Abstract
The posters presented at the 6th International Immunoglobulin Symposium covered a wide range of fields and included both basic science and clinical research. From the abstracts accepted for poster presentation, 12 abstracts were selected for oral presentations in three parallel sessions on immunodeficiencies, autoimmunity and basic research. The immunodeficiency presentations dealt with novel, rare class-switch recombination (CSR) deficiencies, attenuation of adverse events following IVIg treatment, association of immunoglobulin (Ig)G trough levels and protection against acute infection in patients with X-linked agammaglobulinaemia (XLA) and common variable immunodeficiency (CVID), and the reduction of class-switched memory B cells in patients with specific antibody deficiency (SAD). The impact of intravenous immunoglobulin on fetal alloimmune thrombocytopenia, pregnancy and postpartum-related relapses in multiple sclerosis and refractory myositis, as well as experiences with subcutaneous immunoglobulin in patients with multi-focal motor neuropathy, were the topics presented in the autoimmunity session. The interaction of dendritic cell (DC)-SIGN and α2,6-sialylated IgG Fc and its impact on human DCs, the enrichment of sialylated IgG in plasma-derived IgG, as wells as prion surveillance and monitoring of anti-measles titres in immunoglobulin products, were covered in the basic science session. In summary, the presentations illustrated the breadth of immunoglobulin therapy usage and highlighted the progress that is being made in diverse areas of basic and clinical research, extending our understanding of the mechanisms of immunoglobulin action and contributing to improved patient care.
Keywords: antibody, autoimmunity, immunodeficiencies, intravenous immunoglobulin, subcutaneous immunoglobulin
Introduction
In addition to the invited plenary presentations, symposium delegates were invited to submit abstracts for poster presentations. All abstracts submitted were reviewed in a blinded manner by the Scientific Committee, and 48 abstracts were accepted for poster presentation at the Symposium.
The topics addressed in the posters spanned a wide range of fields, covering both basic science and clinical research. Various posters dealt with clinical aspects of immunodeficiencies, including factors that impact upon quality of life, the safety and efficacy of intravenous immunoglobulins (IVIg) in several diseases, the development of consensus guidelines for the use of immunoglobulin replacement therapy in the Asia-Pacific region, the role of specific antibodies and new markers for predicting the outcome of immunoglobulin therapy. Other posters focused upon the use of IVIg in autoimmune disease refractory to corticosteroids or immunosuppressants, such as post-infectious encephalomyelitis and refractory myositis. The clinical management of neuropathies was addressed in a poster on the treatment of ataxic neuropathy in Sjögren's syndrome refractory to immunosuppression and in a poster on diabetic chronic inflammatory demyelinating polyneuropathy (DCIDP) and diabetic lumbosacral plexoradiculoneuropathy (diabetic amyotrophy) (DLPRN), in which inflammation is thought to play a major pathogenic role.
A number of posters focused upon clinical experience with the administration of subcutaneous immunoglobulin (SCIg) in diverse disease states, including common variable immunodeficiency (CVID), severe hyper-IgE syndrome and multi-focal motor neuropathy. Two further posters dealt with in vitro and preclinical characteristics of SCIg stabilized with proline (IgPro20), a highly concentrated (20%) immunoglobulin product developed for subcutaneous administration.
New developments in the production of IVIg products were presented, and data on the safety of immunoglobulin preparation, including the results of prion surveillance in the United Kingdom, were discussed. The effect of sialylation on IVIg function was addressed in two posters, and other topics included the safety and efficacy of therapeutic IgM, identification of target antigens of self-reactive IgG in IVIg preparations and the role of complement inhibition in the anti-inflammatory effects of immunoglobulin products.
The Scientific Committee selected 12 of the poster abstracts for oral presentation in three parallel sessions on immunodeficiencies, autoimmunity and basic research.
Immunodeficiencies
For more than 20 years IVIg has been used in the clinical management of patients with immunodeficiencies, and remains the treatment of choice [1,2]. Advances in the field have contributed to a better understanding of the pathology and aetiology of immunodeficiencies and refinements in the administration of immunoglobulin replacement therapy. This session on immunodeficiencies was chaired by Drs Eduardo Fernandez-Cruz and Hans-Hartmut Peter and featured four outstanding presentations.
