Abstract
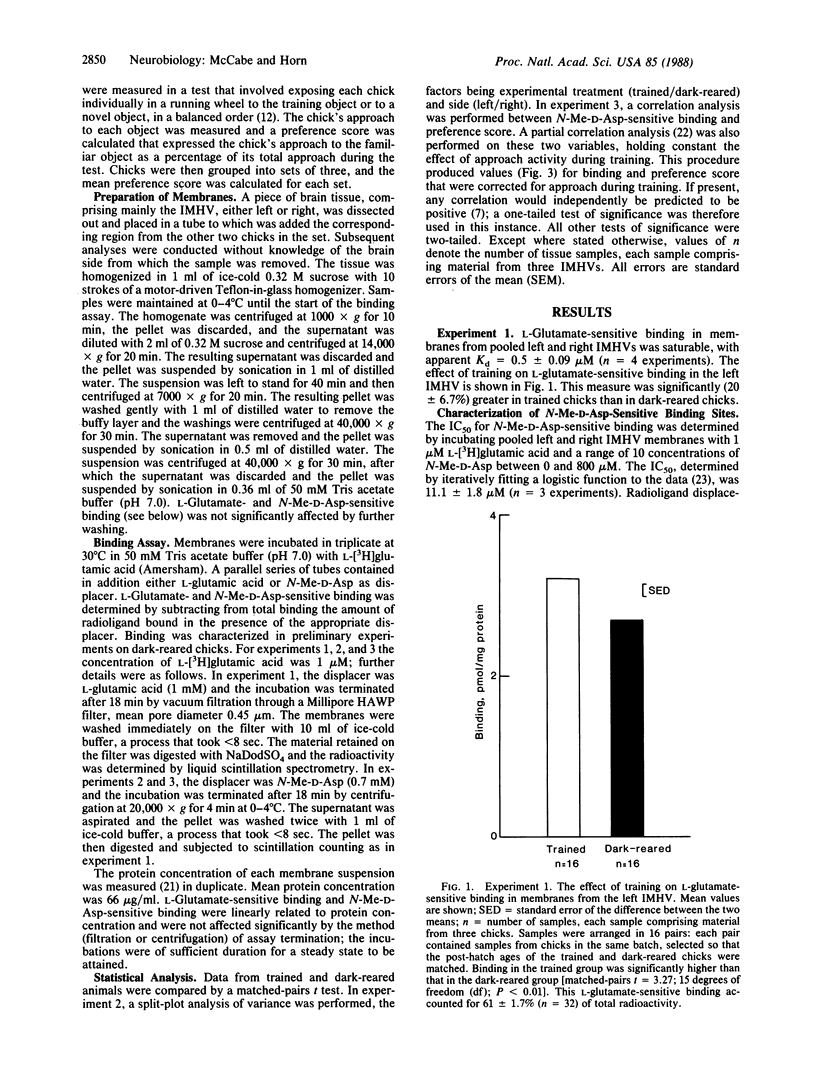
An extensive series of experiments has implicated a restricted region of the chick forebrain in the learning process of imprinting. The region is the intermediate and medial part of the hyperstriatum ventrale (IMHV). Previous studies have shown that training is associated with an increase in the area of the postsynaptic density of axospinous synapses in the left but not the right IMHV. The postsynaptic density is a site of high receptor density, and at least some axospinous synapses are excitatory. We found that imprinting is associated with a 59% increase in N-methyl-D-aspartate-sensitive binding of the excitatory amino acid L-[3H]glutamic acid in the left IMHV. The increase is probably due to an increased number of binding sites. The profile of sensitivity of the sites to a series of amino-, phosphono-substituted carboxylic acids (2-amino-3-phosphonopropionate to 2-amino-8-phosphonooctanoate) is characteristic of N-methyl-D-aspartate-type receptors. There were no significant effects of training on binding in the right IMHV. The effect of training on left IMHV binding could not be attributed to light exposure, arousal, or motor activity per se but was a function of how much the chicks learned. The changes in the left IMHV could increase the effectiveness of synaptic transmission in a region crucial for information storage and so form a neural basis for recognition memory.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateson P. P., Horn G., Rose S. P. Effects of early experience on regional incorporation of precursors into RNA and protein in the chick brain. Brain Res. 1972 Apr 28;39(2):449–465. doi: 10.1016/0006-8993(72)90448-9. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Horn G., Rose S. P. Imprinting: correlations between behaviour and incorporation of (14-C) uracil into chick brain. Brain Res. 1975 Feb 7;84(2):207–220. doi: 10.1016/0006-8993(75)90976-2. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Jaeckel J. B. Imprinting: Correlations between activities of chicks during training and testing. Anim Behav. 1974 Nov;22(4):899–906. doi: 10.1016/0003-3472(74)90013-x. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Rose S. P., Horn G. Imprinting: lasting effects on uracil incorporation into chick brain. Science. 1973 Aug 10;181(4099):576–578. doi: 10.1126/science.181.4099.576. [DOI] [PubMed] [Google Scholar]
- Bradley P., Horn G., Bateson P. Imprinting. An electron microscopic study of chick hyperstriatum ventrale. Exp Brain Res. 1981;41(2):115–120. doi: 10.1007/BF00236600. [DOI] [PubMed] [Google Scholar]
- Cipolla-Neto J., Horn G., McCabe B. J. Hemispheric asymmetry and imprinting: the effect of sequential lesions to the hyperstriatum ventrale. Exp Brain Res. 1982;48(1):22–27. doi: 10.1007/BF00239569. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington M. L., Lynch M. A., Bliss T. V. Long-term potentiation in the dentate gyrus: induction and increased glutamate release are blocked by D(-)aminophosphonovalerate. Neuroscience. 