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacology. 2007;195:357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]
- 11.Moore GJ, Cortese BM, Glitz DA, et al. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009 doi: 10.4088/JCP.07m03745. [Epub ahead of print]; doi 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 12.Haldane M, Frangou S. New insights help define the pathophysiology of bipolar affective disorder: neuroimaging and neuropathology findings. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:943–960. doi: 10.1016/j.pnpbp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 2002;4:105–116. doi: 10.1034/j.1399-5618.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 14.Öngur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambilla P, Stanley JA, Nicoletti MA, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86:61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Konarski J, McIntyre RS, Soczynska JK, Kennedy SH. Neuroimaging approaches in mood disorders: technique and clinical implications. Ann Clin Psychiatry. 2007;19:265–277. doi: 10.1080/10401230701653435. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 18.Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Silverstone PH, McGrath BM, Kim H. Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005;7:1–10. doi: 10.1111/j.1399-5618.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 20.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 21.Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 22.Kato T. Mitochondrial dysfunction as the molecular basis of bipolar disorder: therapeutic implications. CNS Drugs. 2007;21:1–11. doi: 10.2165/00023210-200721010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 24.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biol Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 25.Phillips ML, Travis MJ, Fagiolini A, Kupfer D. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sassi RB, Brambilla P, Hatch JP, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Sassi RB, Nicoletti M, Brambilla P, et al. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 28.Bearden CE, Thompson PM, Dalwani M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bearden CE, Thompson PM, Dutton RA, et al. Three-dimensional mapping of hippocampal anatomy in un-medicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 31.Silverstone PH, Wu RH, O’Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Moore GJ, Bebchuk JM, Parrish JK, et al. Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am J Psychiatry. 1999;156:1902–1908. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]
- 33.Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 34.Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett. 2004;368:123–126. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 35.Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267:16878–16882. [PubMed] [Google Scholar]
- 36.Gould TD, Zarate CA, Manji HK. Glycogen synthase kinase-3: a target for novel bipolar disorder treatments. J Clin Psychiatry. 2004;65:10–21. [PubMed] [Google Scholar]
- 37.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 38.Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chin PC, Majdzadeh N, D’Mello SR. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res Mol Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 41.Jope RS. A bimodal model of the mechanism of action of lithium. Mol Psychiatry. 1999;4:21–25. doi: 10.1038/sj.mp.4000444. [DOI] [PubMed] [Google Scholar]
- 42.Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- 43.Jope RS, Bijur GN. Mood stabilizers, glycogen synthase kinase-3beta and cell survival. Mol Psychiatry. 2002;7:S35–S45. doi: 10.1038/sj.mp.4001017. [DOI] [PubMed] [Google Scholar]
- 44.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 46.Munoz-Montano JR, Moreno FJ, Avila J, Diaz-Nido J. Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett. 1997;411:183–188. doi: 10.1016/s0014-5793(97)00688-1. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien WT, Harper AD, Jove F, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 49.Mendes CT, Mury FB, de Sá Moreira E, et al. Lithium reduces Gsk3b mRNA levels: implications for Alzheimer Disease. Eur Arch Psychiatry Clin Neurosci. 2009;259:16–22. doi: 10.1007/s00406-008-0828-5. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 52.Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 53.Kaidanovich O, Eldar-Finkelman H. The role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Expert Opin Ther Targets. 2002;6:555–561. doi: 10.1517/14728222.6.5.555. [DOI] [PubMed] [Google Scholar]
