Abstract
Natural antibodies are produced by B lymphocytes in the absence of external antigen stimulation. With their ability to recognize self, altered self and foreign antigens, they comprise an important first-line defence against invading pathogens, but are also important for tissue homeostasis. By recognizing oligosaccharides expressed on tumour cells and modified cell surface structures accompanying necrosis, natural antibodies have an important anti-tumorigenic function. IVIg contains a wide spectrum of specificities presented in normal plasma including natural antibodies and has been shown to exert inhibitory effects on tumour cells through a subfraction of anti-vascular endothelial growth factor immunoglobulin (Ig)G antibodies with anti-angiogenic properties. IgA antibodies also have potent immunomodulatory properties, being able to both induce and suppress immune responses. IgA-mediated inhibitory function is able to inhibit several inflammatory diseases including asthma and glomerulonephritis. Autoantibodies of the IgM type, on the other hand, have shown promising results in the treatment of multiple sclerosis. These autoantibodies promote remyelination rather than modulating inflammation. Oxidation-specific epitopes, as found in atherosclerotic lesions and on apoptotic cells, comprise one important target of natural antibodies. By recognizing these epitopes, natural antibodies neutralize proinflammatory responses and mediate atheroprotection.
Keywords: atherosclerosis, cancer, IgA, multiple sclerosis, oxidation-specific epitopes
Introduction
Natural antibodies are defined as those immunoglobulins, preferentially of the IgM isotype, which are produced by B lymphoyctes of the B1 type in the absence of external antigen stimulation. Natural antibodies are characterized by using germline-encoded genes in the variable region (VH and VL) and thus have a stable, restricted but none the less broad repertoire and reactivity pattern. Natural antibodies producing B lymphocytes are found in the marginal zones of the spleen but not in germinal centres of secondary lymphatic follicles, and consequently these natural antibodies do not undergo affinity maturation [1]. Natural antibodies recognize self, altered self and foreign antigens consisting of phospholipids (e.g. phosphatidylcholine), carbohydrate sequences (e.g. gangliosides), single-stranded DNA and peptides (e.g. amyloid beta peptide) or surface glycoproteins (e.g. CD90). They are an important component of the first-line defence against invading pathogens, but also have a homeostatic role in regulating clearance of intracellular molecules or modified cell surface structures of necrotic or apoptotic cells [2]. Their function in waste removal may be beneficial for preventing both autoimmune and inflammatory reactions. T cell major histocompatibility complex (MHC)-mediated help in regulation of B lymphocyte activation apparently does not occur with B1 cells producing natural antibodies; however, it has been observed that interleukin (IL)-4 released by CD1 restricted natural killer T (NK T) cells can stimulate the production of natural antibodies [3]. The majority of natural antibodies consist of IgM with a smaller proportion of IgG and IgA [4]. Therefore, a certain percentage of immunoglobulins of intravenous immunoglobulin (IVIg) preparations has to be regarded as natural antibody.
This session, chaired by Dr Yehuda Shoenfeld and Dr Reinhard Schwartz-Albiez, opened with a presentation by Dr Shoenfeld on the use of natural antibodies in the clinical fields of autoimmunity and cancer followed by Dr Renato Monteiro's presentation on the immunological role of IgA. Dr Moses Rodriguez presented his data on the role of natural IgM in multiple sclerosis (MS) and finally Dr Christoph Binder reported on oxidation-specific epitopes as targets for natural antibodies.
Thus this session concentrated on four pivotal aspects of natural antibodies and their possible therapeutic application, as follows.
Natural antibodies recognize defined cell surface structures, predominantly oligosaccharides, which are expressed in larger quantities in a tissue-specific manner during fetal differentiation but are also expressed on tumour cells (oncofetal antigens). These tumour-reactive natural antibodies can be found in a small percentage of healthy blood donors [5]. In some reports an enhanced proportion of these natural antibodies were also detected in the peripheral blood of tumour patients [6]. Natural antibodies may not only have a direct cytotoxic effect on intact tumour cells, but also a bystander effect during a humoral anti-tumour response. Natural antibodies recognize modified cell surface structures occurring during necrotic processes which accompany tumourigenesis. As components involved in anti-inflammatory reactions, natural antibodies also help to attract other immune cells such as neutrophils to sites of inflammation.
Natural antibodies have a regulatory role in inflammatory reactions. In this way it is noteworthy that a certain percentage of IgA is produced as natural antibodies in the neonatal phase [4]. Secondly, immunoglobulins of the IgA isotype have an inhibitory function towards inflammatory reactions mediated by their interaction with the type 1 Fc receptor (FcαR1/CD89) [7,8]. Interestingly NK T cell activation stimulates IL-4-dependent mucosal IgA production as outlined above [3]. It is still an open question as to what extent natural IgA are involved in these anti-inflammatory processes.
