Abstract
Many species use chemical signals to convey information relevant to social and reproductive status between members of the same species (conspecific), but some chemical signals may also provide information to another species (heterospecific). Both of these types of complex chemical signals may be detected by the vomeronasal organ, which sends projections to the accessory olfactory bulb and on to the medial amygdala. Previous reports in hamster and mouse suggest that the medial amygdala sorts this complex chemosensory information categorically, according to its biological relevance (salience). In the present set of experiments, male mice having undergone vomeronasal removal surgery (VNX) or a sham-operation (SHAM) were exposed to conspecific (male and female mouse urine) or heterospecific (hamster vaginal fluid and worn cat collar) chemical stimuli. Similarly to our previous report with intact male mice (Samuelsen and Meredith, 2009), SHAM mice exhibit different immediate early gene (IEG) expression patterns in the medial amygdala dependent upon the biological relevance of the chemical stimuli. However, regardless of biological relevance, vomeronasal organ removal eliminates all responses in the medial amygdala to any of the chemical stimuli. Interestingly, VNX also disrupts the avoidance of (an unfamiliar) predator odor, worn cat collar. Here we show that the medial amygdala response to the tested chemical signals is dependent upon an intact vomeronasal organ.
Keywords: Olfaction, Pheromone, Fos-related antigens (FRAs), Predator, Behavior
Most mammals employ chemical signals to communicate information pertaining to complex behaviors such as reproductive status, relatedness (strain), social rank (Desjardins et al., 1973) and territorial ownership (Nakamura et al., 2007). The volatile components of chemical signals appear to be detected mainly by receptors in the main olfactory epithelium and the information processed by the main olfactory bulb (Lin et al., 2007; Martel and Baum, 2008), whereas the non-volatile components of chemical signals appear to be detected mainly by receptors in the vomeronasal organ (VNO) with the information processed via the accessory olfactory bulb (Leinders-Zufall et al., 2000; Luo et al., 2003). Both sensory systems can potentially respond to both types of stimuli (Meredith and O’Connell, 1979; Lehman and Winans, 1982; Meredith et al., 1983, O’Connell and Meredith, 1984; Spehr et al., 2006). Inactivation of the VNO, by surgical removal or genetic manipulation, results in behavioral and physiological deficits in reproductive and other behaviors that involve chemical communication (Powers and Winans, 1975; Wysocki et al., 1982; Meredith, 1986; Fewell and Meredith, 2002; Stowers et al., 2002; Westberry and Meredith, 2003(a, b); Pankevich et al., 2004; Pankevich et al., 2006; Keller et al., 2006; Kimchi et al., 2007). Recently, it has been shown that VNO receptors respond differentially to putative pheromones (Leinders-Zufall et al., 2000; Chamero et al., 2007), stimuli from animals differing in gender, strain or disease state (He et al., 2008, Riviere et al., 2009), suggesting that the information necessary to interpret and respond to conspecific biologically relevant chemical signals is present at the level of the VNO epithelium. However, interpretation of this information, which is distributed across several thousand sensory neurons, must occur more centrally.
The amygdala, particularly the medial amygdala (Lehman et al., 1980; Petrulis and Johnston, 1999; Blanchard et al., 2005), is important for the behavioral response to chemosensory information. The medial amygdala is the first site of convergence of afferent main olfactory and vomeronasal information in the brain (Shipley and Adamek, 1984; Pitkanen et al., 1997; Coolen and Wood, 1998; Meredith, 1998; Chamero et al., 2007). Exposure to different salient chemosensory stimuli (reproductive, territorial or predator odors) characteristically changes immediate early gene (IEG) expression in the medial amygdala (Meredith and Westberry, 2004; Samuelsen and Meredith, 2009). Chemical signal input, via the medial amygdala, is thought to be important for the proposed hypothalamic circuits involved in defensive and reproductive behavior (Canteras, 2002; Choi et al., 2005).
Previously we have shown that patterns of IEG expression in posterior medial amygdala depend on the biological relevance of the chemical stimuli. A range of conspecific (same species) chemical signals from males and females, relevant for reproductive or agonistic behavior, all activate both anterior and posterior medial amygdala. A range of non-relevant stimuli from other species (heterospecific) activate anterior medial amygdala, but fail to activate posterior medial amygdala. This categorical difference is seen in mice (Samuelsen and Meredith, 2009) and hamsters (Meredith and Westberry 2004, Meredith et al., 2008). Thus, the pattern of IEG expression in medial amygdala differs categorically according to the biological relevance of the chemical stimuli. Given the importance of the VNO to normal behavioral and physiological function, we reasoned that surgical removal of the VNO would result in a disruption of IEG expression patterns in the medial amygdala to biologically relevant chemosensory stimuli.
Here we show that the VNO is necessary for significant medial amygdala response to critical chemical signals in sexually inexperienced (naïve) male mice. Surgical removal of the vomeronasal organ (VNX) actually eliminates all significant medial amygdala IEG expression above control levels to all tested chemical signals, regardless of biological relevance. The tested stimuli include male and female mouse urine (mMU, fMU), known to convey reproductive and agonistic information to male mice (Hurst and Beynon, 2004; Pankevich et al., 2004; Pankevich et al., 2006; Nakamura et al., 2007), and hamster vaginal fluid (HVF), a heterospecific stimulus known to convey reproductive information from female to male hamsters (Johnston, 1977; Johnston and Brennan, 1982), but presumably irrelevant for mice. In a separate experiment, collars worn by a cat, which mice and rats normally avoid (Dielenberg et al., 1999; Dielenberg et al., 2001; Takahashi et al., 2005; Takahashi et al., 2007; Samuelsen and Meredith, 2009), were used as a biologically relevant heterospecific stimulus.
