Abstract
The directed movement of chromosomes during mitosis and meiosis relies on microtubule-mediated connection between the spindle poles and kinetochores assembled on chromosomes. The molecular basis for the dynamic interaction between microtubules and kinetochores is just beginning to be unveiled. Here, focusing on the mitotic centromere kinase Aurora B, we review our current understanding of the signaling pathways that correct erroneous microtubule attachment at kinetochores. We evaluate several potential models that may explain how maloriented attachments can be recognized and processed by the Aurora B pathway.
Introduction
To direct chromosome movement during mitosis, the kinetochore, a proteinaceous structure assembled on centromeric DNA, dynamically captures microtubules. Importantly, all the microtubules attaching to a kinetochore on one chromatid must link to only one of the two spindle poles, while those attaching to its sister kinetochore must link to the opposite pole. Although the back-to-back orientation of the kinetochore arrangement may contribute to this correct, amphitelic attachment [1*], cells are equipped with machinery to correct the aberrant microtubule attachment geometries found in monotely, syntely and merotely (Figure 1) [2]. Furthermore, some of these aberrant attachments and lack of attachments activate the spindle assembly checkpoint (SAC, or mitotic checkpoint), which delays sister chromatid separation and mitotic exit [3].
Figure 1.
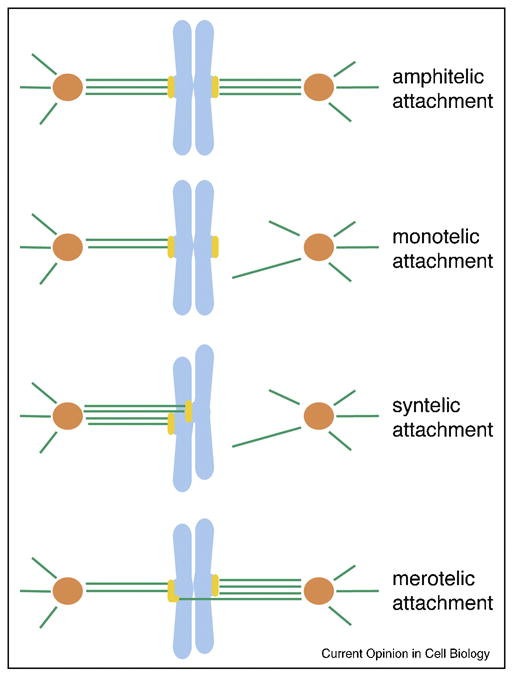
Classification of kinetochore microtubule attachments.
Amphitelic attachment: this is the correct attachment, in which all the microtubules attached to a kinetochore connect one spindle pole, while all those attached to its sister kinetochore link to its opposite pole. Monotelic attachment: a kinetochore attaches to microtubules that link to one spindle pole, while its sister kinetochore does not attach to any microtubules. Syntelic attachment: both sister kinetochores are linked to the same pole by microtubules. Merotelic attachment: a kinetochore attaches to microtubules from more than one spindle pole, a situation that results in a lagging chromosome during anaphase. Kinetochores are in yellow, microtubules in green, chromosomes in light blue and centrosomes in orange.
How is an improper microtubule-kinetochore attachment recognized and repaired? Elegant work in budding yeast and grasshopper spermatocytes has demonstrated that tension at kinetochores/centromeres is a key factor in to stabilizing bi-oriented attachments [4,5]. Upon biorientation during mitosis, tension is generated by microtubules pulling at the kinetochore of each sister chromatid, held together by cohesion near or at centromeres. Indeed, studies in budding and fission yeast have shown that centromeric cohesion is important for accurate mitotic chromosome segregation [6,7]. In the case of a maloriented chromosome where tension is lost, a signaling cascade removes improper attachments, which in turn is believed to activate the SAC [3]. Multiple lines of evidence suggest that centromeric Aurora B kinase activity plays a major role in correcting these improper attachments and in SAC signaling [8,9]. Here, we will restrict our focus to the regulation of Aurora B activation with regard to the correction of improper microtubule attachment, and review several speculative models that may explain how this process is controlled.