Natural antibodies present in healthy individuals recognize antigens of oligodendrocytes and epitopes expressed on axons or neuritis, and may be of therapeutic use in treatment modalities to counteract tissue destruction during MS [9].
One of the major tasks of IgM natural antibodies is to prevent inflammatory processes. It is now well documented that natural IgM antibodies recognize oxidation specific epitopes occurring during, e.g. chronic inflammations [10].
Harnessing the innate immunity to modulate autoimmunity and cancer
During the last 2 decades, IVIg has shown potent immunomodulatory and anti-inflammatory effects in many diseases, from immunodeficiencies, autoimmune diseases and infections to cancer. IVIg is a safe preparation of purified immunoglobulins (>95% IgG), precipitated from the plasma collected from thousands of healthy donors. Therefore, IVIg contains a wide spectrum of specificities present in normal plasma. The specificities include anti-idiotypic antibodies to anti-DNA, anti-β2glycoprotein-I, Arg-Gly-Asp (RGD) motif, anti-Fas, anti-acetylcholine receptor, B cell-activating factor of the tumour necrosis factor (TNF) family (BAFF) and antibodies to variable domain epitopes of the alpha/beta T cell receptor (TCR), human leucocyte antigen (HLA), Fcγ receptor, cytokines, metalloproteinase-9 (MMP9) and others [11,12].
Previous studies have shown that IVIg exhibits an inhibitory effect on different cancer cells (melanoma, colon cancer, breast cancer, sarcoma) with regard to growth and tumour spread in vitro, and tumour spread in animal models [13–15]. Thus, application of IVIg has the potential to be a supportive therapy for treatment of cancer metastases. The anti-tumour activity of IVIg has been explained by its ability to arrest G1 as a result of p21/WAF-1 up-regulation, elevation of p53 expression and anti-BAFF and anti-MMP9 activity [11,16,17].
In order to understand more clearly this activity of IVIg, its effect on vascular endothelial growth factor (VEGF) activity was studied in vitro and in an animal model.
Direct binding of IVIg to VEGF was tested by enzyme-linked immunosorbent assay (ELISA), followed by IVIg anti-VEGF inhibition assays employing anti-human-VEGF monoclonal antibodies as competitors. All these experiments were confirmed by Western blot analyses. The anti-angiogenic activity of IVIg was analysed in a mouse VEGF-mediated ischaemic hind-limb model. Unilateral hind-limb ischaemia was induced by femoral ligation. VEGF was injected intramuscularly to achieve the blood perfusion in the ischaemic limb. Forty-eight hours later, a group of the VEGF-injected mice were treated with 20 mg/mouse (1 g/kg) of IVIg. Hind-limb blood flow was recorded by using laser Doppler perfusion imaging.
IVIg was found to comprise anti-angiogenic activity in vitro and in vivo. IVIg bound VEGF by ELISA in a dose-dependent manner and the half maximal effective concentration (EC50) value was found to be 2·35 mg/ml. The data were confirmed by immunoblot analysis. Moreover, commercial monoclonal anti-human VEGF prevented the binding of IVIg to VEGF by 95%. On the other hand, at a concentration of 5 mg/ml IVIg inhibited the binding of anti-human VEGF monoclonal antibody (mAb) by 37%. These inhibitory binding properties of IVIg and VEGF were confirmed by immunoblot. In parallel, the anti-angiogenic activity of IVIg was tested in the VEGF-mediated ischaemic hind-limb blood flow model, where preliminary results showed that IVIg administration resulted in inhibition of blood perfusion in the VEGF-injected mice (Fig. 1).
Fig. 1.
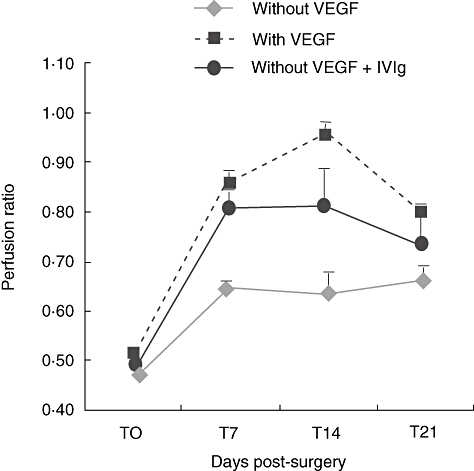
Intravenous immunoglobulin (IVIg) inhibits vascular endothelial growth factor (VEGF)-induced blood perfusion. Blood perfusion in the mouse hind-limb ischaemia model is induced after VEGF application. This effect is inhibited by co-administration of IVIg, demonstrating IVIg's anti-angiogenic activity.