Experimental Procedures
Animals
48 (Exp. 1) and 24 (Exp. 2) sexually naive 3–4 month old male C57 BL/6 mice (Jackson Laboratory) were maintained on a reverse 12/12hr light/dark cycle with food and water ad libitum. All animal procedures were approved by the Florida State University Institutional Animal Care and Use Committee. Animals had no contact with any heterospecific stimuli before the experimental session, no contact with females or female stimuli since weaning and no contact with the male or female conspecific stimulus donors.
Vomeronasal Organ Removal Surgery (VNX)
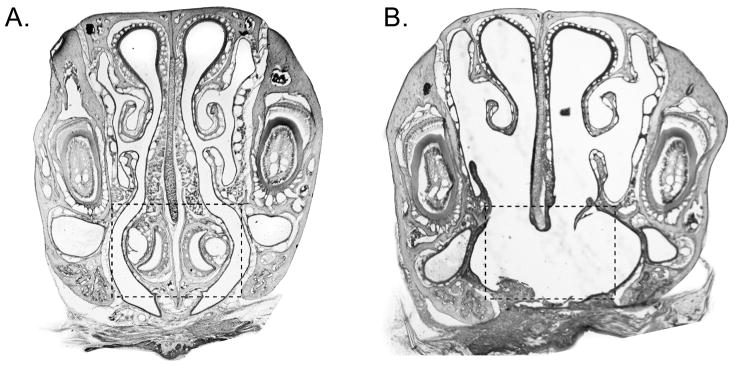
In both SHAM and VNX surgery, a midline incision was made along the palate exposing the VNO capsule. For SHAM surgery, the exposed VNO capsule was examined and then the palatal incision was closed with 2–3 sutures and cyanoacryate adhesive. For VNX, a dental drill was used to separate the VNO capsule from the rostral palatal bones. Using forceps, the medial palatine process of the maxillary bones was broken, separating the VNO capsule from the hard palate at its caudal end. The two VNO capsules were detached with forceps, with care to ensure that the capsule contents were removed with the capsules, and the palatal incision was closed as above. Experiments were conducted 7 days post surgery. To confirm VNX, decalcified noses were cut coronally into 20μm sections, stained with Gills hematoxylin and examined microscopically for intact vomeronasal epithelium (Figure 1). Fifteen animals with residual VNO tissue or excess nasal debris were excluded.
Figure 1.
Representative coronal sections (20μm) through the nose of (A) SHAM and (B) VNX mice. (A) The dashed box denotes the intact VNO. (B) The dashed box shows the bilateral removal of the VNO.
Stimuli
Female mouse urine was collected from 3–4 adult mice placed in a metabolic cage over a 5 day period. Five days of collection was used in order to collect urine from all estrus stages of normally cycling female mice (Champlin and Dorr, 1973). Male mouse urine was collected in a similar manner. Hamster vaginal fluid was collected from 2–4 adult female hamsters in behavioral estrus using a spatula, mixed together and placed in centrifuge tubes. As in our previous experiments (Meredith and Westberry, 2004; Samuelsen and Meredith 2009), all liquid stimuli were diluted 1:10 by weight with distilled water (purified by reverse osmosis and polishing with activated carbon) and centrifuged for 30 min (Fisher clinical centrifuge at medium speed). The supernatant was decanted and held at −20°C until presentation. Heterospecific stimuli are diluted to avoid any neophobia in test animals; conspecific stimuli are diluted equally to match, where possible. Soft nylon cat-collars (CC) (PETCO Single Ply Nylon Collar) were unworn (control) or were worn for 2-weeks by a neutered male house cat. The collars were removed, placed in zip-lock plastic bags and held at −20°C until use.
Testing Procedure and Stimulus Presentation
All mice were single housed for 7 days post-surgery and before testing, minimizing exposure to other male odors. On the day of the experiment, mice were placed in a clean cage with clean corn cob bedding and allowed 2 minutes to acclimate to the surroundings. Experiment 1: Polyester swab-tips (Puritan Medical Products Company) were used to present the stimuli. Clean swabs (control) or swabs containing ~200μl of liquid stimulus were presented in the middle of the cage and replaced every 3 minutes for a total of 15 minutes; a total of five scented swabs or five control swabs per animal. Experiment 2: A 2.5cm × 1.27cm piece of CC worn for two weeks or a piece of clean CC was presented in the middle of the clean cage and left for the entire 15 minute trial. For both experiments, behavior was recorded using a computer program and numbered key pad with each key corresponding to a different behavior. The computer records the latency, the number of presses and total elapsed time each key is depressed. Behaviors recorded were: the number of times the mouse contacted or very closely investigated the stimulus, the duration of investigation of the stimulus, number of rears, time spent rearing and general investigation of the cage. All animals were tested in the first 6 hours of the dark phase of the light cycle in a room lit by red light.