Aurora B is required for correcting erroneous microtubule attachments at kinetochores
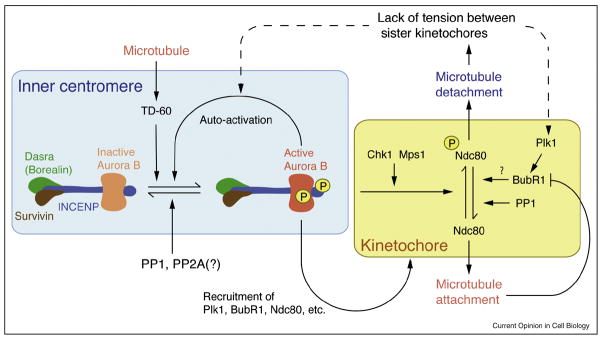
The chromosomal passenger complex (CPC), which contains the kinase Aurora B, INCENP, Dasra (also known as Borealin and CSC-1) and Survivin plays multiple roles at multiple places during mitosis (Figure 2) [10,11**]. Upon entry into mitosis, the CPC is first localized to both chromosome arms and the inner centromere, a region located between sister kinetochores. As the cell cycle progresses to metaphase, the amount of CPC localized to the chromosome arms decreases and it is mainly detected at the inner centromere [12]. When sister chromatids separate in anaphase, the CPC dissociates from centromeres and relocalizes to the spindle midzone.
Figure 2.
Aurora B pathway control of microtubule attachment.
The CPC, composed of Aurora B, INCENP, Dasra (Borealin) and Survivin, is localized to inner centromre, where it signals to correct mal-oriented kinetochore-microtubule attachments. Aurora B can be autoactivated by phosphorylation in trans, but the reaction is inhibited by phosphatases. Upon loss of tension between sister chromatids, inner centromeric Aurora B is activated, where activation may occur directly or indirectly. At the kinetochore, Aurora B phosphorylates Ndc80, leading to destabilization of microtubules at kinetochores. Lack of tension activates the Plk1 dependent phosphorylation of BubR1, a modification which is critical for microtubule attachment [62]. Once microtubule attachment is established, BubR1 is inactivated [63]. Upon bipolar attachment, the Aurora B pathway and the Polo pathway are inactivated, possibly through the action of PP1.
During metaphase, the CPC is critical for the recruitment of a growing number of proteins to the kinetochore and centromere: outer kinetochore proteins, including those involved in SAC signaling (Mad1, Mad2, Bub1, BubR1, Mps1 and Cenp-E) [13–15]; proteins responsible for microtubule-kinetochore interactions (Cenp-E, Ndc80, Knl1, Mis12, Zwilch, p150Glued, MCAK, Dam1 and Plk1) [16–21]; and other inner centromeric proteins such as the Shugoshin family proteins (Sgo1, Sgo2) and MCAK [20,22,23*]. Therefore, the CPC is one of the most upstream regulators of centromere/kinetochore function.
If CPC function is compromised, chromosomes with syntelic and merotelic attachments are frequently observed [14,24,25] due to the failure of improper connections to detach [8,9]. Aurora B-dependent phosphorylation of some key substrates is believed to facilitate the destabilization of aberrant attachments. Accordingly, both the CPC and activated Aurora B are preferentially enriched at merotelically attached kinetochores. This has been determined by using phospho-specific antibodies against the active forms of Aurora B and INCENP [26]. Several studies also indicate that Aurora B-mediated phosphorylation is less abundant on kinetochores at metaphase than at prometaphase when proper attachment has not yet occurred [17,18,27].
The mechanisms by which Aurora B kinase activity destabilizes improper microtubule attachments are not completely understood. However, several key substrates have been elucidated. The Ndc80/Hec1 complex is a major attachment module for microtubules at the outer kinetochore. In the absence of tension, it was proposed that Aurora B phosphorylates Ndc80, which decreases its affinity for microtubules (Figure 2) [28,29]. In addition, Dam1, a protein that allows kinetochores to track depolymerizing plus ends of microtubules in budding yeast, is negatively regulated by Ipl1 (Aurora B)-mediated phosphorylation [30].
Aurora B also regulates the microtubule-depolymerizing enzyme MCAK [17–19]. Interestingly, this appears to be both positive and negative regulation as Aurora B-mediated phosphorylation of MCAK suppresses its depolymerizing activity, but also controls its accumulation at centromeres [17–19]. Although MCAK accumulation can lead to microtubule destabilization, it remains unclear how Aurora B-mediated suppression of MCAK activity contributes to this process [31].