IVIg preparations contain a subfraction of anti-VEGF IgG, which has a biological anti-angiogenic activity. The results demonstrate the anti-metastatic potential of IVIg as an anti-VEGF compound and suggest that IVIg may be used as a supportive therapy in cancer.
Role of IgA
IgA is the most heterogeneous immunoglobulin (Ig) in the body, as it occurs in multiple molecular forms and two subclasses (IgA1 and IgA2), which are distributed differentially between the systemic and mucosal immune systems. Serum IgA, the second most abundant isotype, consists mainly of monomers of the IgA1 subclass, whereas secretory IgA (SIgA) occurs locally as dimers before being transported to mucosal surfaces through epithelial cells by the polymeric Ig receptor.
Pathogens with mucosal tropism induce a local protective SIgA immune response. Serum IgA may also have anti-inflammatory potential. IgA is not usually involved in humoral immune responses and it activates complement poorly. In the absence of antigen, IgA down-regulates IgG-mediated phagocytosis, chemotaxis, bactericidal activity, oxidative burst activity and cytokine release.
Supporting evidence for the regulatory role of IgA comes from patients with selective IgA deficiency. In these patients, both IgA1 and IgA2 are usually reduced markedly or absent, and they may have normal or reduced IgG and IgM levels [18]. In addition to increased infections of the respiratory and gastrointestinal tract, these patients show increased susceptibility to autoimmune and allergic disorders including arthritis, autoimmune endocrinopathies, chronic active hepatitis, ulcerative colitis, Crohn's disease and autoimmune haematological disorders [18].
The molecular basis underlying IgA inhibitory functions is dependent upon the FcαRI/CD89. FcαRI is the only IgA Fc receptor expressed on myeloid cells [7]. It can bind monomeric IgA1 and IgA2 with low affinity (Ka∼106 M-1), and is considered a unique member of the FcR family. The FcαRI gene is not located in the FcR gene cluster, but rather on chromosome 19, inside the leucocyte receptor cluster (LRC). FcαRI is related distantly to other FcRs, being more homologous to LRC-encoded activating and inhibitory receptors.
The anti-inflammatory effects of serum IgA are mediated by FcαRI involving an inhibitory immunoreceptor tyrosine-based activation (ITAM) motif function of the associated FcRγ subunit [8,19] (Fig. 2). Whether the activating or inhibiting function of FcαRI is induced is thought to depend upon whether or not the receptor is engaged by a multimeric or monomeric ligand [20]. Monovalent targeting of FcαRI inhibits markedly IgG-dependent phagocytosis and IgE-induced exocytosis [18]. Upon engagement of FcαRI by a monovalent ligand, such as serum monomeric IgA, the FcRγ ITAM motif is phosphorylated only partially, resulting in the extracellular signal regulated kinase (ERK)-dependent recruitment of the inhibitory phosphatase Src homology phosphatase-1 (SHP-1) and impairment of signalling induced by heterologous receptors.
Fig. 2.
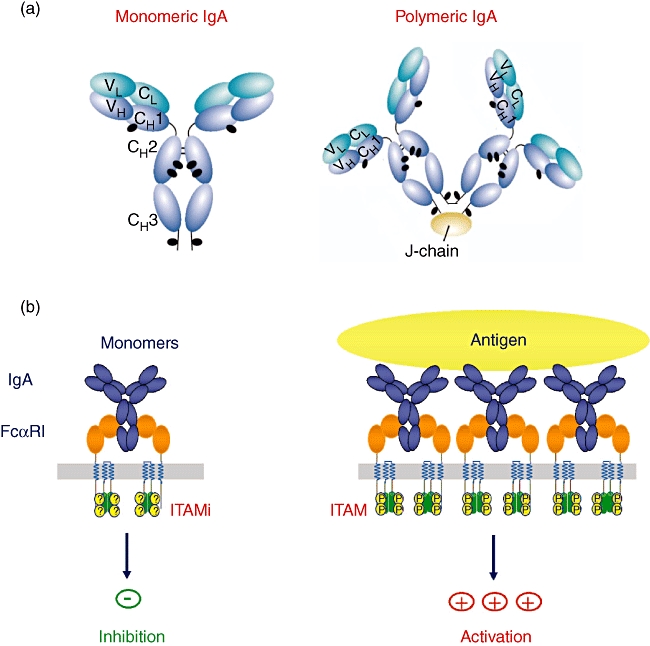
Monovalent or polyvalent binding of immunoglobulin (Ig)A results in either induction of the inhibitory or activating function of the IgA receptor, FcαRI. Monovalent targeting of FcαRI markedly inhibits IgG-dependent phagocytosis and IgE-induced exocytosis. Upon engagement of FcαRI by a monovalent ligand, such as serum monomeric IgA, the FcRγ immunoreceptor tyrosine-based activation (ITAM) motif is phosphorylated only partially, resulting in impairment of signalling induced by heterologous receptors.