Immunocytochemistry
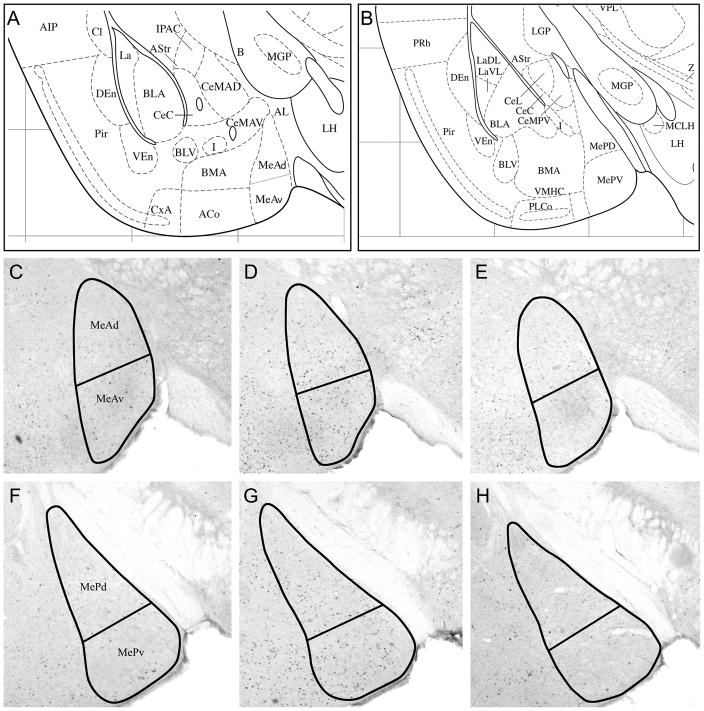
Forty-five minutes after the initial stimulus exposure, mice were anesthetized with Nembutal and perfused with cold 0.1M PBS followed by 4% paraformaldehyde (PFA). Brains were removed and post-fixed overnight in 4% PFA. The next morning brains were placed in 30% sucrose overnight for cyroprotection. Using a freezing microtome, brains were sliced into 40μm sections. Alternate free-floating coronal sections were washed in 0.1M PBS, blocked in a solution of 5% normal goat serum (30 min) and incubated in rabbit anti-FRAs primary antibody solution (SC253 – detects c-Fos, Fos B, Fra-1 and Fra-2; 1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA) for 20–24 hours at room temperature. The next day, sections were washed in 0.1M PBS and incubated in biotinylated goat anti-rabbit secondary antibody solution (1:400; Vector Laboratories, Burlingame, CA) for 2 hours. Sections were processed in ABC reagent (Vector Laboratories, Burlingame, CA) for 1 hour and stained with diamino benzidine (DAB) (Vector Laboratories, Burlingame, CA). FRAs expression was assessed by averaging numbers of densely labeled cell nuclei within areas of interest on both sides of the brain in three adjacent sections per anatomical area. Areas of interest included: 1) Anterior medial amygdala (MeA), which was divided into ventral anterior medial amygdala (MeAv) and dorsal anterior medial amygdala (MeAd); 2) Posterior medial amygdala (MeP), which was divided into ventral posterior medial amygdala (MePv) and dorsal posterior medial amygdala (MePd); both as indicated in the mouse brain atlas (Paxinos and Franklin, 2003; Fig 3). Image analysis software (ImagePro plus, Media Cybernetics, Inc.) was used to count all densely labeled cell nuclei within the borders of the neuroanatomical nucleus of interest. The numbers are presented as means and standard errors. Data are presented separately for MeP as a whole and MeA as a whole, in addition to the data for individual subdivisions, in order to facilitate comparisons with earlier reports that did not provide data for subdivisions.
Figure 3.
Representative coronal sections (40 μM) showing outlines within which FRAs-immunoreactive nuclei were counted. (A, B) Ventrolateral forebrain structures of the coronal sections used to measure IEG response in medial amygdala (from the Paxinos and Franklin (2003) mouse brain atlas): (A) At the level of anterior medial amygdala; Panels C, D, E are approximately at this level (1.06 mm posterior to bregma); (B) Posterior medial amygdala; Panels F, G, H are approximately at this level (1.58 mm posterior to bregma). C, D; F, G: Representative sections showing FRAs-labeled nuclei in anterior medial amygdala (C, D) and posterior medial amygdala (F, G) in SHAM-operated mice exposed to (C, F) clean swabs or to (D, G) female mouse urine (fMU). E and H show the reduced FRAs expression the medial amygdala of a mouse exposed to fMU after bilateral vomeronasal organ removal.
Statistics
IEG expression comparisons were analyzed for each experiment by two-way analysis of variance (ANOVA) with factors surgery (SHAM or VNX) and stimulus (Experiment 1: CON, mMU, fMU and HVF; Experiment 2: Control-CC and worn CC). Post-hoc comparisons were made using the Holm-Sidak test. Behaviors were analyzed using Two-way ANOVAs, with post-hoc comparisons using the Holm-Sidak test. Behavioral responses were tested with factors of surgery (SHAM and VNX) and stimulus (Experiment 1: CON, mMU, fMU and HVF; Experiment 2: Control-CC and worn CC). Reported behaviors include number and cumulative duration of close investigation or contact with the stimulus swab/collar, number of rears, and time spent rearing. Statistical results are reported in detail when p-values are significant or less than twice the critical level of the post-hoc analysis.
