Abstract
Although it is well established that the secretory activity of the corpus luteum absolutely depends on the presence of pituitary-derived luteinizing hormone (LH), it is unknown why the life span of the corpus luteum is extended during early pregnancy by the placental production of chorionic gonadotropin (CG) but regresses in the presence of LH despite the fact that CG and LH have similar actions on the corpus luteum. To compare the responses of the corpus luteum to LH and human CG (hCG), cynomolgus monkeys whose endogenous gonadotropin secretion was blocked during the luteal phase of the menstrual cycle with a gonadotropin-releasing hormone antagonist were i.v. infused with either LH or CG. Infusion of LH at a constant rate overcame the gonadotropin-releasing hormone antagonist-mediated premature luteal regression but failed to prolong the functional life span of the corpus luteum. Continuous infusions of hCG did not effect a pregnancy-like pattern of gonadotropin secretion, but the functional life span of the corpus luteun was extended in two of three animals. Infusion of either LH or hCG in an exponentially increasing manner prolonged the functional life span of the corpus luteum beyond its normal duration. These results indicate that luteal regression at the termination of nonfertile menstrual cycles is caused by a large reduction in the responsiveness of the aging corpus luteum to LH, which can be overcome by elevated concentrations of either LH or CG.
The primate corpus luteum has an inherent 14- to 16-day life span in nonfertile menstrual cycles, but in menstrual cycles in which successful implantation occurs, the prolongation of its functional capacity beyond its usual life span is obligatory for the successful maintenance of the pregnancy until the placenta becomes the principal source for progesterone production. The increase in serum progesterone concentrations that occurs in pregnant rhesus monkeys when spontaneous luteal regression would have normally occurred led Neill et al. (1) to conclude that the corpus luteum is rescued during early pregnancy by the trophoblastic production of chorionic gonadotropin (CG). Although it is indisputable that CG is responsible for the prolongation of luteal function during pregnancy (2–5), why the corpus luteum regresses in the presence of luteinizing hormone (LH) yet its functional life span is extended by CG is not known, especially in view of the fact that both LH and CG interact with the same receptors on luteal cells and both hormones stimulate cAMP (6).
Three hypotheses have been developed to account for the differential responses of the primate corpus luteum to LH and CG. One hypothesis is that the patterns of the secretory dynamics of LH and CG provide different gonadotropic stimuli to the corpus luteum (7). This hypothesis relates to the fact that while plasma concentrations of LH intermittently fall to very low values during the luteal phase, owing to the pulsatile nature of LH secretion by the pituitary gland, plasma concentrations of CG do not fall to these low levels because of both the continuous secretion of CG by the placenta as well as its longer circulatory half-life (8). Thus, it is possible that continuous exposure of the corpus luteum to CG provides a more intense gonadotropic stimulus to the corpus luteum than the episodic stimuli provided by LH.
A second hypothesis is that although CG and LH are structurally similar, the amino acid differences in the β chains of CG and LH as well as differences in the extent of glycosylation and sulfation (9) could result in an inherent difference in biological activity between the two hormones. This hypothesis is based largely on studies of rodent and ovine systems that demonstrated that the time course of steroid production in response to human CG (hCG) was significantly prolonged when compared with ovine LH, suggesting that the dynamics of the interaction of CG with the CG/LH receptor and/or the subsequent activation of intracellular signaling pathways may differ from that of LH (10, 11).
The third hypothesis is that the aging corpus luteum exhibits a diminished responsiveness to gonadotropins such that the ambient concentrations of LH present during the mid- through late luteal phase of the menstrual cycle are unable to sustain its functions (12). In this model, the functional life span of the corpus luteum is prolonged in early pregnancy because the elevated plasma concentrations of CG during early pregnancy overcome the diminishing responsiveness of the aging corpus luteum and sustain its function until the placenta assumes the major role of steroid production.
The purpose of the present study was to compare the responses of the primate corpus luteum in vivo to LH and CG in an attempt to provide insights regarding the physiological mechanism by which CG prolongs the functional life span of the primate corpus luteum. For this purpose, we infused cynomolgus monkeys i.v. with hCG, recombinant macaque LH, or highly purified human LH (hLH) either at a constant rate or an exponentially increasing rate during the mid- through late luteal phase of the menstrual cycle and measured serum progesterone concentrations and the onset of menses as indices of luteal function.
MATERIALS AND METHODS
Animals.
Female cynomolgus monkeys (Macaca fascicularis), 2–3 kg body weight, with normal menstrual cycle histories were used in this study and were housed under standard husbandry conditions at the University of Pittsburgh Primate Research Laboratory. All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Each treatment or reference group consisted of three animals. Daily blood samples were collected by femoral venipuncture under ketamine HCl (10 mg/kg) sedation. The day of ovulation was defined as the day serum estrogen concentrations fell from >150 pg/ml to <50 pg/ml and was followed by rising progesterone concentrations.