Regulators of the Aurora B pathway
The Aurora B pathway can be regulated by controlling the level of kinase activation directly or by controlling the balance of phosphorylation and dephosphorylation of its substrates. Biochemical and structural evidence suggest that for Aurora B to be fully active, it must phosphorylate residues at the C-terminus of INCENP. However, it is predicted that the phosphorylation sites on INCENP are improperly oriented with respect to the active site of the kinase for phosphorylation to occur in cis and that this reaction is carried out in trans [32]. Indeed, high local concentrations of the CPC lead to full activation of Aurora B kinase, even in the presence of counteracting phosphatase activity [33*]. Furthermore, both chromatin and microtubules also lead to activation of the Aurora B kinase pathway, possibly through a similar mechanism of enrichment and trans-autoactivation and/or yet unknown allosteric mechanisms [33*, 34]. Interestingly, another inner centromeric protein, TD-60, was shown to enhance the activation of Aurora B by microtubules [35*]. As detailed in the models below, these activities are key to our understanding of CPC function at the centromere during mitosis (Figure 2).
Two protein kinases recruited to kinetochores can also directly stimulate the kinase activity of Aurora B (Figure 2). Chk1, a protein kinase involved in the DNA damage checkpoint, is transiently localized to kinetochores and controls kinetochore microtubule attachments by activating Aurora B [36*]. Mps1, a protein kinase required for the SAC, controls kinetochore microtubule attachment and correction [37*,38**]. Mps1 stimulates Aurora B activity via phosphorylation of Borealin (Dasra B), modifications critical to error correction of microtubule attachments [38**]. These results suggest that Mps1 and Chk1 stimulate Aurora B activity to correct maloriented attachments in vertebrate cells.
In addition to these positive regulators of Aurora B, a number of negative regulators are believed to be important for limiting Aurora B activity. Several pieces of evidence suggest that protein phosphatase 1 (PP1) is the major counteracting phosphatase of the Aurora B pathway (Figure 2) [16,39,40]. In vertebrates, PP1α and PP1γ xare localized to the outer kinetochore [41,42], where they may remove Aurora B-dependent marks. PP1 probably serves two functions with respect to Aurora B: setting a threshold of kinase activity to counteract random fluctuations in Aurora B activity and allowing for rapid re-attachment of microtubules after removal of incorrectly attached ones.
Another potentially important negative regulator of the Aurora B pathway is the kinetochore kinase, BubR1 (Figure 2). In BubR1-depleted cells, the majority of kinetochores fail to attach to microtubules. However, this attachment defect is due in part to an increase in Aurora B activity upon BubR1-depletion [43,44]. Since Aurora B is required for the phosphorylation and kinetochore localization of BubR1 [14,25,45], BubR1 could act as a negative feedback regulator of the Aurora B pathway.
Potential mechanisms by which Aurora B regulates connections between kinetochores and microtubules
What is the mechanism by which tension and/or microtubule attachment status is sensed and how is this state translated into regulation of Aurora B kinase activity? Here we discuss three mechanisms by which the presence or absence of tension/attachment might modulate the Aurora B kinase pathway, with a focus on correction of aberrant microtubule attachments (Figure 3).
Figure 3.
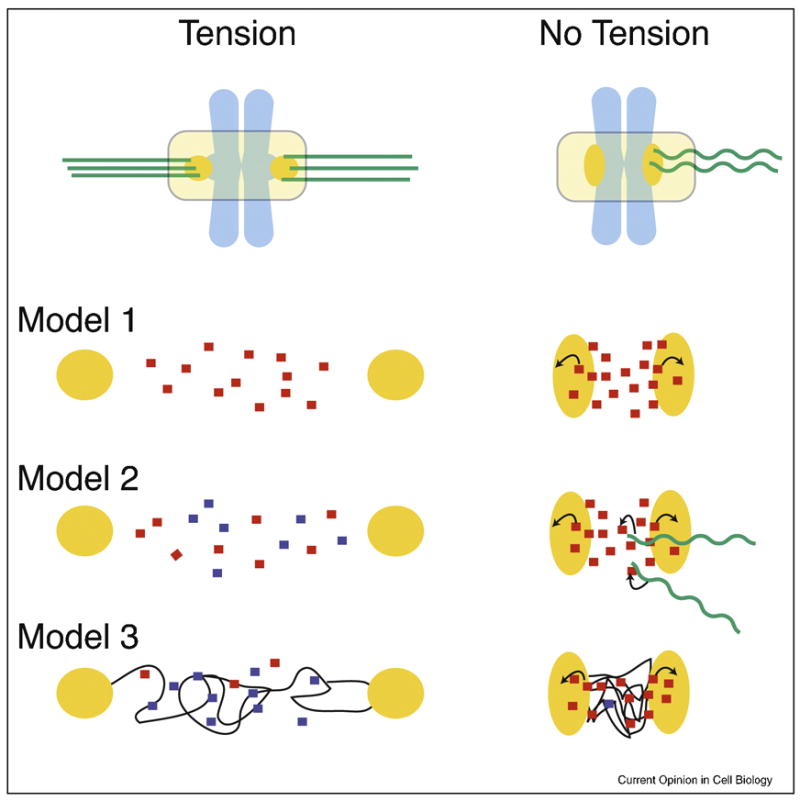
Three models of how the Aurora B pathway is controlled by microtubule attachment or tension.