Results
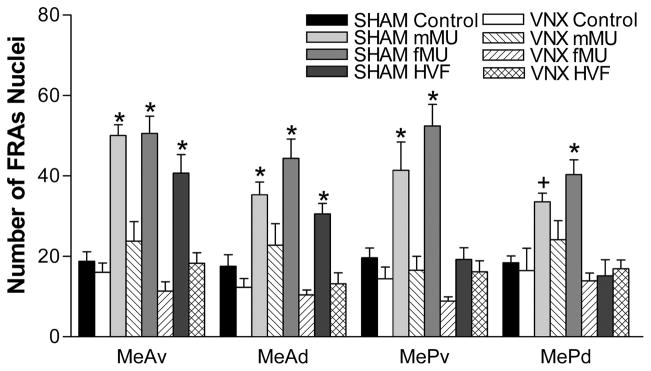
Exposure to mMU, fMU or CC stimuli, all representatives of behaviorally salient stimuli, increased IEG expression in both anterior medial amygdala (MeA) and posterior medial amygdala (MeP) in sham-operated (SHAM) male mice. Hamster vaginal fluid (HVF), selected as a representative non-salient stimulus, increased IEG expression in MeA, but not MeP of SHAM mice. These expression patterns confirm our previous findings, using intact mice, of the characteristic and categorical difference between medial amygdala response to biologically relevant and to non-relevant stimuli (Samuelsen and Meredith, 2009) and conceptually identical results in hamsters (Meredith and Westberry 2004). Data are presented for MeA and MeP as a whole, in addition to data for subdivisions in order to facilitate comparison with these reports. After VNX surgery, none of thestimuli significantly increased IEG expression in medial amygdala of VNX male mice.
Experiment 1
Response to Female Mouse Urine (fMU)
SHAM male mice exposed to fMU had significantly greater FRAs expression in MeA (F(1,39) = 50.7, p<0.001) and MeP (F(1,39) = 35.4, p<0.001), compared to control-SHAM mice exposed to clean-swabs (Supplemental Figure 1). They also had a significant increase in FRAs expression compared to control in all four medial amygdala subdivisions (ventral anterior medial amygdala [MeAv]: F(1,39) = 43.2, p<0.001; dorsal anterior medial amygdala [MeAd]: F(1,39) = 31.2, p<0.001, ventral posterior medial amygdala [MePv]: F(1,39) = 35.0, p<0.001; dorsal posterior medial amygdala [MePd]: F(1,39) = 20.9, p<0.001) (Figure 2). SHAM mice also had significantly greater IEG expression in response to fMU than VNX mice, in MeA and MeP (MeA: F(1,39) = 22.6, p<0.001; MeP: F(1,39) = 58.4, p<0.001), and in each subdivision (MeAv: F(1,39) = 65.6, p<0.001; MeAd: F(1,39) = 50.0, p<0.001; MePv: F(1,39) = 62.0, p<0.001; MePd: F(1,39) = 30.4, p<0.001). Representative brain sections of the FRAs expression in both MeA and MeP to fMU in SHAM and VNX animals are shown in Figure 3.
Figure 2.
Categorical response to chemical signals in the medial amygdala after SHAM or VNX surgery. In SHAM mice, all stimuli increased FRAs expression (mean number of nuclei ± SEM) in MeAv and MeAd, but only the conspecific stimuli (mMU and fMU) increased FRAs expression in MePv and MePd. In VNX-operated mice, no stimuli increased FRAs expression in any subdivision of medial amygdala. * indicates a significant difference from both SHAM-control mice (exposed to clean swabs) and from VNX mice exposed to the stimulus. + indicates a significant difference from control. Refer to the results section for p values.
There was no significant difference in FRAs expression between fMU-exposed VNX mice and SHAM control mice exposed to clean-swabs in any region or subregion of medial amygdala.
Response to Male Mouse Urine (mMU)
SHAM mice exposed to mMU had significantly greater FRAs expression in MeA (F(1,39) = 36.2, p<0.001) and MeP (F(1,39) = 16.5, p<0.001), compared to control SHAM male mice exposed to a clean swab (Supplemental Figure 1). They also had significant FRAs expression compared to control in all four medial amygdala subdivisions (MeAv: F(1,39) = 41.7, p<0.001; MeAd: F(1,39) = 13.7, p<0.001; MePv: F(1,39) = 15.4, p<0.001; MePd: F(1,39) = 10.0, p<0.01) (Figure 2). SHAM mice also had significantly greater IEG expression in response to mMU than VNX mice, in MeA and MeP (MeA: F(1,39) = 79.2, p<0.001; MeP: F(1,39) = 14.3, p<0.001), and in each subdivision except MePd (MeAv: F(1,39) = 29.4, p<0.001; MeAd: F(1,39) = 6.75, p<0.013.
There was no significant difference in FRAs expression between mMU-exposed VNX mice and SHAM control mice exposed to clean-swabs in any region or subregion of medial amygdala.
Response to Hamster vaginal fluid (HVF)
SHAM male mice exposed to the heterospecific odor, HVF, exhibited significantly higher FRAs expression in MeAv, MeAd (Figure 2) and overall MeA (Supplemental Figure 1) as compared to both SHAM control males exposed to clean-swabs (Figure 2) (MeA: F(1,39) = 17.9, p<0.001; MeAv: F(1,39) = 20.6, p<0.001; MeAd: F(1,39) = 7.37, p<0.01) and to VNX males exposed to HVF (MeA: F(1,39) = 22.9, p<0.001; MeAv: F(1,39) = 21.4, p<0.001; MeAd: F(1,39) = 13.1, p<0.001). There was no significant FRAs expression in SHAM mice or VNX mice, after exposure to HVF, in the measured areas of MeP or its subdivisions.
There was no significant difference in FRAs expression between HVF-exposed VNX mice and SHAM control mice exposed to clean-swabs in any region or subregion of medial amygdala.
