Abstract
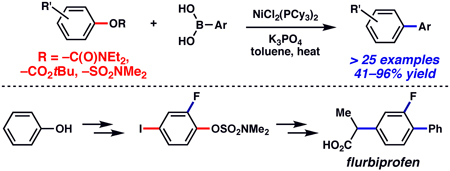
The first Suzuki–Miyaura cross-coupling of carbamates, carbonates, and sulfamates is described. The method presented provides a powerful means to use simple derivatives of phenol as precursors to polysubstituted aromatic compounds, as exemplified by a concise synthesis of the anti-inflammatory drug flurbiprofen.
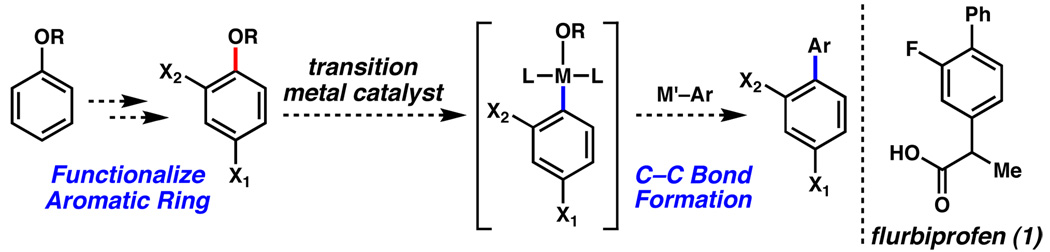
Transition metal-catalyzed cross-coupling reactions continue to play a vital role in modern synthetic chemistry.1 Although cross-couplings of aryl halides and triflates are most common, recent studies have demonstrated the successful cross-coupling of simple and affordable phenolic derivatives. In 2008, notable achievements in this area include the Suzuki–Miyaura coupling of electron deficient aryl methyl ethers by Chatani,2 and the Suzuki–Miyaura coupling of aryl pivalates,3 which was reported simultaneously by our group,4a and the group of Shi.4b A conceptual advantage of these technologies, compared to methodologies involving halides and sulfonates, is the potential to direct the installation of other functional groups onto an aromatic ring prior to cross-coupling (Figure 1). In practice, however, the ability to use methyl ethers (R = Me) and pivalates (R = OC(O)CMe3) in this sense is somewhat limited.5 Given the importance of polyfunctionalized aromatics in medicine, ligands for catalysis, and materials chemistry, we sought to address this problem. In this communication, we describe the first Suzuki–Miyaura couplings of aryl carbamates, carbonates, and sulfamates. Moreover, we disclose a concise synthesis of the anti-inflammatory drug flurbiprofen (1)6 using this methodology.
Figure 1.
Approach to polysubstituted aromatics such as flurbiprofen.
Of the potential phenolic derivatives to be studied, aryl carbamates and sulfamates were considered ideal because of their ready availability and pronounced stability to a variety of reaction conditions. Furthermore, these substrates can be used to direct the installation of functional groups at both the ortho and para positions (via ortho-lithiation chemistry pioneered by Snieckus7 and electrophilic aromatic substitution,8 respectively). Although nickel-catalyzed Kumada couplings of these substrates have been documented,7b,9 cross-coupling under milder, more attractive Suzuki–Miyaura conditions have not been reported. Of note, the oxidative addition of a metal into the aryl C–O bond of an aryl carbamate or sulfamate presents a considerable challenge.
Despite this difficulty, we have found that the Suzuki–Miyaura coupling of aryl carbamates with aryl boronic acids proceeds in the presence of NiCl2(PCy3)2, K3PO4, and heat, with toluene as solvent (Table 1). That NiCl2(PCy3)2 could be used to facilitate the desired transformation is advantageous,10 as this readily available complex shows marked stability to air and water, and can be used on the bench-top rather than in a glovebox.11 The carbamate derivative of 1-naphthol could be converted to the desired biaryl product at 110 °C with 5 mol% Ni complex (entry 1). However, the yield improved substantially by carrying out the reaction at higher temperatures with increased catalyst loading (entry 2). 2-Naphthol derivatives could also be coupled under these conditions (entries 3 and 4). In addition, the reaction proved tolerant of an electron-withdrawing group (–CO2Me, entry 4) and an electron-donating group (–OMe, entry 5) on the naphthyl ring. The corresponding reactions of non-fused aryl carbamates proved more challenging. Nonetheless, carbamates derived from phenol and p-methoxyphenol could be converted to the corresponding cross-coupled products, albeit in modest yields (entries 6 and 7). Aryl t-butylcarbonates were also deemed suitable cross-coupling partners (entries 8–10). Interestingly, the carbonate congener of 2-naphthol delivered the cross-coupled product in significantly higher yield compared to the corresponding carbamate (entry 9 and entry 3).