Each model is a simplified enlargement of the inter-kinetochore regions of chromosomes under tension or in a relaxed state. Kinetochores are in orange, microtubules in green, and chromosomes are in light blue. Arrows indicate activation.
Model 1: Tension-regulated separation of Aurora B from its kinetochore substrates
The distance between active Aurora B at inner centromeres and its kinetochore substrates increases upon bioriented attachment. This increased distance would decrease the likelihood of Aurora B binding kinetochore substrates, which is reinforced by PP1 phosphatase.
Model 2: Microtubule-dependent regulation of Aurora B
Inner centromeric Aurora B on misaligned chromosomes is more accessible to microtubules than at the metaphase plate. Interactions with microtubules stimulate the kinase activity of Aurora B resulting destabilized microtubule attachments.
Model 3: Modulation of Aurora B activity through structural changes in centromeric chromatin
Assuming that the CPC is activated by chromatin through a clustering mechanism, the lack of tension may cause a compaction of chromatin at the centromere leading to kinase activation. However, under tension, the chromatin fibers are stretched resulting in a lower effective concentration of the CPC and thus activity. Chromatin fibers are modeled as thin black lines.
Model 1:Tension-regulated separation of Aurora B from its kinetochore substrates
It has been proposed that it is the physical distance between Aurora B and its kinetochore substrates that determines whether microtubule-kinetochore connections are maintained [8,18]. When sister chromatids are under tension, the distance between pairs of kinetochores is increased relative to a relaxed state. Aurora B remains at the inner centromere, and therefore its kinetochore substrates are no longer co-localized with the kinase (Figure 3) [18]. Under this model, this leads to a situation in which phosphorylation of key kinetochore substrates (e.g. Ndc80) is low and microtubule-kinetochore interactions are stabilized. In turn, PP1, localized to kinetochores in metaphase, dephosphorylates kinetochore substrates to maintain correctly attached microtubules [41,42]. Conversely, when there is little or no tension, Aurora B is physically closer to its substrates and phosphorylation is high, thus leading to destabilization. Reinforcement of this state might occur through the action of kinetochore kinases such as Mps1 and Chk1, which directly activate Aurora B [38**] [36*].
This model assumes that tension-dependent changes in distance between Aurora B and its substrates are enough to prevent interaction. In mammalian cells, differences of roughly 1–3 μm are seen between kinetochores under tension and those in a relaxed state [46]. As the CPC has been shown to be a highly elongated complex with maximum lengths of up to ~40–50 nm [47], it is possible that tension can physically separate Aurora B from its substrates. However, for this model to work, diffusion of the CPC must be very low. As there are conflicting reports on the dynamics of the CPC at the centromere [48–50], this point needs further investigation.
Furthermore, this model may not explain Aurora B-dependent error correction mechanisms during meiosis. In mouse spermatocytes, the CPC remains closely associated with kinetochores during metaphase I and metaphase II [51,52]. Upon progression from prometaphase II to metaphase II, the inner-centromeric fraction of the CPC diminishes while the adjacent kinetochore fraction remains [51]. In addition, this model does not readily explain why full enrichment of the CPC at inner centromeres may not be absolutely essential for the error correction process in vertebrate cells [15,53], while it was proposed that Sgo2-dependent centromeric localization of the CPC is critical for this process in fission yeast [23*]. To further validate this model, it is important to quantify the spatial distribution of Aurora B-dependent phosphorylation at centromeres and kinetochores in the absence and presence of tension. Recent developments such as the Aurora B activity FRET sensor and chromatin micro patterning will be helpful in addressing this issue [54**].