Behavioral Response to Stimuli
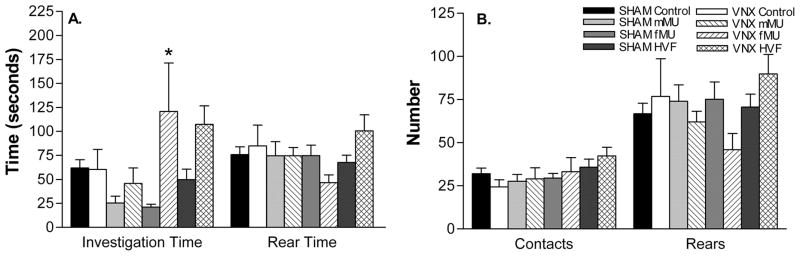
In Experiment 1, there was a significant main effect of surgery (F(1,39) = 7.9, p<0.01), but no significant main effect of stimulation, and no significant interaction VNX mice exposed to fMU spent significantly more time investigating the stimulus swab than their SHAM-operated counterparts (F(1,39) = 10.4, p<0.005). Although not significant, VNX mice spent more time than SHAM mice investigating HVF (F(1,39) = 3.5, p = 0.07). There were no significant differences in any other of the measured behaviors (Figure 3).
Experiment 2
Responses to Cat-collar (CC)
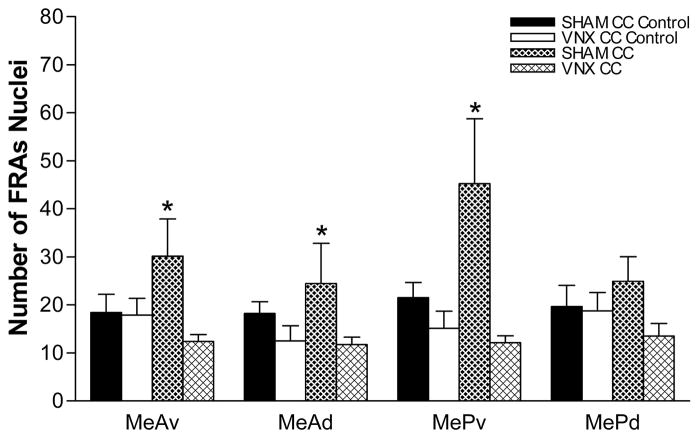
SHAM mice exposed to a 2.5 cm piece of CC (worn for 2 weeks) had significantly greater FRAs expression in MeA (F(1,23) = 20.0, p<0.001) and MeP (F(1,23) = 22.1, p<0.001) compared to SHAM mice exposed to a piece of clean collar (Supplemental Figure 2). They also have increased FRAs expression in the subregions MeAv (F(1,23) = 17.2, p<0.001), MeAd (F(1,23) = 17.3, p<0.001) and MePv (F(1,23) = 44.9, p<0.001) (Figure 5). There was no significant difference in the MePd subregion with CC exposure. SHAM mice also had greater FRAs expression in response to CC than VNX mice in MeA (F(1,23) = 38.6, p<0.001) and MeP (F(1,23) = 40.2, p<0.001), and in each subregion except MePd (MeAv: F(1,23) = 31.8, p<0.001; MeAd: F(1,23) = 33.8, p<0.001; MePv: F(1,23) = 69.1, p<0.001). In MePd, only the difference between SHAM and VNX mice exposed to CC was significant (F(1,23) = 7.81, p<0.011).
Figure 5.
IEG expression in the medial amygdala in response to predator stimuli after SHAM or VNX surgery. SHAM mice exposed to cat collar (CC) worn for two weeks had increased FRAs expression in both anterior medial amygdala subdivisions, MeAv and MeA. CC exposure results in a robust increase in MePv, but no difference in MePd. In VNX mice, this stimulus did not increase FRAs expression in any medial amygdala subdivision. * Significantly different from control. Refer to the results section for p values. (mean number of nuclei ± SEM).
There was no significant difference in FRAs expression between CC-exposed VNX mice and SHAM control mice exposed to clean-swabs in any region or subregion of medial amygdala.
Behavioral Response to CC Stimuli
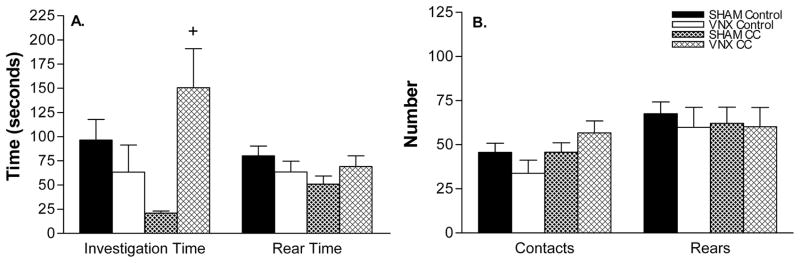
There were no main effects of surgery or stimulus in Experiment 2, but there was a significant interaction (p<0.01), indicating a difference in response to the stimulus between SHAM and VNX mice. Although SHAM mice spent less time investigating worn CC than control CC, the difference was not significant. VNX mice exposed to CC spent significantly more time investigating the stimulus collar than either VNX clean-collar controls (F(1,23) = 5.3, p<0.035) or SHAM CC exposed mice (F(1,23) = 11.7, p<0.005). Although not significant, SHAM mice spent less time investigating CC than those which were exposed to control CC (F(1,23) = 4.0, p = 0.06). There was no difference in any of the other measured behaviors (Figure 6).
Figure 6.