Table 1.
Cross-coupling of aryl carbamates and carbonates with arylboronic acids 2a or 2b.a
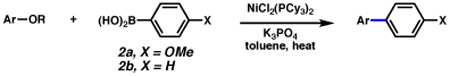 | ||||
|---|---|---|---|---|
| entry | Ar–OR | (HO)2B–Ar | product | yieldc |
| 1b | 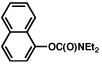 |
2a | 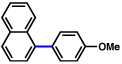 |
51% |
| 2 | 86% | |||
| 3 | 2a | 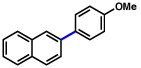 |
47% | |
| 4 | 2b | 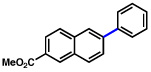 |
54% | |
| 5 | 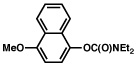 |
2b | 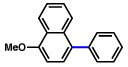 |
77% |
| 6 | 2a | 52% | ||
| 7 | 2b | 41% | ||
| 8 | 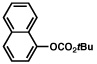 |
2a | 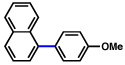 |
72% |
| 9 | 2a | 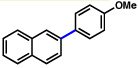 |
85% | |
| 10 | 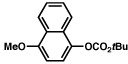 |
2b | 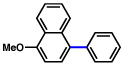 |
65% |
Conditions: NiCl2(PCy3)2 (10 mol%), ArB(OH)2 (4 equiv), K3PO4 (7.2 equiv), toluene (0.3 M), 130 °C for 24 h.
Conditions: NiCl2(PCy3)2 (5 mol%), ArB(OH)2 (2.5 equiv), K3PO4 (4.5 equiv), toluene (0.3 M), 110 °C, 24 h.
Isolated yields.
As shown in Table 2, aryl sulfamates serve as superior coupling partners in the Ni-catalyzed Suzuki–Miyaura reaction. Both naphthyl and non-fused aromatic substrates could be converted to biaryl products in excellent yield (entries 1–11). Electron-withdrawing (entries 2 and 9) and electron-donating groups were tolerated (entries 3, 10, and 11). In addition to para, meta, and ortho-methyl substituents (entries 5–7), a sterically congested 2,6-disubstituted substrate also participated in the desired cross-coupling process (entry 8). A heteroaromatic sulfamate (entry 12) and a vinyl sulfamate (entry 13) proved to be competent substrates. Finally, a range of o-substituted sulfamates, prepared by o-lithiation / functionalization of phenyl dimethylsulfamate,12 underwent smooth cross-coupling in excellent yields (entries 14–17).
Table 2.
Cross-coupling of aryl sulfamates.a
| entry | R–OSO2NMe2 | (HO)2B–Ar | product | yieldb |
|---|---|---|---|---|
| 1 | 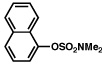 |
2a | 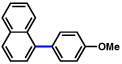 |
95% |
| 2 | 2b | 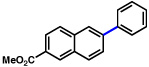 |
72% | |
| 3 | 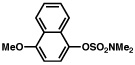 |
2b | 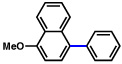 |
92% |
| 4 | 2a | 87% | ||
| 5 | 2a | 89% | ||
| 6 | 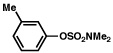 |
2a | 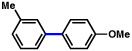 |
91% |
| 7 | 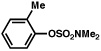 |
2a | 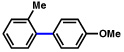 |
92% |
| 8 | 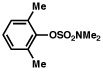 |
2a | 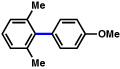 |
63% |
| 9 | 2b | 81% | ||
| 10 | 2b | 80% | ||
| 11 | 2b | 76% | ||
| 12 | 2b | 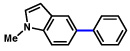 |
75% | |
| 13 | 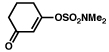 |
2b | 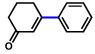 |
75% |
| 14c |  |
2a | 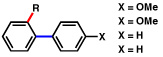 |
92% |
| 15 | 2a | 93% | ||
| 16 | 2b | 90% | ||
| 17c | 2b | 85% |
Conditions: NiCl2(PCy3)2 (5 mol%), ArB(OH)2 (2.5 equiv), K3PO4 (4.5 equiv), toluene (0.3 M), 110 °C, 24 h.
Isolated yields.