Model 2: Microtubule-dependent regulation of Aurora B
Microtubule-mediated regulation of Aurora B may play a major role in transducing the force of microtubule-kinetochore connections to Aurora B activity [55]. In budding yeast, there is recent evidence that the CPC may act as a molecular bridge between microtubules and the centromere. This suggests that the CPC is both the tension-sensor and the master-regulator of error correction [55], though it remains possible that the microtubule-binding domain may have other functions. In this model, under conditions of low tension, Aurora B activity is high leading to a weakening of microtubule attachments. Upon correct bi-orientation, tension is now applied to the CPC “bridge” which in turn causes a conformational change that leads to inhibition of the kinase.
This model is simple and direct in that there is a discrete conformational change that is transformed into a chemical signal that regulates microtubule attachment. However, it is not clear whether this mechanism is feasible in metazoans where the CPC is localized to the inner centromere and therefore may not directly interact with microtubules at the outer kinetochore. One possibility is that the forces of tension are transduced through multiple components that ultimately impinge upon the CPC and activate it in the manner detailed above. Alternatively, the simple act of binding of microtubules to the CPC could be responsible for Aurora B activation, assuming that microtubules interact with the CPC specifically at centromeres with maloriented kinetochores (Figure 3) [35]. Merotelic attachments, although still localized near the metaphase plate, have been suggested to cause an increased frequency of microtubule plus ends at the inner centromere [56].
Interestingly, removal of the domain of INCENP that is required for microtubule-mediated Aurora B activation (Boo Shan Tseng and H. F., unpublished results) does not affect its ability to correct improper attachments in HeLa cells [57*]. Rather, this mutant is defective in activating the SAC in response to unattached kinetochores [57*]. To make sense of this conflicting data, it is important to show that the CPC-microtubule interaction occurs at maloriented attachments in vivo and that this interaction plays a role in error correction. In addition, biophysical studies are needed to demonstrate whether Aurora B kinase activity can be regulated by microtubule-dependent tension.
Model 3: Modulation of Aurora B activity through structural changes in centromeric chromatin
Here we consider a third model, in which changes in the centromeric chromatin structure regulate Aurora B activity, as previously indicated [8]. Tension generated by microtubules pulling on kinetochores can provide enough force to potentially unwind nucleosomes at the centromere (discussed in [58]). Therefore, tension might affect the distribution of CPC molecules at the centromere. We propose that under low tension, chromatin is in a compact state resulting in a high effective concentration of the CPC. This may increase the likelihood that one CPC molecule phosphorylates another, which has been shown to lead to sustained activation of the kinase. When the centromere is under tension, this mechanism may be suppressed due to a decreased local concentration of the CPC and/or physical disruption of its oligomerization state. Monitoring the dynamics of CPC inter-molecular interactions under conditions of both high and low tension will aid in the validation of this model.
Alternatively, chromatin-mediated activation of Aurora B may be sensitive to the structure or the topological orientation of the DNA. The fact that topoisomerase II is required for Aurora B-mediated phosphorylation indicates that unresolved topological constraints could interfere with Aurora B activation [59**]. Strikingly, the inner centromeric ATPase PICH controls topoisomerase II-dependent decatenation of centromeric DNA during mitosis, and is required for proper chromosome alignment [60*,61**] (Lily Wang and Erich Nigg, personal communication). Thus, the topological status of centromeric DNA may be carefully regulated during mitotic progression. A reconstituted CPC-chromatin system is needed to elucidate whether changes in chromatin structure/topology can alter Aurora B kinase activity.
Conclusions
It is readily apparent that Aurora B represents a hub, in which many pathways converge to transduce the mechanical forces imposed by microtubule-kinetochore attachments into chemical signals that regulate such attachments. We have yet to understand the molecular nature by which improper attachments are removed and reestablished, but we have some valuable clues. It is important to remember that the Aurora B kinase pathway interacts with several centromeric/kinetochore kinases (Bub1, BubR1, Mps1 and Plk1) as well as phosphatases (PP2A-Sgo1, PP2A-Sgo2 and PP1) (Figure 2). The extent to which each of these effectors regulates Aurora B kinase activity and localization also depends on the nature of the microtubule-kinetochore connection. Therefore, a small perturbance to this system could result in an amplified Aurora B response due a complex network of feedback loops. A full understanding of the kinetic parameters governing each interaction in this complex network, in conjunction with computational modeling, will be key to tackling this exciting problem.