On day 7 of the luteal phase, under ketamine anesthesia (50 mg/kg), animals were fitted with either a jugular or femoral vein catheter that was exteriorized through the skin in the scapular region. The catheter was protected by a vest and a flexible stainless steel cable and was connected to a swivel device (Spalding Medical Products, Arroyo Grand, CA) that was mounted to the roof of the cage. Immediately after attaching the catheter to the swivel device, animals received an i.v. infusion of either LH or hCG as described below. The gonadotropin infusion was controlled by a Sage infusion pump (Sage Instruments, Cambridge MA). On day 8 of the luteal phase, 1 day after beginning the gonadotropin infusion, animals received daily intramuscular injections of a gonadotropin-releasing hormone (GnRH) antagonist [Ac-D2Na1, DCpa2, DTrp3, DArg6, DAla10]-GnRH (4 mg/kg, emulsified in peanut oil). This GnRH antagonist was synthesized at the Salk Institute under Contract NO1-HD-0–2906 with the National Institutes of Health and was made available by the Contraceptive Development Branch, Center for Population Research, National Institute of Child Health and Human Development. Treatment with the GnRH antagonist continued for 7 days. Daily blood samples were collected by venipuncture until day 22 postovulation or until menses was observed. Serum was stored at −20°C until used for gonadotropin and progesterone assays. Serum collected from cynomolgus monkeys with documented pregnancies was generously provided by Keith Gordon, Eastern Virginia Medical School, Norfolk, VA.
Hormones.
The following hormone preparations were used in these studies. hCG (CR 123; 13,500 units/mg) was generously provided by Stephen Birkin, Columbia College of Physicians and Surgeons, New York. Recombinant cynomolgus LH (6936A, 12,000 units/mg) and hLH (AFP 7572B, 4,500 units/mg) were provided by the National Hormone and Pituitary Program, National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health.
Gonadotropin Infusions.
The objective of these studies was to achieve comparable bioactive plasma concentrations of LH and hCG by i.v. infusion. Because each of the hormone preparations differed in biopotency and circulatory half-life, preliminary studies were conducted to establish infusion rates of each hormone that would achieve comparable steady-state serum bioactive gonadotropin concentrations. Gonadotropins were dissolved in PBS containing 1% cynomolgus monkey serum as a protein carrier and 100 units/ml of penicillin and 100 μg/ml of streptomycin. All infusates were sterilized by passage through a 0.2-μm filter (Gelman Sciences, Ann Arbor, MI). The rate of infusion was set at 15 ml/day, and the concentration of gonadotropin in the infusate was adjusted to achieve the desired plasma gonadotropin concentrations.
For the continuous LH infusion regimen, animals received 180 μg of recombinant macaque LH per day. For the continuous CG infusion regimen, animals received 12 μg of hCG per day. For the exponentially increasing CG infusion regimen, animals received 4.8 μg of hCG per day for the first 5 days (days 7–11 of the luteal phase), and thereafter the concentration of hCG in the infusate was increased 1.44× per day. For the exponentially increasing LH infusion regimen, each animal received 80 μg of hLH per day for the first 5 days (days 7–11) and thereafter the concentration of hLH in the infusate was increased 1.44× per day.
Hormone Assays.
Serum estrogen and progesterone concentrations were measured by RIA as previously described (13). Serum from animals infused with gonadotropins as well as serum from monkeys collected during the spontaneous luteal phase and during early pregnancy were measured for LH/CG bioactivity by using the mouse interstitial cell bioassay of Van Damme et al. (14), modified as described previously (12). The recombinant macaque LH was used as a standard, and all data are expressed as bioactive equivalent masses of this preparation. The intra- and inter-assay coefficients of variation of eight assays for a macaque serum pool, which measured 2.8 ng/ml, were 9.7% and 22.1%, respectively.
Data Analysis.
Cumulative serum progesterone levels (areas under the curve, AUCs) from control, pregnant and gonadotropin-infused animals were calculated by adding the daily progesterone concentrations in each animal from days 0–7 and days 8–22 postovulation, which represents the maximum duration of the gonadotropin infusions. In some animals (i.e., control animals and animals that received continuous infusions of LH or hCG, progesterone levels fell below the limits of detection before day 22. For these animals, a progesterone value of 0.2 ng/ml, which represents the minimal serum progesterone concentration of the RIA, was used for all days in which progesterone levels were undetectable. Values for the AUCs for each treatment and control group were assessed for statistical significance by AVOVA, and comparison of group means was accomplished by Duncan’s multiple range analyses (15).