- 54.Bhat RV, Budd SL. GSK3beta signalling: casting a wide net in Alzheimer’s disease. Neurosignals. 2002;11:251–261. doi: 10.1159/000067423. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez G, Muñoz-Montaño JR, Satrústegui J, Avila J, Bogónez E, Díaz-Nido J. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 2002;4:153–165. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Sato S, Murayama O, et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321:61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- 57.Su Y, Ryder J, Li B, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- 58.Chuang DM, Chen RW, Chalecka-Franaszek E, et al. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 59.Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci U S A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhat RV, Shanley J, Correll MP, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 62.Kerkela R, Kockeritz L, Macaulay K, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008;118:3609–3618. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gould TD, Manji HK. Signaling networks in the pathophysiology and treatment of mood disorders. J Psychosom Res. 2002;53:687–697. doi: 10.1016/s0022-3999(02)00426-9. [DOI] [PubMed] [Google Scholar]
- 64.Quiroz JA, Gray NA, Kato T, Manji HK. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- 65.Casabona G. Intracellular signal modulation: a pivotal role for protein kinase C. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:407–425. doi: 10.1016/s0278-5846(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 67.Serova M, Ghoul A, Benhadji KA, et al. Preclinical and clinical development of novel agents that target the protein kinase C family. Semin Oncol. 2006;33:466–478. doi: 10.1053/j.seminoncol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 69.Friedman E, Hoau Y-W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry. 1993;33:520–525. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 70.Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biol Psychiatry. 1996;40:568–575. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- 71.Lenox RH, Watson DG, Patel J, Ellis J. Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain Res. 1992;570:333–340. doi: 10.1016/0006-8993(92)90598-4. [DOI] [PubMed] [Google Scholar]
- 72.Watson DG, Lenox RH. Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J Neurochem. 1996;67:767–777. doi: 10.1046/j.1471-4159.1996.67020767.x. [DOI] [PubMed] [Google Scholar]
- 73.Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993;268:1501–1504. [PubMed] [Google Scholar]
- 74.Gani D, Downes CP, Batty I, Bramham J. Lithium and myo-inositol homeostasis. Biochim Biophys Acta. 1993;1177:253–269. doi: 10.1016/0167-4889(93)90121-5. [DOI] [PubMed] [Google Scholar]
- 75.Kendall DA, Nahorski SR. Acute and chronic lithium treatments influence agonist and depolarization-stimulated inositol phospholipid hydrolysis in rat cerebral cortex. J Pharmacol Exp Ther. 1987;241:1023–1027. [PubMed] [Google Scholar]
- 76.Kennedy ED, Challiss RA, Nahorski SR. Lithium reduces the accumulation of inositol polyphosphate second messengers following cholinergic stimulation of cerebral cortex slices. J Neurochem. 1989;53:1652–1655. doi: 10.1111/j.1471-4159.1989.tb08566.x. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy ED, Challiss RA, Ragan CI, Nahorski SR. Reduced inositol polyphosphate accumulation and inositol supply induced by lithium in stimulated cerebral cortex slices. Biochem J. 1990;267:781–786. doi: 10.1042/bj2670781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kofman O, Belmaker RH, Grisaru N, et al. Myo-inositol attenuates two specific behavioral effects of acute lithium in rats. Psychopharmacol Bull. 1991;27:185–190. [PubMed] [Google Scholar]
- 79.Tricklebank MD, Singh L, Jackson A, Oles RJ. Evidence that a proconvulsant action of lithium is mediated by inhibition of myo-inositol phosphatase in mouse brain. Brain Res. 1991;558:145–148. doi: 10.1016/0006-8993(91)90732-b. [DOI] [PubMed] [Google Scholar]