1987 Jan;20(1):279–284. doi: 10.1016/0306-4522(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Comparison of L-[3H]glutamate, D-[3H]aspartate, DL-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur J Pharmacol. 1987 Jan 20;133(3):291–300. doi: 10.1016/0014-2999(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Horn G., Bradley P., McCabe B. J. Changes in the structure of synapses associated with learning. J Neurosci. 1985 Dec;5(12):3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G., McCabe B. J., Bateson P. P. An autoradiographic study of the chick brain after imprinting. Brain Res. 1979 May 25;168(2):361–373. doi: 10.1016/0006-8993(79)90176-8. [DOI] [PubMed] [Google Scholar]
- Horn G., Rose S. P., Bateson P. P. Experience and plasticity in the central nervous system. Science. 1973 Aug 10;181(4099):506–514. doi: 10.1126/science.181.4099.506. [DOI] [PubMed] [Google Scholar]
- Horn G., Rose S. P., Bateson P. P. Monocular imprinting and regional incorporation of tritiated uracil into the brains of intact and "split-brain" chicks. Brain Res. 1973 Jun 29;56:227–237. doi: 10.1016/0006-8993(73)90337-5. [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Horn G. Dissociation of recognition memory and associative learning by a restricted lesion of the chick forebrain. Neuropsychologia. 1986;24(3):329–340. doi: 10.1016/0028-3932(86)90018-7. [DOI] [PubMed] [Google Scholar]
- Kohsaka S. I., Takamatsu K., Aoki E., Tsukada Y. Metabolic mapping of chick brain after imprinting using [14C]2-deoxyglucose technique. Brain Res. 1979 Aug 31;172(3):539–544. doi: 10.1016/0006-8993(79)90585-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mamounas L. A., Thompson R. F., Lynch G., Baudry M. Classical conditioning of the rabbit eyelid response increases glutamate receptor binding in hippocampal synaptic membranes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2548–2552. doi: 10.1073/pnas.81.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. J., Cipolla-Neto J., Horn G., Bateson P. Amnesic effects of bilateral lesions placed in the hyperstriatum ventrale of the chick after imprinting. Exp Brain Res. 1982;48(1):13–21. doi: 10.1007/BF00239568. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G., Bateson P. P. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981 Jan 26;205(1):29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7532–7536. doi: 10.1073/pnas.83.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nafstad P. H. An electron microscope study on the termination of the perforant path fibres in the hippocampus and the fascia dentata. Z Zellforsch Mikrosk Anat. 1967;76(4):532–542. doi: 10.1007/BF00339754. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Patterson T. A., Alvarado M. C., Warner I. T., Bennett E. L., Rosenzweig M. R. Memory stages and brain asymmetry in chick learning. Behav Neurosci. 1986 Dec;100(6):856–865. doi: 10.1037//0735-7044.100.6.856. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Hahn S. Ketamine-xylazine anaesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987 Mar 12;326(6109):183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]