RESULTS
Effect of GnRH Antagonist on Corpus Luteum Function.
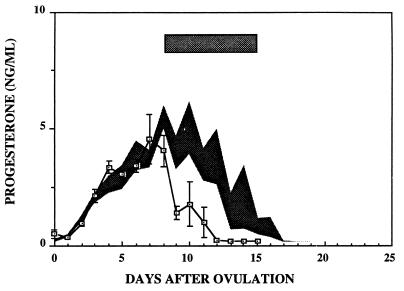
Fig. 1 illustrates serum progesterone concentrations in three monkeys during spontaneous luteal phases (shaded area) and in three monkeys that were treated with the GnRH antagonist for 7 days, beginning on day 8 of the luteal phase. As demonstrated previously (16, 17), serum progesterone concentrations fell rapidly upon administration of GnRH antagonist and were undetectable 4–5 days after the initiation of treatment. Menses occurred on days 12–14 after ovulation in the GnRH antagonist-treated animals as compared with days 15, 16, and 19 in the spontaneously cycling animals.
Figure 1.
Results show mean ± 1 SEM of serum progesterone concentrations from three cynomolgus monkeys treated with a GnRH antagonist as described in Materials and Methods. The duration of antagonist treatment is shown by the shaded rectangle. The shaded area encompasses the mean ± 1 SEM of serum progesterone levels during luteal phases of three control monkeys.
Continuous Infusions of LH or hCG.
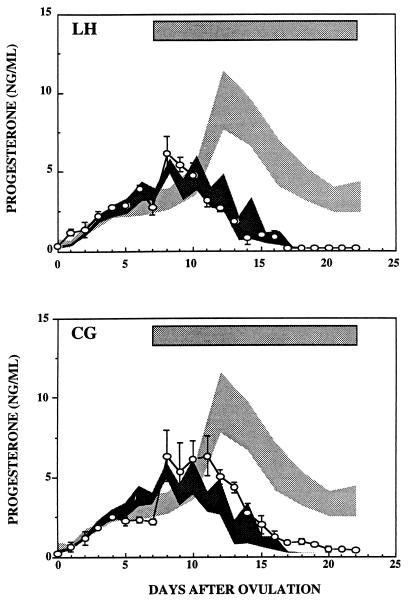
Fig. 2 (Upper) illustrates serum progesterone concentrations in GnRH antagonist-treated monkeys that received a continuous infusion of recombinant macaque LH at an infusion rate of 180 μg/day. Shown for comparison are serum progesterone concentrations during the spontaneous luteal phase (dark shading) as well as serum progesterone concentrations in three pregnant monkeys (light shading) at comparable times after ovulation. The duration of LH treatment is shown by the shaded bar at the top of the figure. The continuous infusion of LH overcame the GnRH-antagonist mediated premature regression of the corpus luteum. However, progesterone production in these LH-infused animals continued only until the expected time of luteal regression and became undetectable thereafter despite the continued infusion of LH. Menses in the LH-infused animals occurred on days 15, 16, and 18 after ovulation, similar to times seen in the spontaneously cycling animals presented in Fig. 1.
Figure 2.
Results show serum progesterone concentrations (means ± 1 SEM) in GnRH antagonist-treated monkeys that received continuous infusions of recombinant cynomolgus LH (Upper) or hCG (Lower) as described in Materials and Methods. The duration of each gonadotropin treatment is shown by the shaded rectangles. The dark shaded areas encompass the means ± 1 SEM of serum progesterone levels during luteal phases of three control monkeys and the light shaded areas encompass the means ± 1 SEM of serum progesterone levels during luteal phases of three pregnant monkeys.
Fig. 2 (Lower) illustrates serum progesterone concentrations in GnRH-antagonist-treated monkeys that received a continuous infusion of hCG (12 μg/day). Serum progesterone concentrations during CG treatment were slightly greater than those seen during spontaneous luteal phases (dark shading) but, like that seen in seen in LH-infused animals, subsequently fell to low levels (<1 ng/ml) at the expected time of luteal regression despite the continued infusion of the gonadotropin. However, unlike the animals that received the continuous infusion of LH, serum progesterone concentrations did not fall to undetectable levels, and the onset of menses was delayed in two of the three animals that received continuous infusions of CG (days 18, 23, and 24 after ovulation).
Exponentially Increasing Infusions of LH or hCG.