SHAM and VNX mouse behavioral response to predator odor. (A) VNX mice exposed to CC, spent significantly more time investigating the stimulus compared to both control and SHAM CC, (B) but contacted them a similar number of times. + indicates significant difference from control and SHAM CC. Refer to the results section for p values, and the discussion for interpretation of SHAM animals’ apparent avoidance of worn cat collar.
Discussion
In this report, we provide evidence that IEG response to biologically relevant chemical signals in the medial amygdala depends on an intact VNO. We have previously reported that the pattern of IEG expression in medial amygdala to biologically relevant (mainly conspecific) chemical signals is categorically different from the pattern of most heterospecific stimuli. These non-relevant heterospecific chemosensory stimuli do elicit an amygdala response, but have little or no effect on behavior and may be unimportant to the responding animals. In particular, biologically relevant stimuli increased IEG expression in both MeA and MeP, but stimuli with no apparent relevance increased expression only in MeA. This is the case for both mice (Samuelsen and Meredith, 2009) and hamsters (Meredith and Westberry, 2004). As mouse VNO neurons selectively respond to biologically relevant features of conspecific urine stimuli (Leinders-Zufall et al., 2000; Chamero et al., 2008; He et al., 2008), we expected the responses to conspecific signals in the medial amygdala to be disrupted by VNX. Although, we did not test a wide range of stimuli here, our evidence suggests that surgical removal of the VNO eliminates significant IEG response in the medial amygdala in male mice, regardless of the biological relevance (or species of origin) of the chemical stimulus.
Immediate early gene expression in the medial amygdala in response to chemical signals was essentially unaffected by SHAM surgery. The only stimulus/area that exhibited a different pattern of IEG expression than in our previous report on intact animals (Samuelsen and Meredith, 2009) was a significant increase in IEG expression in the MePd upon exposure to mMU. In our previous report, the response in MePd was slightly higher, but not significantly different from control. This variation may be due to small individual differences in male mice and the larger number of mice in the previous experimental group. MePd is thought to be an important contributor to reproductive circuits in the hypothalamus (Choi et al., 2005), so an increase in FRAs expression to mMU is unexpected; although the phenotype and projections of the responding cells in each case are not yet known and may be different.
Similar to our demonstration here, Pankevitch et al. (2006) reported a decrease of medial-amygdala IEG-expression following VNX in male mice, but in response to the volatile components of fMU. In male hamsters, we have repeatedly found that VNX eliminates medial-amygdala IEG-response to HVF, an important chemosignal for hamsters (Fernandez-Fewell and Meredith, 1994; Westberry and Meredith, 2003b), as well as responses to other chemosensory stimuli (J.M. Westberry and M. Meredith, unpublished observations).
The behavioral responses of SHAM mice were essentially similar to those previously reported in intact male mice (Samuelsen and Meredith, 2009). However, when analyzed by two-way ANOVA, the higher levels of investigation of clean control swabs, compared to scented swabs, was not significant for SHAM mice. All tested mice appear to treat clean swabs as potential bedding material and spend time shredding the tip in a characteristic behavior. This behavior was not seen with scented swabs, in either SHAM or VNX mice. Thus, the increased time VNX mice spent investigating scented swabs was not due to time spent shredding. VNX mice clearly can detect/discriminate male and female mouse urine (Pankevich et al., 2004; Pankevich et al., 2006) and probably all the stimuli used here, by olfaction. The increased investigation times after VNX may indicate an attempt to stimulate the VNO, whether to obtain more complete information or for the proposed reinforcing properties of VNO input (Martínez-Ricós et al., 2007). These animals had no prior contact with any heterospecific stimuli or any of the donors of conspecific stimuli, but they had experience with urine of mature males and also with urine of a mature female (prior to weaning). The increased investigation of the conspecific stimuli after VNX can be interpreted as a response to the novelty of an olfactory “signature” in the absence of the corresponding VNO input; i.e. a novel sensory experience for a stimulus otherwise belonging to a familiar class. This explanation does not immediately appear to be likely for the heterospecific stimuli. However, these are complex stimuli and it is possible that a heterospecific stimulus may contain some component in common with a salient chemosensory stimulus, which cannot be easily distinguished without vomeronasal input, thus eliciting additional investigation. Manipulation of a complex perceptual signature, by adding rather than subtracting components, may underlie the increase of aggression in male mice when androstenone, a pheromonal component of boar saliva, is added to a mouse urine stimulus. A similar increase of aggression does not occur when androstenone is presented in water (Ingersoll and Launay, 1986).
Under many circumstances, in sexually naïve hamsters and mice, previous experiments have shown that a functional VNO is necessary for normal social (Wekesa et al., 1994; Stowers et al., 2002; Kobayakawa et al., 2007) and sexual behaviors (Meredith, 1986; Keller et al., 2006; Kimchi et al., 2007) as well as preferences for social odors (Johnston and Peng, 2000; Pankevich et al., 2004; Woodly et al., 2004; Pankevich et al., 2006). However, a recent report suggests that female mating behavior is not affected by VNX (Martel and Baum, 2009).