In summary, serum monomeric IgA has powerful immunomodulatory roles, being able to both induce and suppress immune responses. IgA-mediated inhibitory function is able to inhibit several inflammatory diseases, including asthma and glomerulonephritis [8,21]. Early studies show a promising role of IgA in immunotherapy and further studies are awaited eagerly.
Role of IgM in MS: the paradox of using natural autoantibodies to treat a presumptive autoimmune disease
Current MS treatments assume a major role for the immune system in the destruction of oligodendrocytes and axons. Some treatments have had a partial response. However, many patients suffer a progressive course and severe, permanent disability. Alternatively, those cells in the central nervous system (CNS) that play a critical role in the disease, such as oligodendrocytes, neurones and axons, may be targeted. Specific therapies are being designed which either activate oligodendrocytes to produce new myelin or activate neurones to extend their processes. It has been discovered that natural autoantibodies present in the serum of healthy individuals contain immunoglobulins directed against surface molecules on oligodendrocytes [10,22]. One human monoclonal antibody, HIgM22, derived from the repertoire of a natural antibody, is directed seemingly against integrins on the cell surface of oligodendrocytes. HIgM22 binds to lipid rafts on oligodendrocytes and induces a calcium influx mediated through an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA)-mediated channel, which results in the phosphorylation of specific molecules to induce remyelination.
A second set of antibodies, HIgM12 and HIgM42, are directed against axons or neurites and appear to be directed against sialic acid-containing compounds on gangliosides. These human natural autoantibodies induce neurone outgrowth similar to that observed in neurones grown on laminin substrate. Experiments have demonstrated that the remyelinating and the neurite-binding antibodies enter the CNS of animals infected chronically with Theiler's virus and result in reversal of persistent neurological deficits.
Based on these observations, mouse and human mAbs that would enhance remyelination were generated. The goal was to generate a mAb that would result in a physiological function, rather than search for a mAb directed against a specific antigen or protein. To test the extent of remyelination, animals were infected with Theiler's virus. Severe, extensive demyelination developed after 6–9 months. The animals were treated intraperitoneally with mAbs for 5 weeks and then spinal cord remyelination was evaluated which, when it occurred, was almost complete within 5 weeks [23].
Eight different mouse mAbs that promoted remyelination were identified [24]. All remyelination-promoting mAbs reacted to surface antigens (lipids and proteins) on oligodendrocytes, the myelin-producing cells [25]. The mouse mAbs were found to have relatively conserved germline DNA sequences, implying they were natural autoantibodies. Therefore we surmised that similar natural autoantibodies directed against oligodendrocytes would be present in the normal human population.
To identify natural autoantibodies present in the human population, we sought patients with disease processes that caused them to make their own mAbs, specifically patients with multiple myeloma, Waldenstrom's syndrome and monoclonal gammopathy of unknown significance. Two mAbs (sHIgM22 and sHIgM46) demonstrated extensive remyelination [26] in Theiler's virus and in lysolecithin-induced demyelination [27] (Fig. 3).
Fig. 3.
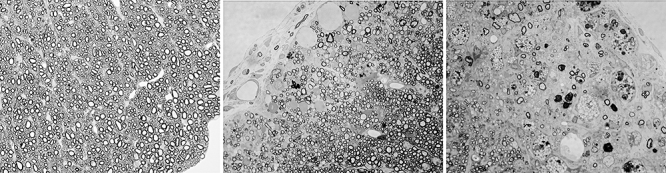
rHIgM22 promotes remyelination in mice infected with Theiler's virus. (a) Normal myelin in a mouse not infected with Theiler's virus. (b) Remyelination by oligodendrocytes characterized by thinner myelin sheath compared to sheaths in (a). This mouse was infected with Theiler's virus and 6 months later treated with a single injection of rHIgM22. The section was taken 5 weeks following treatment. (c) Extensive demyelination without remyelination in a mouse treated with phosphate-buffered saline (PBS). Similar absence of remyelination is observed in mice treated with isotype control human immunoglobulin (Ig)M antibody. All sections are from the lateral spinal cord and were perfused with Trump's fixative. Spinal cord blocks were osmicated and embedded in Araldite plastic. Sections, each 1 micron thick, were stained with paraphenylenediamine (PPD) to darken the myelin (magnification 600×).