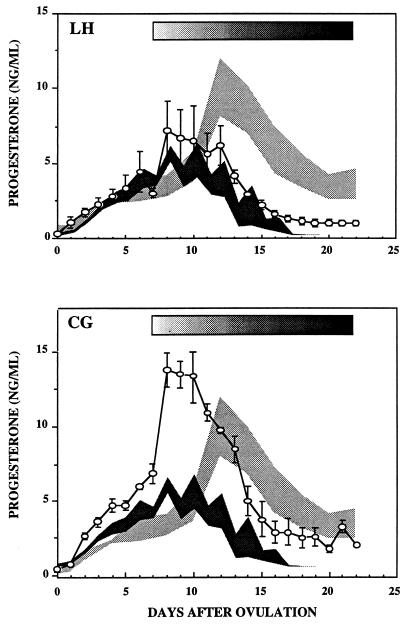
Fig. 3 (Upper) illustrates serum progesterone concentration in animals that received hLH in a exponentially increasing manner as described in Materials and Methods. For these studies it was necessary to use purified hLH rather than recombinant macaque LH because sufficient quantities of the macaque LH were not available. Progesterone production in response to the progressively increasing LH infusions was sustained beyond the typical 14- to 16-day life span of the corpus luteum, and menses was delayed in two animals (days 25 and 27 after ovulation). The third animal did not exhibit obvious menses for at least 1 week after the cessation of gonadotropin treatment.
Figure 3.
Results show serum progesterone concentrations (means ± 1 SEM) in GnRH antagonist-treated cynomolgus monkeys that received exponentially increasing infusions of recombinant LH (Upper) or hCG (Lower) as described in Materials and Methods. The duration of each gonadotropin treatment is shown by the shaded rectangles. The dark shaded areas encompass the means ± 1 SEM of serum progesterone levels during luteal phases of three control monkeys and the light shaded areas encompass the means ± 1 SEM of serum progesterone levels during luteal phases of three pregnant monkeys.
Fig. 3 (Lower) illustrates serum progesterone concentrations in animals that received hCG in an exponentially increasing manner. The absolute magnitude of serum progesterone concentrations was increased as well as the duration of its production when compared with the spontaneous luteal phase. The pattern of serum progesterone concentrations in these CG-infused animals paralleled that seen during spontaneous pregnancy and was similar to that seen in the pregnant animals when the data were adjusted to the initial rise in progesterone seen in the pregnant animals (i.e., data from CG-infused animals right-shifted by 3 days). The timing of the onset of menses was delayed in two of the animals (both on days 23 after ovulation), and obvious menses was not observed in the third animal for at least 1 week after the termination of the gonadotropin infusion.
Cumulative Progesterone Production During Gonadotropin Infusions.
Table 1 presents the AUCs of serum progesterone concentrations on days 0–7 postovulation and from days 8–22 after ovulation during the spontaneous luteal phase, in response to exogenous gonadotropin treatments and during early pregnancy. There was no statistical difference (P > 0.05) between the day 8–22 AUCs of progesterone levels in control animals and animals that received the continuous infusions of LH. Cumulative progesterone levels in animals that received either a continuous infusion of hCG or an exponentially increasing infusion of hLH were greater than control animals and animals that received continuous infusions of recombinant macaque LH (P < 0.05). The cumulative progesterone levels in animals that received the exponential infusion of hCG were significantly greater (P < 0.05) than control animals and those that received either continuous infusions of either LH or hCG as well as those that received the exponentially increasing regimen of hLH. Comparison of the AUCs of the individual treatment groups before the initiation of the gonadotropin infusions (days 0–7) revealed that the cumulative production rates of progesterone of the animals that subsequently were treated with the exponential infusion regimen of CG were, for unexplained reasons, greater than those of the other treatment and control groups, hence the extent to which the absolute amounts of progesterone seen in this group during gonadotropin treatment may have been biased by the elevated pretreatment values is uncertain. When the data from these animals were normalized to those that received the exponentially increasing LH regimen by multiplying the day 8–22 AUCs by the ratio of their cumulative progesterone levels observed during days 0–7 (i.e., day 0–7 LHe/day 0–7 CGe), the resultant normalized values for the animals that received the exponentially increasing CG regimen (61.3 ± 1.76) remained statistically greater than the animals that received the exponentially increasing LH regimen but were not statistically different from the pregnant animals.
Table 1.
Cumulative progesterone levels
| Group | Days 0–7 | Days 8–22 |
|---|---|---|
| Control | 15.7 ± 1.55a | 21.4 ± 1.68a |
| LH continuous | 17.6 ± 1.59a | 26.6 ± 0.28a |
| hCG continuous | 13.4 ± 0.74a | 43.7 ± 5.06b |
| LH exponential | 19.2 ± 4.05a | 49.7 ± 9.03b |
| hCG exponential | 29.7 ± 2.08b | 94.9 ± 4.06c |
| Pregnant | 15.1 ± 1.68a | 69.4 ± 7.15d |
Values within each column with different superscript letters differ significantly (P < 0.05).