- 80.Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 81.Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 82.Yildiz A, Demopulos CM, Moore CM, Renshaw PF, Sachs GS. Effect of lithium on phosphoinositide metabolism in human brain: a proton decoupled (31)P magnetic resonance spectroscopy study. Biol Psychiatry. 2001;50:3–7. doi: 10.1016/s0006-3223(01)01069-1. [DOI] [PubMed] [Google Scholar]
- 83.Forester BP, Finn CT, Berlow YA, Wardrop M, Renshaw PF, Moore CM. Brain lithium, N-acetyl aspartate and myo-inositol levels in older adults with bipolar disorder treated with lithium: a lithium-7 and proton magnetic resonance spectroscopy study. Bipolar Disord. 2008;10:691–700. doi: 10.1111/j.1399-5618.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berridge MJ. The Albert Lasker Medical Awards. Inositol trisphosphate, calcium, lithium, and cell signaling. JAMA. 1989;262:1834–1841. [PubMed] [Google Scholar]
- 85.Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971;233:267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- 86.Berry GT, Buccafusca R, Greer JJ, Eccleston E. Phosphoinositide deficiency due to inositol depletion is not a mechanism of lithium action in brain. Mol Genet Metab. 2004;82:87–92. doi: 10.1016/j.ymgme.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Manji HK, Etcheberrigaray R, Chen G, Olds JL. Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J Neurochem. 1993;61:2303–2310. doi: 10.1111/j.1471-4159.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 88.Chen G, Manji HK, Hawver DB, Wright CB, Potter WZ. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J Neurochem. 1994;63:2361–2364. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- 89.Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1999;1:81–86. doi: 10.1034/j.1399-5618.1999.010204.x. [DOI] [PubMed] [Google Scholar]
- 90.Seelan RS, Khalyfa A, Lakshmanan J, Casanova MF, Parthasarathy RN. Deciphering the lithium transcriptome: microarray profiling of lithium-modulated gene expression in human neuronal cells. Neuroscience. 2008;151:1184–1197. doi: 10.1016/j.neuroscience.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 91.Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 92.Baum AE, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watson DG, Watterson JM, Lenox RH. Sodium valproate down-regulates the myristoylated alanine-rich C kinase substrate (MARCKS) in immortalized hippocampal cells: a property of protein kinase C-mediated mood stabilizers. J Pharmacol Exp Ther. 1998;285:307–316. [PubMed] [Google Scholar]
- 94.Mocchetti I, Brown M. Targeting neurotrophin receptors in the central nervous system. CNS Neurol Disord Drug Targets. 2008;7:71–82. doi: 10.2174/187152708783885138. [DOI] [PubMed] [Google Scholar]
- 95.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 96.Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA. Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: implications for stress-related cortical pathology? Eur J Neurosci. 2002;15:1681–1691. doi: 10.1046/j.1460-9568.2002.02000.x. [DOI] [PubMed] [Google Scholar]
- 97.Kapczinski F, Frey BN, Kauer-Sant’Anna M, Grassi-Oliveira R. Brain-derived neurotrophic factor and neuroplasticity in bipolar disorder. Expert Rev Neurother. 2008;8:1101–1113. doi: 10.1586/14737175.8.7.1101. [DOI] [PubMed] [Google Scholar]
- 98.Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- 99.Einat H, Yuan P, Gould TD, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 101.Ozaki N, Chuang DM. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J Neurochem. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- 102.Tardito D, Tiraboschi E, Kasahara J, Racagni G, Popoli M. Reduced CREB phosphorylation after chronic lithium treatment is associated with down-regulation of CaM kinase IV in rat hippocampus. Int J Neuropsychopharmacol. 2007;10:491–496. doi: 10.1017/S1461145706007140. [DOI] [PubMed] [Google Scholar]