Autoradiography and magnetic resonance imaging (MRI) studies showed antibodies crossing the blood–brain barrier (BBB) in animals infected with Theiler's virus or experimental autoimmune encephalopathy (EAE) [28] and entering the CNS. In contrast, the mAb did not accumulate in the CNS of uninfected animals or animals without demyelination [29]. Thus, IgM mAbs cross the BBB and, if directed to a CNS antigen, find their target (in this case, oligodendrocytes and myelin).
Animals infected with Theiler's virus were given a single dose of human mAb. After 5 weeks, animals treated with rHIgM22 showed a remarkable decrease in lesion volume load in the spinal cord [29], as detected by MRI after just a single dose of human mAb.
Two mAbs, sHIgM12 and sHIgM42, that bound to the surface of neurones and enhanced neuronal outgrowth and neurite extensions, were discovered inadvertently [30]. The level of neurite extension was the same as that of neurones grown on laminin substrate. Mice infected with Theiler's virus, showing extensive axonal degeneration and loss during the chronic phase of the disease, were treated [31]. Neurological function improved at 2 weeks post-treatment and persisted until the experiment was terminated 8 weeks later. The mechanism of action of this class of mAbs appears to be very similar, irrespective of whether the mAb promotes remyelination or neurite outgrowth.
In contrast to current therapies for MS aimed at modulating inflammation, remyelination-promoting IgMs are the first potential therapeutic molecules designed to induce tissue repair by acting within the CNS at sites of damage on the cells responsible for myelin synthesis, with the aim of initiating a long-term reparative effect on the central nervous system.
Oxidation-specific epitopes are a dominant target of natural IgM antibodies
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of oxidized lipoproteins and apoptotic cells, both of which contain various oxidation-specific neoepitopes. The atherosclerotic disease process is modulated strongly by both innate and adaptive immunity, and accumulating evidence suggests that oxidation-specific epitopes are recognized by different innate receptors, such as scavenger receptors on macrophages, plasma proteins, such as C-reactive protein (CRP) and natural antibodies, which are selected by evolution and constitute the humoral arc of innate immunity [32,33].
IgM antibodies dominate the humoral response to epitopes of oxidized low-density lipoproteins (OxLDL) in mice, and mouse models of atherosclerosis, such as cholesterol-fed Ldlr–/– mice and apoE–/– mice, display high titres of these IgM antibodies. This enabled the cloning of IgM secreting hybridomas from the spleens of cholesterol-fed apoE–/– mice with specificity for various epitopes of OxLDL [34]. One prototypic anti-OxLDL clone, termed EO6, was found to bind specifically to the phosphocholine (PC) headgroup of oxidized phospholipids (OxPL), such as 1-palmitoyl-2-(5′-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), but not to the PC of native PL [35]. In addition, EO6 was also found to bind to the surface of apoptotic cells and an increased content of OxPL was documented in the membranes of apoptotic cells by mass spectroscopy [36,37]. If not cleared promptly, such apoptotic cells are likely to be proinflammatory [37,38]. A detailed analysis of the sequence encoding its CDR regions revealed EO6 to be genetically identical to a well-characterized B-1 cell clone, T15 [39]. T15 natural antibody binds to PC linked covalently to the cell wall polysaccharide (C-PS) of pathogens and provides optimal protection to mice from lethal infection with Streptococcus pneumoniae[40]. Indeed, immunization of cholesterol-fed Ldlr–/– mice with heat-killed S. pneumoniae led to a near monoclonal expansion of EO6/T15 natural antibodies and conferred atheroprotection [41]. Thus, the same germline natural antibody is able to provide first-line defence against microbial infections as well as housekeeping functions to protect from atherosclerosis. These data suggest strongly that the PC moiety of OxPL, apoptotic cells and the cell wall of bacteria constitute a pathogen-associated molecular pattern (PAMP) recognized by EO6/T15 and that each could exert positive selective pressure.
A variety of such oxidation-specific epitopes, besides PC of OxPL, are likely to occur in abundance not only on apoptotic cells, but on shed microparticles, and in general on membranes and even bacteria during inflammatory responses. They might constitute a previously unrecognized but important class of PAMPs, and in turn would be a major target of innate natural antibodies (see Fig. 4). Therefore, it was hypothesized that T15/EO6 is a representative of many germline-encoded natural antibodies with specificities for oxidation-specific epitopes.
Fig. 4.