Serum Gonadotropin Concentrations.
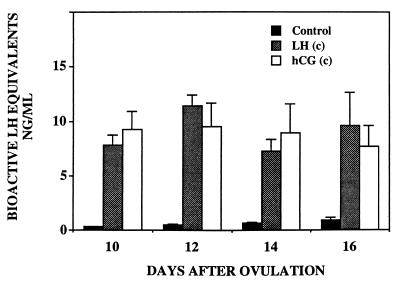
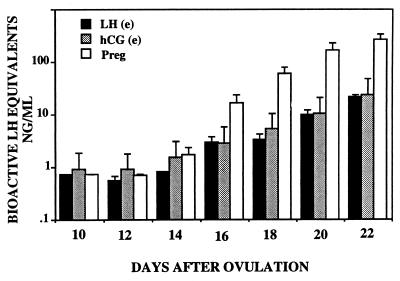
Fig. 4 shows mean serum bioactive gonadotropin concentrations (LH or CG) during the spontaneous luteal phase and during continuous infusions of recombinant macaque LH and hCG. In all gonadotropin-infused animals, steady-state concentrations of gonadotropins were achieved within the first 24 hr after the initiation of the infusions. Bioactive serum gonadotropin concentrations were similar in the LH-and CG-infused animals and were approximately 10-fold greater than those measured during the spontaneous luteal phase. Fig. 5 compares bioactive gonadotropin concentrations in serum from monkeys exponentially infused with hLH and hCG with those seen at comparable times in spontaneously pregnant monkeys. Results are plotted on a logarithmic scale to accommodate the temporal increase in CG levels during spontaneous pregnancy.
Figure 4.
Results show serum bioactive LH/hCG levels (mean ± 1 SEM) in control animals and animals that received continuous infusions of gonadotropins.
Figure 5.
Results compare bioactive serum gonadotropin levels in monkeys that received either an exponentially increasing infusion of hLH or hCG with those of pregnant animals at comparable times of the luteal phase (mean ± 1 SEM).
DISCUSSION
Although it is well known that the extension of the life span of the primate corpus luteum during early pregnancy is caused by the production of CG by the embryonic trophoblast, the reason the corpus luteum regresses in the presence of pituitary-derived LH, yet is rescued by placental CG, remains a mystery. To address this question, an i.v. infusion paradigm in cynomolgus monkeys was used in which comparable bioactive plasma concentrations of LH and CG were achieved either during continuous infusion or exponentially increasing infusion regimens. In agreement with the findings of others (2–5), our results indicate that luteal function can be prolonged beyond its usual life span by the administration of either exogenous LH or CG. The current studies extend these earlier observations and provide additional information regarding the nature of the hormonal signal that is required to extend the functional life span of the corpus luteum during early pregnancy as well as the physiological mechanism responsible for the regression of the corpus luteum at the termination of nonfertile menstrual cycles.
As described in the Introduction, one hypothesis that could explain why the corpus luteum regresses in the presence of LH but is rescued by CG is that the switch from the pulsatile pattern of plasma LH concentrations seen during the nonfertile luteal phase to the continuous pattern of CG concentrations seen during early pregnancy may provide a more intense stimulus to the corpus luteum and thereby extend its functional life span. This hypothesis can be rejected outright based on results in Fig. 2, which show that timely luteal regression was observed in animals that received a continuous (i.e., nonpulsatile) infusion of recombinant macaque LH.