- 103.Stewart RJ, Chen B, Dowlatshahi D, MacQueen GM, Young LT. Abnormalities in the cAMP signaling pathway in post-mortem brain tissue from the Stanley Neuropathology Consortium. Brain Res Bull. 2001;55:625–629. doi: 10.1016/s0361-9230(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 104.Young LT, Bezchlibnyk YB, Chen B, Wang JF, MacQueen GM. Amygdala cyclic adenosine monophosphate response element binding protein phosphorylation in patients with mood disorders: effects of diagnosis, suicide, and drug treatment. Biol Psychiatry. 2004;55:570–577. doi: 10.1016/j.biopsych.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 105.Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen G, Hasanat KA, Bebchuk JM, Moore GJ, Glitz D, Manji HK. Regulation of signal transduction pathways and gene expression by mood stabilizers and antidepressants. Psychosom Med. 1999;61:599–617. doi: 10.1097/00006842-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 107.Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- 108.Frey BN, Andreazza AC, Rosa AR, et al. Lithium increases nerve growth factor levels in the rat hippocampus in an animal model of mania. Behav Pharmacol. 2006;17:311–318. doi: 10.1097/01.fbp.0000205013.59455.09. [DOI] [PubMed] [Google Scholar]
- 109.Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024:183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 110.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 111.McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics. 2007;17:605–617. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- 112.Hammonds MD, Shim SS, Feng P, Calabrese JR. Effects of subchronic lithium treatment on levels of BDNF, Bcl-2 and phospho-CREB in the rat hippocampus. Basic Clin Pharmacol Toxicol. 2007;100:356–359. doi: 10.1111/j.1742-7843.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- 113.Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002;43:1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 114.Tseng M, Alda M, Xu L, et al. BDNF protein levels are decreased in transformed lymphoblasts from lithium-responsive patients with bipolar disorder. J Psychiatry Neurosci. 2008;33:449–453. [PMC free article] [PubMed] [Google Scholar]
- 115.Rybakowski JK, Suwalska A, Skibinska M, et al. Prophylactic lithium response and polymorphism of the brain-derived neurotrophic factor gene. Pharmacopsychiatry. 2005;38:166–170. doi: 10.1055/s-2005-871239. [DOI] [PubMed] [Google Scholar]
- 116.Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA. Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in a rat model of depression. Int J Neuropsychopharmacol. 2003;6:225–231. doi: 10.1017/S1461145703003468. [DOI] [PubMed] [Google Scholar]
- 117.Hellweg R, Lang UE, Nagel M, Baumgartner A. Subchronic treatment with lithium increases nerve growth factor content in distinct brain regions of adult rats. Mol Psychiatry. 2002;7:604–608. doi: 10.1038/sj.mp.4001042. [DOI] [PubMed] [Google Scholar]
- 118.Walz JC, Frey BN, Andreazza AC, et al. Effects of lithium and valproate on serum and hippocampal neurotrophin-3 levels in an animal model of mania. J Psychiatr Res. 2008;42:416–421. doi: 10.1016/j.jpsychires.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 119.Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silva R, Martins L, Longatto-Filho A, Almeida OF, Sousa N. Lithium prevents stress-induced reduction of vascular endothelium growth factor levels. Neurosci Lett. 2007;429:33–38. doi: 10.1016/j.neulet.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 121.Warsh JJ, Andreopoulos S, Li PP. Role of intracellular calcium signaling in the pathophysiology and pharmacotherapy of bipolar disorder: current status. Clin Neurosci Res. 2004;4:201–213. [Google Scholar]
- 122.Andreazza AC, Cassini C, Rosa AR, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res. 2007;41:523–529. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 123.Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–175. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 124.Machado-Vieira R, Andreazza AC, Viale CI, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett. 2007;421:33–36. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 125.DeVries AC, Joh HD, Bernard O, et al. Social stress exacerbates stroke outcome by suppressing Bcl-2 expression. Proc Natl Acad Sci U S A. 2001;98:11824–11828. doi: 10.1073/pnas.201215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ghribi O, Herman MM, Spaulding NK, Savory J. Lithium inhibits aluminum-induced apoptosis in rabbit hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, Bax elevation and caspase-3 activation. J Neurochem. 2002;82:137–145. doi: 10.1046/j.1471-4159.2002.00957.x. [DOI] [PubMed] [Google Scholar]
- 127.Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yeste M, Alvira D, Verdaguer E, et al. Evaluation of acute antiapoptotic effects of Li+ in neuronal cell cultures. J Neural Transm. 2007;114:405–416. doi: 10.1007/s00702-006-0557-8. [DOI] [PubMed] [Google Scholar]