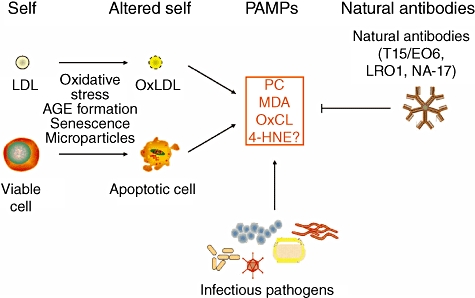
Oxidation-specific epitopes present an oxidized LDL and apoptopic cells represent a novel class of pathogen associated molecular pattern (PAMP) and equivalent epitopes can be present on microbes. These oxidation-specific epitopes may exert selective pressure to expand the pool of natural antibodies, which in turn play an important role in mediating homeostatic functions consequent to inflammation and cell death. Figure modified from [33].
Multiple lines of evidence have been provided that oxidation-specific epitopes constitute a dominant, previously unrecognized target of natural antibodies in both mice and humans [42]. Even naive wild-type C57BL/6 mice held under specific pathogen-free conditions have significant plasma titres of IgM antibodies against various oxidation-specific epitopes, such as PC of oxidized phospholipids, malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), suggesting that natural antibodies against different oxidation-specific epitopes exist. Consistently, equally high IgM titres against these specificities were found in germ-free mice, and some of these titres increased following reconstitution with gut bacteria. To demonstrate directly that B-1 cells, which are considered the major source of natural antibodies in mice, secrete IgM antibodies against oxidation-specific epitopes, B-1 cells were isolated from naive mice and stimulated in vitro with different stimuli. Toll-like receptor-2 (TLR-2) as well as TLR-4 agonists and IL-5 stimulated strongly the production of IgM antibodies against OxLDL, MDA-LDL and 4-HNE-LDL, with MDA-specific IgM being most dominant (∼30% of total IgM). In general, the levels of IgM with specificity for oxidation-specific epitopes were much higher than those against the prototypic B-1 cell antigen α1,3-dextran. These findings were confirmed in reconstituted mice solely expressing IgM natural antibodies, in which Rag–/– mice that lack T and B cells were reconstituted selectively with purified B-1 cells from wild-type mice. Ten weeks following the adoptive transfer, reconstituted mice developed high IgM titres to oxidation-specific epitopes with MDA-specific IgM again displaying the most prominent titres. Remarkably, detailed analyses of the recipient plasma by absorption studies revealed that more than 30% of all IgM in the plasma of reconstituted mice had specificity for various oxidation-specific epitopes. Moreover, IgM-secreting cells (ISCs) were detectable in the spleens of reconstituted mice, and MDA-specific ISCs accounted for ∼12% of all ISCs, which was found to be comparable to the frequencies of MDA-specific ISCs in naive wild-type mice. Moreover, the existence of natural IgM antibodies with such specificities was confirmed when a monoclonal IgM (NA-17) with specificity for MDA-LDL from the spleens of B-1 cell reconstituted mice was cloned. This mAb displayed complete germline gene usage of the VH rearrangement and only one nucleotide insertion (C) at the splice site of the VL and joining (JL) germline gene segments. Thus, oxidation-specific epitopes are dominant targets of natural IgM antibodies in mice. To demonstrate whether the natural antibody repertoire in humans displays similar specificities, IgM antibodies in human umbilical cord blood, which are exclusively from the infant and represent the human equivalent of naive natural antibodies, were evaluated. Umbilical cord blood IgM contained prominent titres against MDA-LDL and OxLDL, but not against native LDL or keyhole limpet haemocyanin (KLH). In addition, when compared with the IgM titres found in matched maternal plasma samples, an enrichment of oxidation-specific IgM in umbilical cord blood could be observed. Therefore, oxidation epitope-specific natural antibodies also appear to be dominant in humans.
Oxidation-specific epitopes are found in numerous inflammatory settings and are present in OxLDL and on apoptotic cells. Because oxidative processes are ubiquitous, it can be hypothesized that these epitopes exert selective pressure to expand natural antibodies, which in turn play an important role in mediating homeostatic functions consequent to inflammation and cell death. For example, the prototypic anti-OxLDL natural IgM EO6 recognizes epitopes on apoptotic cells and in atherosclerotic lesions, neutralizes proinflammatory responses of endothelial cells and macrophages induced by oxidized phospholipids [38,43] and mediates atheroprotection [41,44]. In analogy, the entire set of B-1 cell-derived natural antibodies described above was also found to recognize epitopes in atherosclerotic lesions and on apoptotic cells and circulating microparticles, which have been implicated in coronary artery disease. Importantly, the MDA-specific natural IgM NA-17 also stained atherosclerotic lesions and bound to apoptotic cells. Finally, B-1 cell-derived IgM as a whole and NA-17 specifically, but not a KLH-specific monoclonal IgM, increased clearance of apoptotic cells in vivo significantly.
The findings regarding the importance of oxidation-specific epitopes as targets of natural antibodies provide novel insights into the functions of natural antibodies in mediating host homeostasis, and into their roles in health and diseases, such as chronic inflammatory diseases and atherosclerosis.