In this experiment, we report that the behavioral response to a predator odor is disrupted by VNX. VNX mice spent significantly more time investigating a piece of worn CC as compared to both VNX mice exposed to a clean collar control and SHAM mice exposed to worn CC. In a previous experiment (Samuelsen and Meredith, 2009), intact males, as sham-operated males here, appeared to avoid worn CC pieces. Both intact and SHAM mice use the “stretch-attend” posture (Yang et al., 2004) in investigating CC stimuli. The intact animals spent significantly less time investigating CC stimuli than clean collar control stimuli, although, as with sham-operated animals here, there was no difference in the number of investigatory contacts. Although the SHAM mice had much shorter investigation times for worn CC, when analyzed in a two-way ANOVA with the VNX data, they were not significantly different from investigation of clean collars. This difference was significant if the SHAM data were analyzed separately by one way ANOVA, as in the previous experiment with intact animals. The two-way analysis does confirm a significant difference between investigation time for SHAM and VNX animals. The behavioral response of VNX mice suggests that they do not recognize the biological relevance of these chemical signals.
An overall increase in IEG expression in MeP, as well as in MeA, is characteristic of all biologically relevant stimuli tested, in both mice (Samuelsen and Meredith, 2009) and hamsters (Meredith and Westberry, 2004). While each biologically relevant chemosensory stimulus carries a potentially different message, the medial amygdala response is “categorical” because the presence or absence of an overall MeP response separates clearly relevant stimuli from those with no obvious biological relevance. Stimuli with no clear biological relevance, which evoke IEG expression only in MeA, include, in hamsters; artificial activation of amygdala input (Meredith and Westberry, 2004; Nolte and Meredith, 2005) as well as male and female mouse signals (Meredith and Westberry 2004). In mice, they include steer urine and hamster stimuli (Samuelsen and Meredith 2009). All the stimuli used here are unlearned in that the animals had no previous experience with the individual stimulus donors, but they may be familiar with male and female mouse odors as categories of stimuli (see above). An interesting next step will be to examine how the medial amygdala response is modified by experience with particular individual signals.
Removal of vomeronasal sensory input to medial amygdala is one obvious consequence of VNX, but a loss of subsequent hormonal responses could also contribute to the results observed here. For example, intact, but not VNX, male mice increase LH secretion in response to female urine (Coquelin et al., 1984), presumably a result of increased GnRH release into the pituitary portal vessels. There is a subsequent increase in circulating testosterone over the next half hour (Wysocki et al., 1983). This thirty minute delay means testosterone changes are not major factors in the behavioral responses and probably not in IEG responses reported here. However, GnRH is also released into the brain and intraventricular infusion of GnRH can alter behavior (Fernandez-Fewell and Meredith, 1995) as well as IEG responses in the amygdala (Westberry and Meredith, 2003a). Endogenous GnRH release directly at the site of action in the brain should be faster-acting than exogenous GnRH infused in the cerebral ventricles, and could be fast enough to feed-back onto amygdala circuits and influence results here. There is also evidence for estrogen effects on conspecific-signal recognition and behavioral response to predator odors (Pierman et al 2006; Kavaliers et al 2008), so a rapid change in aromatization of testosterone to estrogen could also influence brain circuits. Changes in steroid receptor function within the time-course of these experiments are also possible (Blake and Meredith, submitted).
The categorization of biologically relevant conspecific and heterospecific chemical signals in the mouse medial amygdala, whether learned or unlearned, depends upon a functional VNO. Even though there are direct projections from the main olfactory bulb to the medial amygdala (Pro-Sistiaga et al., 2007; Kang et al., 2009), our results suggest that main olfactory input alone is not sufficient to activate the characteristic categorical IEG expression pattern of the medial amygdala to biologically relevant chemical signals.
Supplementary Material
Categorical response to chemical signals in the medial amygdala after SHAM or VNX surgery. In SHAM-operated mice, all stimuli elicited increased FRAs expression (mean number of nuclei ± SEM) overall in MeA, but only conspecific stimuli (mMU and fMU) increased FRAs expression overall in MeP. In VNX-operated mice, no stimuli increased FRAs expression in either MeA or MeP.
IEG expression in the medial amygdala in response to predator stimuli after SHAM or VNX surgery. SHAM mice exposed to a cat collar (CC) worn for two weeks had increased FRAs expression compared to control mice in MeA and MeP (a response characteristic of biological relevant stimuli). In VNX-operated mice, no stimuli increased FRAs expression in either MeA or MeP.
Figure 4.
SHAM and VNX mouse behavioral response to conspecific and heterospecific chemical signals. (A) VNX mice exposed to fMU spent significantly more time (mean seconds ± SEM) than control investigating the stimulus swabs. (B) There were no differences in other measured behaviors. * indicates significant difference from control. Refer to the results section for p values.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC 005813 and T32 DC00044(M.M.) and fellowship F31 DC08062 (C.L.S.).