- 129.Hiroi T, Wei H, Hough C, Leeds P, Chuang DM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J. 2005;5:102–111. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- 130.Shao L, Sun X, Xu L, Young LT, Wang JF. Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci. 2006;78:1317–1323. doi: 10.1016/j.lfs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 131.Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 132.Cui J, Shao L, Young LT, Wang JF. Role of glutathione in neuroprotective effects of mood stabilizing drugs. Neuroscience. 2007;144:1447–1453. doi: 10.1016/j.neuroscience.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry. 2005;58:879–884. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 134.Shao L, Cui J, Young LT, Wang JF. The effect of mood stabilizer lithium on expression and activity of glutathione s-transferase isoenzymes. Neuroscience. 2008;151:518–524. doi: 10.1016/j.neuroscience.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 135.Chen G, Zeng WZ, Yuan PX, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 136.Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 137.Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 139.Ma J, Zhang GY. Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia. Neurosci Lett. 2003;348:185–189. doi: 10.1016/s0304-3940(03)00784-5. [DOI] [PubMed] [Google Scholar]
- 140.Chen RW, Qin ZH, Ren M, et al. Regulation of c-Jun N-terminal kinase, p38 kinase and AP-1 DNA binding in cultured brain neurons: roles in glutamate excitotoxicity and lithium neuroprotection. J Neurochem. 2003;84:566–575. doi: 10.1046/j.1471-4159.2003.01548.x. [DOI] [PubMed] [Google Scholar]
- 141.Bown CD, Wang JF, Young LT. Attenuation of N-methyl-D-aspartate-mediated cytoplasmic vacuolization in primary rat hippocampal neurons by mood stabilizers. Neuroscience. 2003;117:949–955. doi: 10.1016/s0306-4522(02)00743-1. [DOI] [PubMed] [Google Scholar]
- 142.Centeno F, Mora A, Fuentes JM, Soler G, Claro E. Partial lithium-associated protection against apoptosis induced by C2-ceramide in cerebellar granule neurons. Neuroreport. 1998;9:4199–4203. doi: 10.1097/00001756-199812210-00036. [DOI] [PubMed] [Google Scholar]
- 143.Mora A, Sabio G, Risco AM, et al. Lithium blocks the PKB and GSK3 dephosphorylation induced by ceramide through protein phosphatase-2A. Cell Signal. 2002;14:557–562. doi: 10.1016/s0898-6568(01)00282-0. [DOI] [PubMed] [Google Scholar]
- 144.Cimarosti H, Rodnight R, Tavares A, et al. An investigation of the neuroprotective effect of lithium in organotypic slice cultures of rat hippocampus exposed to oxygen and glucose deprivation. Neurosci Lett. 2001;315:33–36. doi: 10.1016/s0304-3940(01)02310-2. [DOI] [PubMed] [Google Scholar]
- 145.Hennion JP, el-Masri MA, Huff MO, el-Mallakh RS. Evaluation of neuroprotection by lithium and valproic acid against ouabain-induced cell damage. Bipolar Disord. 2002;4:201–206. doi: 10.1034/j.1399-5618.2002.01162.x. [DOI] [PubMed] [Google Scholar]
- 146.Jordà EG, Verdaguer E, Morano A, et al. Lithium prevents colchicine-induced apoptosis in rat cerebellar granule neurons. Bipolar Disord. 2004;6:144–149. doi: 10.1046/j.1399-5618.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 147.Tseng WP, Lin-Shiau SY. Long-term lithium treatment prevents neurotoxic effects of beta-bungarotoxin in primary cultured neurons. J Neurosci Res. 2002;69:633–641. doi: 10.1002/jnr.10318. [DOI] [PubMed] [Google Scholar]
- 148.Kang HJ, Noh JS, Bae YS, Gwag BJ. Calcium-dependent prevention of neuronal apoptosis by lithium ion: essential role of phosphoinositide 3-kinase and phospholipase Cgamma. Mol Pharmacol. 2003;64:228–234. doi: 10.1124/mol.64.2.228. [DOI] [PubMed] [Google Scholar]
- 149.Wilot LC, Bernardi A, Frozza RL, et al. Lithium and valproate protect hippocampal slices against ATP-induced cell death. Neurochem Res. 2007;32:1539–1546. doi: 10.1007/s11064-007-9348-3. [DOI] [PubMed] [Google Scholar]
- 150.Mora A, Sabio G, Alonso JC, Soler G, Centeno F. Different dependence of lithium and valproate on PI3K/PKB pathway. Bipolar Disord. 2002;4:195–200. doi: 10.1034/j.1399-5618.2002.40301.x. [DOI] [PubMed] [Google Scholar]