Conclusions
It becomes increasingly evident that natural antibody can no longer be regarded as negligible bystander elements of humoral immunity but rather as important first-line defenders against pathogens and as decisive regulatory elements in acute or chronic inflammation. Many aspects with regard to regulation of natural antibody production and details of their regulatory function need to be studied in more detail. In particular, provided that natural antibody may have overlapping reactivities against pathogens and self-antigens, the question arises as to how harmful autoimmune processes are prevented by the immune system. Do natural antibodies have a role in tumour surveillance, given the fact that we find anti-tumour cytotoxic natural antibodies in healthy people? This demonstrates clearly that research on natural antibodies is a challenging field of modern immunology. For a long time IVIg treatment was considered to be evidence-based medicine. As exemplified here, we are now already at the verge of exploiting our current knowledge to design new therapeutic applications of natural antibodies.
Disclosures
MR states that this work was supported by grants from the National Institutes of Health (R01 NS 24180, R01 NS 32129, P01 NS 38468, R01 CA104996, R01 CA096859), the National Multiple Sclerosis Society (RG 317 2-B-8, CA 1011 A8-3), the Multiple Sclerosis Society of Canada, the Applebaum Foundation, the Hilton Foundation, the Peterson Foundation and Acorda Therapeutics, Inc. (Hawthorne, NY). A non-provisional patent application has been filed under the title ‘Human Monoclonal Antibodies for CNS Remyelination,’ Mayo Clinic case #2003-03, and the technology has been licensed to a third party. No royalties have accrued to the authors or Mayo Clinic to date.
YS is a consultant for another IVIg company. All other authors have declared that they have no conflicts of interest.
References
- 1.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and diseases. Trends Immunol. 2009;30:43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Simon HU, Späth PJ. IVIG-mechanisms of action. Allergy. 2003;58:543–52. doi: 10.1034/j.1398-9995.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- 3.Kamijuku H, Nagata Y, Jiang X, et al. Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1:208–18. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- 4.Cukrowska B, Sinkora J, Mandel L, et al. Thymic B cells of pig fetuses and germ-free pigs spontaneously produce IgM, IgG and IgA: detection by Elispot method. Immunology. 1996;87:487–92. doi: 10.1046/j.1365-2567.1996.499573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz-Albiez R, Laban S, Eichmueller S, et al. Cytotoxic natural antibodies against human tumours: an option for anti-cancer immunotherapy? Autoimmunity Rev. 2008;7:491–95. doi: 10.1016/j.autrev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Pal S, Chatterjee M, Bhattecharya DK, et al. Identification and purification of cytolytic antibodies directed against O-acetylated sialic acid in childhood acute lymphoblastic leukemia. Glycobiology. 2000;10:539–49. doi: 10.1093/glycob/10.6.539. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro R, van de Winkel JG. IgA Fc receptors. Ann Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 8.Pasquier B, Launay P, Kanamaru Y, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Bieber AJ, Warrington A, Pease LR, Rodriguez M. Humoral autoimmunity as a mediator of CNS repair. Trends Neurosci. 2001;24:39–44. doi: 10.1016/s0166-2236(00)01991-3. [DOI] [PubMed] [Google Scholar]
- 10.Binder C, Chou J, Fogelstrand MY, et al. Natural antibodies in murine atherosclerosis. Curr Drug Targets. 2008;9:190–5. doi: 10.2174/138945008783755520. [DOI] [PubMed] [Google Scholar]
- 11.Shoenfeld Y, Fishman P. Gamma-globulin inhibits tumour spread in mice. Int Immunol. 1999;11:1247–51. doi: 10.1093/intimm/11.8.1247. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro S, Shoenfeld Y, Gilburd B, et al. Intravenous gamma globulin inhibits the production of matrix metalloproteinase-9 in macrophages. Cancer. 2002;95:2032–7. doi: 10.1002/cncr.10905. [DOI] [PubMed] [Google Scholar]
- 13.Sapir T, Blank M, Shoenfeld Y, et al. Immunomodulatory effects of intravenous immunoglobulins as a treatment for autoimmune diseases, cancer, and recurrent pregnancy loss. Ann NY Acad Sci. 2005;1051:743–78. doi: 10.1196/annals.1361.118. [DOI] [PubMed] [Google Scholar]
- 14.Shoenfeld Y, Krause I. IVIG for autoimmune, fibrosis, and malignant conditions: our experience with 200 patients. J Clin Immunol. 2004;24:107–14. doi: 10.1023/b:joci.0000019809.55787.ec. [DOI] [PubMed] [Google Scholar]
- 15.Shoenfeld Y, Levy Y, Fishman P. Shrinkage of melanoma metastases following high doses intravenous immunoglobulin treatment. Isr Med Assoc J. 2001;3:863. [PubMed] [Google Scholar]