Conditions: NiCl2(PCy3)2 (10 mol%), ArB(OH)2 (4 equiv), K3PO4 (7.2 equiv), toluene (0.3 M), 130 °C, 24 h.
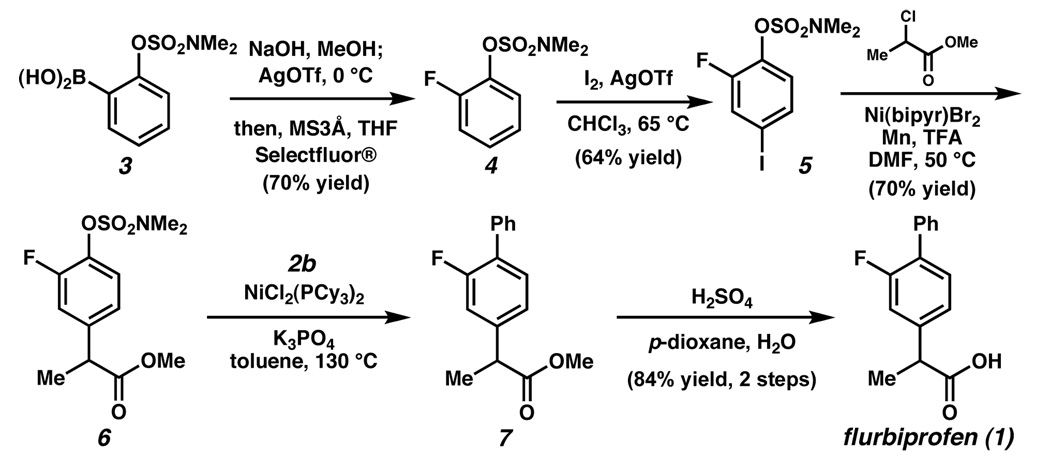
To further probe the scope and utility of the sulfamate cross-coupling methodology, a synthesis of the anti-inflammatory drug flurbiprofen6 was performed (Figure 2). Boronic acid 3, derived from o-lithiation / borylation of phenyl dimethylsulfamate,12 was fluorinated using the conditions described by Furuya and Ritter13 to provide fluorosulfamate 4. Selective iodination of 4 para to the sulfamate furnished 5 in 64% yield. Of note, both the fluoride and sulfamate of 5 were deemed unreactive toward Pd(0). As the aryl iodide displayed orthogonal reactivity, we carried out a site-selective enolate coupling to install the necessary propionate side chain. Whereas enolate coupling of aryl iodide 5 under Buchwald’s Pd-based conditions was feasible,14 higher yields of 6 were obtained using a Ni-catalyzed variant.15 Although the sulfamate was not disturbed in this process, exposure of 6 to our Ni-catalyzed Suzuki–Miyaura conditions facilitated the key sulfamate cross-coupling. Acid-mediated hydrolysis furnished flurbiprofen (1) in 84% yield, over the two steps. It should be emphasized that the aryl fluoride of 6 was chemically inert under our nickel-catalyzed cross-coupling conditions.16
Figure 2.
Synthesis of flurbiprofen using orthogonal cross-couplings.
In summary, we have discovered the first Suzuki–Miyaura coupling reactions of aryl carbamates, carbonates, and sulfamates. The method relies on the use of a readily available, air-stable Ni(II) complex to facilitate the desired transformations. Furthermore, the technology presented herein allows for the installation of multiple functional groups onto an aromatic ring prior to the cross-coupling event, as demonstrated by a concise synthesis of the anti-inflammatory drug flurbiprofen.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to the NIH-NIGMS (R00 GM079922), the University of California, Los Angeles, the Amgen Scholars Program (undergraduate fellowship to M.R.) and Boehringer Ingelheim for financial support. We thank Takeru Furuya (Harvard University), Professor Ritter (Harvard University), and Professor Snieckus (Queen’s University) for pertinent discussions, the Garcia-Garibay laboratory (UCLA) for access to instrumentation, and Dr. John Greaves (UC Irvine) for mass spectra.