A second hypothesis is that there may be inherent differences in the biological activities of the two hormones such that CG on a mass basis may provide a greater stimulus to the corpus luteum than LH. Results of our studies indicate that qualitatively similar responses were observed in animals infused with LH and CG. Neither hormone, when provided in a continuous mode, maintained a pattern of serum progesterone concentrations typical of those seen during spontaneous pregnancy. Likewise, exponentially increasing regimens of both hormones prolonged luteal function beyond the expected time of spontaneous luteal regression. Although the responses to LH and CG were qualitatively similar, comparison of the cumulative progesterone levels (Table 1) between the animals that received either continuous or exponentially increasing infusions of the gonadotropins revealed that the absolute serum progesterone concentrations seen in the LH-infused animals (both continuous and exponentially increasing) were approximately 40% lower than those seen in the animals that received hCG despite the fact that bioactive plasma concentrations of both gonadotropins were comparable. Further, as shown in Fig. 2, although the decline in serum progesterone concentrations and the onset of menses occurred at the expected time of spontaneous luteal regression in the animals that received the continuous infusions of macaque LH, serum progesterone concentrations in the CG-infused animals persisted at low levels beyond the expected time of spontaneous luteal regression, and the onset of menses was delayed in two of three animals. A similar difference between the biological activities of LH and CG was observed by Molskness et al. (18) who demonstrated that while the time course of progesterone production in vitro by luteinizing macaque granulosa cells to recombinant LH was qualitatively similar to that of highly purified hCG, the absolute magnitude of progesterone secretion in response to CG was greater than that seen in response to comparable concentrations of LH. These observations are similar to earlier findings of Hanson et al. (2), which demonstrated that although administration of comparable doses of both LH and CG to humans stimulated progesterone production, the absolute amounts of progesterone seen in the CG-treated subjects exceeded those that were treated with LH. However, as plasma concentrations of LH and CG were not analyzed in this study, it remains uncertain as to whether the differences in the responses between the two hormones were caused by inherent differences in the biological activities of the two gonadotropins or differences in their resultant plasma concentrations. Although our observations would suggest that there may be differences in biological efficacies of these two structurally similar hormones, results of other studies which compared the potencies of LH and CG do not support this conclusion. By using 293 cells that were stably transfected with the hLH/CG receptor, Jia et al. (6) found that on a mass basis LH and CG have comparable receptor affinities as well as equivalent potencies in stimulating cAMP production. Although an explanation for the apparent differences in the ovarian responses to LH and CG observed in the current study as well as those previously described by Molskness et al. (18) is not immediately at hand, it does not appear that these differences per se can account for the prolongation of luteal function that occurs during early pregnancy because results presented in Figs. 2 and 4 demonstrate that continuous infusions of LH that resulted in plasma concentrations that were at least 10-fold greater than those present during the spontaneous luteal phase were unable to prolong the functional life span of the corpus luteum. However, a quantitative difference in the efficacy of CG could explain the initial spike in progesterone levels that occurs at the time of implantation when CG first appears in the circulation (1, 19).
The third hypothesis for the maintenance of luteal function during early pregnancy is that the corpus luteum undergoes an age-related decrease in its responsiveness to LH such that the ambient concentrations of LH seen during the late luteal phase are insufficient to maintain its function, and luteal rescue can be achieved only by the elevated concentrations of CG seen during early pregnancy. Our current results are entirely consistent with this hypothesis. Figs. 1, 2, and 4 demonstrate that while concentrations of recombinant macaque LH and hCG that were approximately 10-fold greater than endogenous LH concentrations during spontaneous menstrual cycles were able to overcome the GnRH antagonist-induced premature luteal regression, they were unable to maintain a pattern of progesterone concentrations typical of spontaneous pregnancy. These results indicate that the corpus luteum experiences a substantial reduction in its responsiveness to LH between the mid- and late luteal phases of the menstrual cycle and thereby confirm the conclusion made more than 50 years ago by Frederick Hisaw that “menstruation is not due necessarily to a lack or absence of pituitary gonadotropin but, rather, to a failure of the corpus luteum” (20). That prolongation of luteal progesterone production to an extent that was seen during spontaneous pregnancy occurred in the group of animals that received the exponentially increasing infusions of LH and CG is entirely consistent with the hypothesis that the diminished responsiveness of the aging corpus luteum can be overcome only by the elevated concentrations of gonadotropins achieved by the placental production of CG. A major unresolved question relates to whether an exponential rise in plasma gonadotropin concentrations is required to prolong the functional life span of the corpus luteum or whether the extension of luteal function during pregnancy is simply the result of the attainment of gonadotropin concentrations that are sufficiently high enough to overcome the diminished responsiveness of the aging corpus luteum to LH. Our current data and those of others (4, 5) suggest that an exponential rise in gonadotropins may be required to extend the functional life span of the corpus luteum. However, the earlier findings of Neill and Knobil (3), in which pregnancy-like patterns of progesterone concentrations were observed in rhesus monkeys that were treated with daily intramuscular injections of a fixed amount of CG, suggest that the absolute concentration of gonadotropin rather than the pattern of its production is likely the principal determinant for the prolongation of luteal function during early pregnancy.