Acknowledgments
Our group is supported by National Institute of Health (R01 GM075249) and the Rockefeller University. A. E. K. is supported by a Charles H. Revson Biomedical Fellowship. We thank Adam Douglass, Todd Stukenberg, Tomo Tanaka and Linda Wordeman for stimulating discussions, Lily Wang and Erich Nigg for sharing unpublished data, and Boo Shan Tseng and Christian Zierhut for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1*.Indjeian VB, Murray AW. Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr Biol. 2007;17:1837–1846. doi: 10.1016/j.cub.2007.09.056. The authors demonstrate that budding yeast Sgo1 is not only required for the tension-sensitive SAC, but also for correction of erroneous kinetochore attachments. Since bipolar attachment is more severely affected in sgo1 mutants with unseparated spindle pole bodies (SPBs) than those with separated SPBs, the authors conclude that yeast chromosomes have an intrinsic geometrical bias to bi-orientation. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka TU. Bi-orienting chromosomes: acrobatics on the mitotic spindle. Chromosoma. 2008 doi: 10.1007/s00412-008-0173-5. [DOI] [PubMed] [Google Scholar]
- 3.Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Dewar H, Tanaka K, Nasmyth K, Tanaka TU. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature. 2004 doi: 10.1038/nature02328. [DOI] [PubMed] [Google Scholar]
- 5.King JM, Nicklas RB. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. Journal of Cell Science. 2000;113(Pt 21):3815–3823. doi: 10.1242/jcs.113.21.3815. [DOI] [PubMed] [Google Scholar]
- 6.Yamagishi Y, Sakuno T, Shimura M, Watanabe Y. Heterochromatin links to centromeric protection by recruiting shugoshin. Nature. 2008;455:251–255. doi: 10.1038/nature07217. [DOI] [PubMed] [Google Scholar]
- 7.Eckert CA, Gravdahl DJ, Megee PC. The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev. 2007;21:278–291. doi: 10.1101/gad.1498707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora Kinase-INCENP) Complex Promotes Chromosome Bi-orientation by Altering Kinetochore-Spindle Pole Connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 9.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 10.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 11**.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. The crystal structure of Survivin, N-terminal INCENP, and N-terminal Borealin reveals a three-helix bundle. The authors show that this module can recruit the CPC to the midbody during anaphase, but not to the centromere during metaphase, suggesting that the C-terminus of Borealin contributes to centromere targeting. [DOI] [PubMed] [Google Scholar]
- 12.Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci. 2004;117:4033–4042. doi: 10.1242/jcs.01242. [DOI] [PubMed] [Google Scholar]
- 13.Vigneron S, Prieto S, Bernis C, Labbe JC, Castro A, Lorca T. Kinetochore Localization of Spindle Checkpoint Proteins: Who Controls Whom? Mol Biol Cell. 2004;15:4584–4596. doi: 10.1091/mbc.E04-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. Embo J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CS, Stukenberg PT. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B Phosphorylates Centromeric MCAK and Regulates Its Localization and Microtubule Depolymerization Activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 18.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 19.Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouwels J, Kukkonen AM, Lan W, Daum JR, Gorbsky GJ, Stukenberg T, Kallio MJ. Shugoshin 1 plays a central role in kinetochore assembly and is required for kinetochore targeting of Plk1. Cell Cycle. 2007;6:1579–1585. doi: 10.4161/cc.6.13.4442. [DOI] [PubMed] [Google Scholar]
- 21.Goto H, Kiyono T, Tomono Y, Kawajiri A, Urano T, Furukawa K, Nigg EA, Inagaki M. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nat Cell Biol. 2006;8:180–187. doi: 10.1038/ncb1350. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Feng J, Famulski J, Rattner JB, Liu ST, Kao GD, Muschel R, Chan GK, Yen TJ. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J Cell Biol. 2007;177:413–424. doi: 10.1083/jcb.200701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21:420–435. doi: 10.1101/gad.1497307. This paper shows that fission yeast Sgo2 interacts with Survivin and recruits the CPC to pericentromeres. By artificially targeting the CPC to centromeres, the authors demonstrate that the Sgo2-mediated CPC localization of the centromere is important for correct microtubule attachment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 27.Zeitlin SG, Barber CM, Allis CD, Sullivan KF. Differential regulation of CENP-A and histone H3 phosphorylation in G2/M. J Cell Sci. 2001;114:653–661. doi: 10.1242/jcs.114.4.653. [DOI] [PubMed] [Google Scholar]