- 16.Damianovich M, Solomon AS, Blank M, Shoenfeld Y. Attenuation of colon carcinoma tumour spread by intravenous immunoglobulin. Ann NY Acad Sci. 2007;1110:567–77. doi: 10.1196/annals.1423.061. [DOI] [PubMed] [Google Scholar]
- 17.Schachter J, Katz U, Mahrer A, et al. Efficacy and safety of intravenous immunoglobulin in patients with metastatic melanoma. Ann NY Acad Sci. 2007;1110:305–14. doi: 10.1196/annals.1423.032. [DOI] [PubMed] [Google Scholar]
- 18.Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109:581–91. doi: 10.1067/mai.2002.122466. [DOI] [PubMed] [Google Scholar]
- 19.Olas K, Butterweck H, Teschner W, et al. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005;140:478–90. doi: 10.1111/j.1365-2249.2005.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinheiro da Silva F, Aloulou M, Benhamou M, Monteiro RC. Inhibitory ITAMs: a matter of life and death. Trends Immunol. 2008;29:366–73. doi: 10.1016/j.it.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Kanamaru Y, Pfirsch S, Aloulou M, et al. Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008;180:2669–78. doi: 10.4049/jimmunol.180.4.2669. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez M, Lennon VA, Benveniste EN, Merrill JE. Remyelination by oligodendrocytes stimulated by antiserum to spinal cord. J Neuropathol Exp Neurol. 1987;46:84–95. doi: 10.1097/00005072-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lang W, Rodriguez M, Lennon VA, Lampert PW. Demyelination and remyelination in murine viral encephalomyelitis. Ann NY Acad Sci. 1984;436:98–102. doi: 10.1111/j.1749-6632.1984.tb14779.x. [DOI] [PubMed] [Google Scholar]
- 24.Warrington AE, Bieber AJ, Ciric B, et al. A recombinant human IgM promotes myelin repair after a single, very low dose. J Neurosci Res. 2007;85:967–76. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]
- 25.Asakura K, Miller DJ, Murray K, et al. Monoclonal autoantibody SCH94.03, which promotes central nervous system remyelination, recognizes an antigen on the surface of oligodendrocytes. J Neurosci Res. 1996;43:273–81. doi: 10.1002/(SICI)1097-4547(19960201)43:3<273::AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Asakura K, Miller DJ, Pease LR, Rodriguez M. Targeting of IgMkappa antibodies to oligodendrocytes promotes CNS remyelination. J Neurosci. 1998;18:7700–08. doi: 10.1523/JNEUROSCI.18-19-07700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrington AE, Asakura K, Bieber AJ, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci USA. 2000;97:6820–5. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter SF, Miller DJ, Rodriguez M. Monoclonal remyelination-promoting natural autoantibody SCH 94·03: pharmacokinetics and in vivo targets within demyelinated spinal cord in a mouse model of multiple sclerosis. J Neurol Sci. 1997;150:103–13. doi: 10.1016/s0022-510x(97)00080-4. [DOI] [PubMed] [Google Scholar]
- 29.Pirko I, Ciric B, Gamez J, et al. A human antibody that promotes remyelination enters the CNS and decreases lesion load as detected by T2-weighted spinal cord MRI in a virus-induced murine model of MS. FASEB J. 2004;18:1577–79. doi: 10.1096/fj.04-2026fje. [DOI] [PubMed] [Google Scholar]
- 30.Warrington AE, Bieber AJ, Van Keulen V, et al. Neuron-binding human monoclonal antibodies support central nervous system neurite extension. J Neuropathol Exp Neurol. 2004;63:461–73. doi: 10.1093/jnen/63.5.461. [DOI] [PubMed] [Google Scholar]
- 31.McGavern DB, Murray PD, Rivera-Quinones C, et al. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123:519–31. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 33.Binder CJ, Shaw PX, Chang MK, et al. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–63. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–14. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hörkkö S, Bird DA, Miller E, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–28. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang MK, Binder CJ, Miller YI, et al. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200:1359–70. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang MK, Bergmark C, Laurila A, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–58. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 39.Shaw PX, Hörkkö S, Chang MK, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. Clin Invest. 2000;105:1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. Exp Med. 1982;156:1177–85. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binder CJ, Hörkkö S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidised LDL. Nat Med. 2003;9:736–43. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 42.Chou MY, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies. J Clin Invest. 2009;119:1335–49. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faria-Neto JR, Chyu KY, Li X, et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]