A complete understanding of the regression of the corpus luteum and the prolongation of its function during early pregnancy will require the identification of the mechanisms responsible for the diminishing responsiveness of the aging corpus luteum to LH. In general terms, the declining responsiveness of the corpus luteum could result from age-related alterations in the cellular signaling pathway that governs steroidogenesis and/or intraluteal vascular changes that impinge on the delivery of gonadotropin to the aging tissue. With respect to changes in the LH signaling pathway, the previous observations made by Stouffer and coworkers (21, 22) that neither the number of cell surface LH receptors nor LH-responsive adenylyl cyclase activity decline until after serum progesterone levels begin to fall usually have been interpreted to suggest that changes in the transmembrane signaling system for LH do not appear to be causal to the onset of luteal regression. However as shown in Fig. 3 and as previously reported by others (2–5), both during spontaneous pregnancy and during treatment with exogenous hCG, serum progesterone concentrations progressively fall despite the exponentially increasing plasma concentrations of CG. These observations indicate that a steady decline in luteal function occurs even as the life span of the corpus luteum is being prolonged by the elevated gonadotropin concentrations. According to laws of mass action, the concentration of occupied hormone receptors is proportional to both the number of total receptors as well as the plasma hormone concentration (23). A decrease in receptor concentration could be compensated for by an increase in free hormone concentration (i.e., CG), which would increase the fractional occupancy of the remaining LH/CG receptors and thereby sustain luteal function. Indeed, previous studies (24) demonstrated that treatment of rhesus monkeys with exogenous hCG to mimic early pregnancy resulted in a progressive increase in the fractional occupancy of luteal LH/CG receptors such that after 10 days of gonadotropin treatment >80% of the total receptors were occupied by hormone. With respect to changes in microvascularity, previous results demonstrated that the expression of mRNA for vascular endothelial growth factor declines as the corpus luteum ages (25). If this angiogenic factor contributes to the maintenance of the microvasculature of the corpus luteum as it may do in other endocrine-responsive tissues (26), a decline in its production could diminish microvascular perfusion and reduce gonadotropin delivery to the aging corpus luteum, which could be compensated for by the elevated gonadotropin concentrations during early pregnancy.
During spontaneous pregnancy, as well as in response to exogenous gonadotropin, progesterone production by the corpus luteum steadily declines despite the progressively rising concentrations of CG. This observation led Neill and Knobil (3) to propose that after its initial response to CG, the corpus luteum becomes refractory to further stimulation. Our current findings that the corpus luteum undergoes a dramatic decrease in its responsiveness to LH during the late luteal phase of the menstrual cycle suggests that the progressive decline in luteal function during pregnancy simply may represent the continuation of its terminal aging process. Thus, previous studies have shown that the mRNAs that encode for enzymes involved in progesterone biosynthesis steadily decline throughout the luteal phase and that this decline is not attenuated either by administration of exogenous hCG or during spontaneous pregnancy (27–29). Further, cellular proliferation in the corpus luteum, as assessed by Ki-67 immunostaining, ceases shortly after luteinization and is not restored by exogenous treatment with hCG (30). Collectively, these observations indicate that the heroic concentrations of CG achieved during early pregnancy do not result in the restoration of the secretory capacity of the corpus luteum but rather cause the maximal stimulation of progesterone secretion by the continuously declining tissue.
Acknowledgments
I am grateful for the generous assistance of Mrs. Deborah Bolette, Drs. Suresh Ramaswamy and Tony M. Plant for their assistance in the maintenance of the tethered monkeys, Michael Cicco and Robert Beidler for collecting daily blood samples, and Mrs. Lynda Little-Ihrig for performing the hormone assays. This work was supported by National Institutes of Health Grants HD 08610, HD 16842, and HD 16851.