Footnotes
Supporting Information Available: Detailed experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.a) Diederich F, Meijere A, editors. Metal-Catalyzed Cross-Coupling Reactions. Weinheim: Wiley-VCH; 2004. [Google Scholar]; b) Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; c) Miyaura N, editor. Topics in Current Chemistry. Vol. 219. New York: Springer-Verlag; 2002. [Google Scholar]; d) Corbet J, Mignani G. Chem. Rev. 2006;106:2651–2710. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]; e) Negishi E. Bull. Chem. Soc. Jpn. 2007;80:233–257. [Google Scholar]
- 2.Tobisu M, Shimasaki T, Chatani N. Angew. Chem. Int. Ed. 2008;47:4866–4869. doi: 10.1002/anie.200801447. [DOI] [PubMed] [Google Scholar]
- 3.For reviews, see: Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2009;48:3565–3568. doi: 10.1002/anie.200900465.Gooβen LJ, Gooβen K, Stanciu C. Angew. Chem. Int. Ed. 2009;48:3569–3571. doi: 10.1002/anie.200900329.Knochel P, Gavryushin A. Synfacts. 2009;2:200..
- 4.a) Quasdorf KW, Tian X, Garg NK. J. Am. Chem. Soc. 2008;130:14422–14423. doi: 10.1021/ja806244b. [DOI] [PubMed] [Google Scholar]; b) Guan B-T, Wang Y, Li B-J, Yu D-G, Shi Z-J. J. Am. Chem. Soc. 2008;130:14468–14470. doi: 10.1021/ja8056503. [DOI] [PubMed] [Google Scholar]
- 5.The ortho-directing ability of methyl ethers is modest (see ref 7a and refs therein), whereas ortho substitution of aryl pivalates presents a considerable challenge.
- 6.For pertinent reviews, see: Richy F, Rabehnda V, Mawet A, Reginster J-Y. Int. J. Clin. Pract. 2007;61:1396–1406. doi: 10.1111/j.1742-1241.2007.01452.x.Kumar P, Pathak PK, Gupta VK, Srivastava BK, Kushwaha BS. Asian J. Chem. 2004;16:558–562..
- 7.a) For a review, see: Snieckus V. Chem. Rev. 1990;90:879–933.. b) For the use of sulfamates in directed metallation reactions and Kumada couplings, see: Macklin TK, Snieckus V. Org. Lett. 2005;7:2519–2522. doi: 10.1021/ol050393c..
- 8.Arenes that possess an –OC(O)R substituent are well known to undergo electrophilic aromatic substitution to predominantly afford para substituted products; see: Smith MB, March J. March’s Advanced Organic Chemistry. 6th ed. New Jersey: John Wiley & Sons Inc.; 2007. p. 668..
- 9.a) Sengupta S, Leite M, Raslan DS, Quesnelle C, Snieckus V. J. Org. Chem. 1992;57:4066–4068. [Google Scholar]; b) Dallaire C, Kolber I, Gingras M. Org. Synth. 2002;78:42. [Google Scholar]; c) Yoshikai N, Matsuda H, Nakamura E. J. Am. Chem. Soc. 2009;131:9590–9599. doi: 10.1021/ja903091g. [DOI] [PubMed] [Google Scholar]; d) Wehn PM, Du Bois J. Org. Lett. 2005;21:4685–4688. doi: 10.1021/ol051896l. [DOI] [PubMed] [Google Scholar]
- 10.NiCl2(PCy3)2, is now commercially available from Strem Chemicals Inc. (catalog #28-0091), or can be prepared in multigram quantities following a simple one-step protocol; see: Stone PJ, Dori Z. Inorg. Chim. Acta. 1971;5:434–438.Barnett KW. J. Chem. Educ. 1974;51:422–423..
- 11.In the presence of excess arylboronic acid, NiCl2(PCy3)2 is thought to undergo reduction to an active Ni(0) catalyst.
- 12.See Supporting Information for details.
- 13.Furuya T, Ritter T. Org. Lett. 2009;11:2860–2863. doi: 10.1021/ol901113t. [DOI] [PubMed] [Google Scholar]
- 14.Moradi WA, Buchwald SL. J. Am. Chem. Soc. 2001;123:7996–8002. doi: 10.1021/ja010797+. [DOI] [PubMed] [Google Scholar]
- 15.Durandetti M, Gosmini C, Périchon J. Tetrahedron. 2007;63:1146–1153. [Google Scholar]
- 16.For nickel-catalyzed Kumada and Suzuki–Miyaura couplings of aryl fluorides, see: Yoshikai N, Mashima H, Nakamura E. J. Am. Chem. Soc. 2005;127:17978–17979. doi: 10.1021/ja056327n.Dankwardt JW. J. Organomet. Chem. 2005;690:932–938.Schaub T, Backes M, Radius U. J. Am. Chem. Soc. 2006;128:15964–15965. doi: 10.1021/ja064068b..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.