Abbreviations
- CC
cat collar
- fMU
female mouse urine
- FRAs
Fos-related antigens
- HVF
hamster vaginal fluid
- IEG
immediate early gene
- MeAv/d
anterior medial amygdala, ventral/dorsal divisions
- MePv/d
posterior medial amygdala, ventral/dorsal divisions
- mMU
male mouse urine
- VNO
vomeronasal organ
- VNX
vomeronasal organ removal
Literature References
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29(8):1243–53. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450(7171):899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4(9):2230–6. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182(115):939–41. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Arnold JC, McGregor IS. Low-dose midazolam attenuates predatory odor avoidance in rats. Pharmacol Biochem Behav. 1999;62:197–201. doi: 10.1016/s0091-3057(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Facilitation of mating behavior in male hamsters by LHRH and AcLHRH5–10: interaction with the vomeronasal system. Physiol Behav. 1995;57(2):213–21. doi: 10.1016/0031-9384(94)00276-b. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941(1–2):91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;25;320(5875):535–8. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll DW, Launay J. Murine aggression induced by a boar chemosignal: a stimulus presentation dependency. Physiol Behav. 1986;36(2):263–9. doi: 10.1016/0031-9384(86)90014-4. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Peng M. The vomeronasal organ is involved in discrimination of individual odors by males but not by females in golden hamsters. Physiol Behav. 2000;70(5):537–49. doi: 10.1016/s0031-9384(00)00304-8. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Devidze N, Choleris E, Fudge M, Gustafsson JA, Korach KS, Pfaff DW, Ogawa S. Estrogen receptors alpha and beta mediate different aspects of the facilitatory effects of female cues on male risk taking. Psychoneuroendocrinology. 2008;33(5):634–42. doi: 10.1016/j.psyneuen.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23(2):521–30. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448(7157):1009–14. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210(4469):557–60. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240(1):27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 2000;15;405(6788):792–6. doi: 10.1038/35015572. [DOI] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci U S A. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299(5610):1196–201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2008;29(2):368–76. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009;29(24):7658–66. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Ricós J, Agustín-Pavón C, Lanuza E, Martínez-García F. Intraspecific communication through chemical signals in female mice: reinforcing properties of involatile male sexual pheromones. Chem Senses. 2007;32(2):139–48. doi: 10.1093/chemse/bjl039. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36(4):737–43. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M, Graziadei PP, Graziadei GA, Rashotte ME, Smith JC. Olfactory function after bulbectomy. Science. 1983;222(4629):1254–5. doi: 10.1126/science.6648533. [DOI] [PubMed] [Google Scholar]
- Meredith M, O’Connell RJ. Efferent control of stimulus access to the hamster vomeronasal organ. J Physiol. 1979;286:301–16. doi: 10.1113/jphysiol.1979.sp012620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann N Y Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Meredith M, Samuelsen CL, Blake C, Westberry J. Selective responses of medial amygdala subregions to reproductive and defensive chemosignals from conspecific and heterospecific species. In: Hurst JL, editor. Chemical signals in Vertebrates. Vol. 11. Springer; 2008. pp. 367–378. [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kikusui T, Takeuchi Y, Mori Y. The critical role of familiar urine odor in diminishing territorial aggression toward a castrated intruder in mice. Physiol Behav. 2007;90:512–517. doi: 10.1016/j.physbeh.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Nolte CM, Meredith M. mGluR2 activation of medial amygdala input impairs vomeronasal organ-mediated behavior. Physiol Behav. 2005;86(3):314–23. doi: 10.1016/j.physbeh.2005.08.036. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Meredith M. Effects of volatile and nonvolatile chemical signals on male sex behaviors mediated by the main and accessory olfactory systems. Behav Neurosci. 1984;98(6):1083–93. doi: 10.1037//0735-7044.98.6.1083. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006;120:925–936. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates: Compact Second Edition. Pub. Elsevier Science & Technology Books; 2003. [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;13(2):345–57. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Balthazart J, Baum MJ, Bakker J. Attraction thresholds and sex discrimination of urinary odorants in male and female aromatase knockout (ArKO) mice. Horm Behav. 2006;49(1):96–104. doi: 10.1016/j.yhbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science. 1975;187(4180):961–3. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Bañon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Pérula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;1;504(4):346–62. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Research. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Adamek GD. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull. 1984;12:669–688. doi: 10.1016/0361-9230(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295(5559):1493–500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Wekesa KS, Lepri JJ. Removal of the vomeronasal organ reduces reproductive performance and aggression in male prairie voles. Chem Senses. 1994;19(1):35–45. doi: 10.1093/chemse/19.1.35. [DOI] [PubMed] [Google Scholar]
- Westberry J, Meredith M. The influence of chemosensory input and gonadotropin releasing hormone on mating behavior circuits in male hamsters. Brain Res. 2003a;974(1–2):1–16. doi: 10.1016/s0006-8993(03)02535-6. [DOI] [PubMed] [Google Scholar]
- Westberry J, Meredith M. Pre-exposure to female chemosignals or intracerebral GnRH restores mating behavior in naive male hamsters with vomeronasal organ lesions. Chem Senses. 2003b;28(3):191–6. doi: 10.1093/chemse/28.3.191. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Cloe AL, Waters P, Baum MJ. Effects of vomeronasal organ removal on olfactory sex discrimination and odor preferences of female ferrets. Chem Senses. 2004;29(8):659–69. doi: 10.1093/chemse/bjh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CJ, Katz Y, Bernhard R. Male vomeronasal organ mediates female-induced testosterone surges in mice. Biol Reprod. 1983;(4):917–22. doi: 10.1095/biolreprod28.4.917. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav. 1984;29(2):315–27. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC. The rat exposure test: a model of mouse defensive behaviors. Physiol Behav. 2004;81(3):465–73. doi: 10.1016/j.physbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Categorical response to chemical signals in the medial amygdala after SHAM or VNX surgery. In SHAM-operated mice, all stimuli elicited increased FRAs expression (mean number of nuclei ± SEM) overall in MeA, but only conspecific stimuli (mMU and fMU) increased FRAs expression overall in MeP. In VNX-operated mice, no stimuli increased FRAs expression in either MeA or MeP.
IEG expression in the medial amygdala in response to predator stimuli after SHAM or VNX surgery. SHAM mice exposed to a cat collar (CC) worn for two weeks had increased FRAs expression compared to control mice in MeA and MeP (a response characteristic of biological relevant stimuli). In VNX-operated mice, no stimuli increased FRAs expression in either MeA or MeP.