ABBREVIATIONS
- CG
chorionic gonadotropin
- hCG
human CG
- LH
luteinizing hormone
- hLH
human LH
- GnRH
gonadotropin-releasing hormone
- AUC
area under the curve
References
- 1.Neill J D, Johansson E D B, Knobil E. Endocrinology. 1969;84:45–48. doi: 10.1210/endo-84-1-45. [DOI] [PubMed] [Google Scholar]
- 2.Hanson F W, Powell J E, Stevens V C. J Clin Endocrinol. 1971;32:211–215. doi: 10.1210/jcem-32-2-211. [DOI] [PubMed] [Google Scholar]
- 3.Neill J D, Knobil E. Endocrinology. 1972;90:34–38. doi: 10.1210/endo-90-1-34. [DOI] [PubMed] [Google Scholar]
- 4.Wilks J, Noble A S. Endocrinology. 1983;112:1256–1266. doi: 10.1210/endo-112-4-1256. [DOI] [PubMed] [Google Scholar]
- 5.Ottobre J S, Stouffer R L. Endocrinology. 1984;114:2175–2182. doi: 10.1210/endo-114-6-2175. [DOI] [PubMed] [Google Scholar]
- 6.Jia X C, Oikawa B M, Tanaka T, Ny T, Boime I, Hsueh A J. Mol Endocrinol. 1991;5:759–768. doi: 10.1210/mend-5-6-759. [DOI] [PubMed] [Google Scholar]
- 7.Stouffer R L, Ottobre J S, VandeVoort C A. In: The Primate Ovary. Stouffer R L, editor. New York: Plenum; 1987. pp. 207–220. [Google Scholar]
- 8.Monfort S L, Hess D L, Hendrickx A G, Lasley B L. Endocrinology. 1989;125:1766–1773. doi: 10.1210/endo-125-4-1766. [DOI] [PubMed] [Google Scholar]
- 9.Strickland T W, Parsons T F, Pierce J G. In: Luteinizing Hormone Actions and Receptors. Ascoli M, editor. Boca Raton, FL: CRC; 1985. pp. 1–15. [Google Scholar]
- 10.Segaloff D L, Puett D, Ascoli M. Endocrinology. 1981;108:632–638. doi: 10.1210/endo-108-2-632. [DOI] [PubMed] [Google Scholar]
- 11.Bourdage R J, Fitz T A, Niswender G D. Proc Soc Exp Biol Med. 1984;175:483–486. doi: 10.3181/00379727-175-41824. [DOI] [PubMed] [Google Scholar]
- 12.Zeleznik A J, Little-Ihrig L. Endocrinology. 1990;126:2237–2244. doi: 10.1210/endo-126-5-2237. [DOI] [PubMed] [Google Scholar]
- 13.Zeleznik A J, Resko J A. Endocrinology. 1980;186:1820–1826. doi: 10.1210/endo-106-6-1820. [DOI] [PubMed] [Google Scholar]
- 14.Van Damme M-P, Robertson D M, Diczfalusy E. Acta Endocrinol. 1974;77:655–671. doi: 10.1530/acta.0.0770655. [DOI] [PubMed] [Google Scholar]
- 15.Woolf C M. Principles of Biometry. Princeton: Van Nostrand; 1968. pp. 83–113. [Google Scholar]
- 16.Ravindranath N, Little-Ihrig L, Benyo D F, Zeleznik A J. Endocrinology. 1992;131:2065–2070. doi: 10.1210/endo.131.5.1425410. [DOI] [PubMed] [Google Scholar]
- 17.Fraser H M, Nestor J J, Vickery B H. Endocrinology. 1987;121:612–618. doi: 10.1210/endo-121-2-612. [DOI] [PubMed] [Google Scholar]
- 18.Molskness T A, Zelinski-Wooten M B, Hild-Petito S A, Stouffer R L. Biol Reprod. 1991;45:273–281. doi: 10.1095/biolreprod45.2.273. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson L E, Hotchkiss J, Fritz G R, Surve A H, Neill J D, Knobil E. Biol Reprod. 1975;12:335–345. doi: 10.1095/biolreprod12.3.335. [DOI] [PubMed] [Google Scholar]
- 20.Hisaw F L. Yale J Biol Med. 1944;17:121–137. [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron J L, Stouffer R L. Endocrinology. 1982;110:2068–2073. doi: 10.1210/endo-110-6-2068. [DOI] [PubMed] [Google Scholar]
- 22.Eyster K M, Ottobre J S, Stouffer R L. Endocrinology. 1985;117:1571–1577. doi: 10.1210/endo-117-4-1571. [DOI] [PubMed] [Google Scholar]
- 23.Roth J, Grunfeld C. In: Textbook of Endocrinology. Williams R H, editor. Philadelphia: Saunders; 1981. pp. 15–72. [Google Scholar]
- 24.Stouffer R L, Ottobre J S, VandeVoort C A. In: The Primate Ovary. Stouffer R L, editor. New York: Plenum; 1987. pp. 207–220. [Google Scholar]
- 25.Ravindranath N, Phillips H, Ferrara N, Zeleznik A J. Endocrinology. 1992;131:254–260. doi: 10.1210/endo.131.1.1612003. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Endocrinology. 1998;139:441–442. doi: 10.1210/endo.139.2.5858. [DOI] [PubMed] [Google Scholar]
- 27.Bassett S G, Little-Ihrig L L, Mason J I, Zeleznik A J. J Clin Endocrinol Metab. 1991;72:362–366. doi: 10.1210/jcem-72-2-362. [DOI] [PubMed] [Google Scholar]
- 28.Benyo D F, Little-Ihrig L, Zeleznik A J. Endocrinology. 1993;133:699–704. doi: 10.1210/endo.133.2.8344208. [DOI] [PubMed] [Google Scholar]
- 29.Hild-Petito S, Fazleabas A T. J Clin Endocrinol Metab. 1997;82:955–962. doi: 10.1210/jcem.82.3.3813. [DOI] [PubMed] [Google Scholar]
- 30.Christenson L K, Stouffer R L. Endocrinology. 1996;137:367–374. doi: 10.1210/endo.137.1.8536637. [DOI] [PubMed] [Google Scholar]