- 28.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 29.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 30.Gestaut DR, Graczyk B, Cooper J, Widlund PO, Zelter A, Wordeman L, Asbury CL, Davis TN. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat Cell Biol. 2008;10:407–414. doi: 10.1038/ncb1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wordeman L, Wagenbach M, von Dassow G. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. J Cell Biol. 2007;179:869–879. doi: 10.1083/jcb.200707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 33*.Kelly AE, Sampath SC, Maniar TA, Woo EM, Chait BT, Funabiki H. Chromosomal enrichment and activation of the aurora B pathway are coupled to spatially regulate spindle assembly. Dev Cell. 2007;12:31–43. doi: 10.1016/j.devcel.2006.11.001. This paper demonstrates that the Aurora B pathway is suppressed in the cytoplasm of metaphase Xenopus egg extracts by phosphatase activity, but that chromatin, stabilized microtubules, centrosomes and forced clustering of the CPC can stimulate kinase activation. The authors propose a model detailing how the Aurora B pathway mediates spindle assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadea BB, Ruderman JV. Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc Natl Acad Sci U S A. 2006;103:4493–4498. doi: 10.1073/pnas.0600702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. Dephosphorylated CPC cannot be activated efficiently unless microtubules and TD-60 are present. The results suggest that Aurora B substrates can inhibit Aurora B activity, and that priming by centromeric kinases (Haspin or Plk1) could alleviate this, revealing a possible mechanism for regulation of Aurora B at the centromere. [DOI] [PubMed] [Google Scholar]
- 36*.Zachos G, Black EJ, Walker M, Scott MT, Vagnarelli P, Earnshaw WC, Gillespie DA. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. Using a conditional knockdown of Chk1 in chicken DT40 cells, the authors convincingly demonstrate that Chk1, which plays an important role in the DNA-damage checkpoint, can directly activate Aurora B, is required for Aurora B-mediated phosphorylation of Cenp-A and proper chromosome segregation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Maure JF, Kitamura E, Tanaka TU. Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr Biol. 2007;17:2175–2182. doi: 10.1016/j.cub.2007.11.032. This paper clearly demonstrates that Mps1 is required for error correction process of microtubule attachment in budding yeast. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Jelluma N, Brenkman AB, van den Broek NJ, Cruijsen CW, van Osch MH, Lens SM, Medema RH, Kops GJ. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–246. doi: 10.1016/j.cell.2007.11.046. By RNAi-mediated knockdown of Mps1 in HeLa cells, the authors show that Mps1 is required for error correction process of microtubule attachment. Mps1 can phosphorylate Borealin and stimulate Aurora B activity in the CPC. Strikingly, a phospho-mimetic mutant of Borealin can rescue the chromosome misalignment but not spindle checkpoint defect of Mps1 knockdown. [DOI] [PubMed] [Google Scholar]
- 39.Francisco L, Wang W, Chan CS. Type 1 protein phosphatase acts in opposition to IpL1 protein kinase in regulating yeast chromosome segregation. Mol Cell Biol. 1994;14:4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 41.Trinkle-Mulcahy L, Andersen J, Lam YW, Moorhead G, Mann M, Lamond AI. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J Cell Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinkle-Mulcahy L, Andrews PD, Wickramasinghe S, Sleeman J, Prescott A, Lam YW, Lyon C, Swedlow JR, Lamond AI. Time-lapse imaging reveals dynamic relocalization of PP1gamma throughout the mammalian cell cycle. Mol Biol Cell. 2003;14:107–117. doi: 10.1091/mbc.E02-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logarinho E, Resende T, Torres C, Bousbaa H. The human spindle assembly checkpoint protein bub3 is required for the establishment of efficient kinetochore-microtubule attachments. Mol Biol Cell. 2008;19:1798–1813. doi: 10.1091/mbc.E07-07-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lampson MA, Kapoor TM. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat Cell Biol. 2005;7:93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- 45.Morrow CJ, Tighe A, Johnson VL, Scott MI, Ditchfield C, Taylor SS. Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J Cell Sci. 2005;118:3639–3652. doi: 10.1242/jcs.02487. [DOI] [PubMed] [Google Scholar]