- 151.Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- 152.Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 153.Omata N, Murata T, Takamatsu S, et al. Neuroprotective effect of chronic lithium treatment against hypoxia in specific brain regions with upregulation of cAMP response element binding protein and brain-derived neurotrophic factor but not nerve growth factor: comparison with acute lithium treatment. Bipolar Disord. 2008;10:360–368. doi: 10.1111/j.1399-5618.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 154.Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 155.Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 1999;453:260–264. doi: 10.1016/s0014-5793(99)00685-7. [DOI] [PubMed] [Google Scholar]
- 156.Tajes M, Gutierrez-Cuesta J, Folch J, et al. Lithium treatment decreases activities of tau kinases in a murine model of senescence. J Neuropathol Exp Neurol. 2008;67:612–623. doi: 10.1097/NEN.0b013e3181776293. [DOI] [PubMed] [Google Scholar]
- 157.Rockenstein E, Torrance M, Adame A, et al. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhong J, Lee WH. Lithium: a novel treatment for Alzheimer’s disease? Expert Opin Drug Saf. 2007;6:375–383. doi: 10.1517/14740338.6.4.375. [DOI] [PubMed] [Google Scholar]
- 159.Rametti A, Esclaire F, Yardin C, Cogne N, Terro F. Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci Lett. 2008;434:93–98. doi: 10.1016/j.neulet.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 160.Angst J, Gamma A, Gerber-Werder R, Zarate CA, Jr, Manji HK. Does long-term medication with lithium, clozapine or antidepressants prevent or attenuate dementia in bipolar and depressed patients? Int J Psychiatry Clin Pract. 2007;11:2–8. doi: 10.1080/13651500600810133. [DOI] [PubMed] [Google Scholar]
- 161.Kessing LV, Sondergard L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008;65:1331–1335. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- 162.Wei H, Qin ZH, Senatorov VV, et al. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 163.Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington’s disease. Mol Psychiatry. 2004;9:371–385. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- 164.Crespo-Biel N, Camins A, Pallas M, Canudas AM. Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro. Neuropharmacology. 2009;56:422–428. doi: 10.1016/j.neuropharm.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 165.Wood NI, Morton AJ. Chronic lithium chloride treatment has variable effects on motor behaviour and survival of mice transgenic for the Huntington’s disease mutation. Brain Res Bull. 2003;61:375–383. doi: 10.1016/s0361-9230(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 166.Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 167.Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008;155:567–572. doi: 10.1016/j.neuroscience.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fornai F, Longone P, Cafaro L, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl cad Sci U S A. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Everall IP, Bell C, Mallory M, et al. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- 170.Dou H, Ellison B, Bradley J, et al. Neuroprotective mechanisms of lithium in murine human immunodeficiency virus-1 encephalitis. J Neurosci. 2005;25:8375–8385. doi: 10.1523/JNEUROSCI.2164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Letendre SL, Woods SP, Ellis RJ, et al. Lithium improves HIV-associated neurocognitive impairment. AIDS. 2006;20:1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- 172.Watase K, Gatchel JR, Sun Y, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4:e182. doi: 10.1371/journal.pmed.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 174.Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schuettauf F, Rejdak R, Thaler S, et al. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp Eye Res. 2006;83:1128–1134. doi: 10.1016/j.exer.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 176.Huang X, Wu DY, Chen G, Manji H, Chen DF. Support of retinal ganglion cell survival and axon regeneration by lithium through a Bcl-2-dependent mechanism. Invest Ophthalmol Vis Sci. 2003;44:347–354. doi: 10.1167/iovs.02-0198. [DOI] [PubMed] [Google Scholar]
- 177.Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol Psychiatry. 2008;13:585–596. doi: 10.1038/mp.2008.31. [DOI] [PubMed] [Google Scholar]
- 178.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 179.Ikeda A, Kato T. Biological predictors of lithium response in bipolar disorder. Psychiatry Clin Neurosci. 2003;57:243–250. doi: 10.1046/j.1440-1819.2003.01112.x. [DOI] [PubMed] [Google Scholar]