- 46.Waters JC, Chen RH, Murray AW, Salmon ED. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. Journal of Cell Biology. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell. 2002;13:3064–3077. doi: 10.1091/mbc.E02-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murata-Hori M, Wang YL. Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J Cell Biol. 2002;159:45–53. doi: 10.1083/jcb.200207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delacour-Larose M, Molla A, Skoufias DA, Margolis RL, Dimitrov S. Distinct dynamics of Aurora B and Survivin during mitosis. Cell Cycle. 2004;3:1418–1426. doi: 10.4161/cc.3.11.1203. [DOI] [PubMed] [Google Scholar]
- 50.Delacour-Larose M, Thi MN, Dimitrov S, Molla A. Role of survivin phosphorylation by aurora B in mitosis. Cell Cycle. 2007;6:1878–1885. doi: 10.4161/cc.6.15.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parra MT, Gomez R, Viera A, Page J, Calvente A, Wordeman L, Rufas JS, Suja JA. A perikinetochoric ring defined by MCAK and Aurora-B as a novel centromere domain. PLoS Genet. 2006;2:e84. doi: 10.1371/journal.pgen.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parra MT, Viera A, Gomez R, Page J, Carmena M, Earnshaw WC, Rufas JS, Suja JA. Dynamic relocalization of the chromosomal passenger complex proteins inner centromere protein (INCENP) and aurora-B kinase during male mouse meiosis. J Cell Sci. 2003;116:961–974. doi: 10.1242/jcs.00330. [DOI] [PubMed] [Google Scholar]
- 53.Yue Z, Carvalho A, Xu Z, Yuan X, Cardinale S, Ribeiro S, Lai F, Ogawa H, Gudmundsdottir E, Gassmann R, et al. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J Cell Biol. 2008;183:279–296. doi: 10.1083/jcb.200806118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. The authors develop an Aurora B-phosphorylation sensitive FRET sensor, which reveals that Aurora B-mediated phosphorylation remains higher on chromosomes near the anaphase spindle midzone than on those near spindle poles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandall S, Severin F, McLeod IX, Yates JR, 3rd, Oegema K, Hyman A, Desai A. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell. 2006;127:1179–1191. doi: 10.1016/j.cell.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cimini D. Detection and correction of merotelic kinetochore orientation by Aurora B and its partners. Cell Cycle. 2007;6:1558–1564. doi: 10.4161/cc.6.13.4452. [DOI] [PubMed] [Google Scholar]
- 57*.Vader G, Cruijsen CW, van Harn T, Vromans MJ, Medema RH, Lens SM. The chromosomal passenger complex controls spindle checkpoint function independent from its role in correcting microtubule kinetochore interactions. Mol Biol Cell. 2007;18:4553–4564. doi: 10.1091/mbc.E07-04-0328. The authors demonstrate in human tissue culture cells that deletion of the putative coiled-coil domain of INCENP leads to inactivation of SAC function of the CPC without affecting its role in error correction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bloom KS. Beyond the code: the mechanical properties of DNA as they relate to mitosis. Chromosoma. 2008;117:103–110. doi: 10.1007/s00412-007-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Coelho PA, Queiroz-Machado J, Carmo AM, Moutinho-Pereira S, Maiato H, Sunkel CE. Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol. 2008;6:e207. doi: 10.1371/journal.pbio.0060207. This paper demonstrates that RNAi-mediated depletion or ICRF-187-mediated inhibition of topo II in Drosophila S2 cells leads to the accumulation of syntelic attachments and loss of Aurora B-dependent histone H3 phosphorylation. Treatment of HeLa cells with ICRF-187 also causes syntelic attachments and decreases Aurora B-dependent Cenp-A phosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Wang LH, Schwarzbraun T, Speicher MR, Nigg EA. Persistence of DNA threads in human anaphase cells suggests late completion of sister chromatid decatenation. Chromosoma. 2008;117:123–135. doi: 10.1007/s00412-007-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Baumann C, Korner R, Hofmann K, Nigg EA. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. doi: 10.1016/j.cell.2006.11.041. These two papers show that centromeric alphoid DNA remains connected in a thread-like structure between chromatids during anaphase. These threads are coated with a novel centromeric ATPase, PICH, and must be decatenated by topo II for completion of sister chromatid separation. PICH is also required for metaphase chromosome alignment and SAC activation. [DOI] [PubMed] [Google Scholar]
- 62.Elowe S, Hummer S, Uldschmid A, Li X, Nigg EA. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 2007;21:2205–2219. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mao Y, Desai A, Cleveland DW. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J Cell Biol. 2005;170:873–880. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]