The session opened with a presentation from Dr Anne Durandy, who received the Poster Award from the Scientific Committee for her scientific contribution. Dr Durandy reported the findings of her group on a novel, rare class-switch recombination (CSR) deficiency, which results from deleterious, homozygous mutations in the gene encoding the post-meiotic segregation 2 (PMS2) component of the mismatch repair machinery [3]. This disease is characterized by the occurrence of malignancies from early childhood onwards as well as the development of severe bacterial infections, due to a defect in immunoglobulin production in some of them. Four patients with this condition were examined. The B cells from all four patients expressed high levels of IgM and IgD, but not IgA nor IgG, suggesting that the CSR mechanism was defective. In vitro analysis revealed that although the patients' B cells proliferated normally, class-switching to IgE and IgA after appropriate stimulation [with soluble CD40 ligand and either interleukin (IL)-4 or IL-10, respectively] was defective. It was further shown that double-stranded DNA breaks, which are necessary for class-switching, were defective in the PMS2-deficient patients (Fig. 1), suggesting that PMS2 acts at the step of double-strand DNA break generation. Based on the observation that a very severe Ig-CSR defect is observed in patients lacking uracil-DNA glycosylase (UNG) [4], it is likely that PMS2-initiated mismatch repair does not represent an alternative pathway for base excision repair in human CSR, but instead functions downstream of UNG in the same pathway. PMS2 has been shown previously to induce DNA cleavage at the mismatch, allowing exonuclease 1 to exert its 5′-3′exonucleasic activity [5]. Thus, a defect in this endonuclease activity of PMS2 may account for the decreased frequency of S region DNA cleavage observed in PMS2-deficient patients.
Fig. 1.
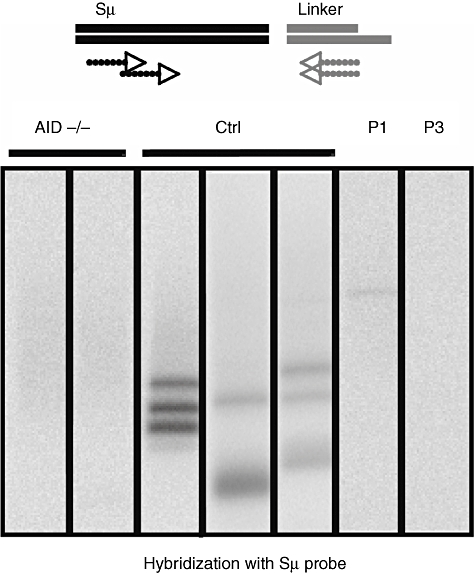
Post-meiotic segregation 2 (PMS2) acts on double-strand DNA breaks during class-switch recombination. B cells were stimulated by soluble CD40 ligand and interleukin-4 for 5 days for class-switch recombination. Double-strand breaks (DSB) in the DNA of switch regions (Sµ) were detected by a ligated-mediated polymerase chain reaction (PCR). PCR products were transferred to agarose gels and hybridized with a labelled Sµ probe. Very few double-strand breaks were observed in the B cells from the two PMS2-deficient patients (P1, P3) compared with healthy controls (Ctrl). AID−/−: B cells from activation-induced cytidine deaminase patients (negative control).
The development of some adverse events after IVIg administration has been associated with activation of the complement cascade [6]. Dr Nathan Cantoni reported here on a prospective study investigating this link in 20 patients receiving IVIg infusions after stem cell transplantation. IgG, IgM, IgA, C3, C4, C1 inhibitor and sC5b-9 levels were monitored, and adverse reactions were recorded using a questionnaire. Binary logistic regression was used to assess the association between adverse reactions and the following covariates: complement activation; age; gender; graft-versus-host disease; premedication; long-term immunosuppression; C-reactive protein; white blood cell counts; previous IVIg exposure; and IVIg dose. IVIg-associated adverse reactions were recorded in eight of 20 patients (40%). An association between increasing concentrations of circulating sC5b-9 and adverse reactions was noted [relative risk 14·0, 95% confidence interval (CI) 1·25–156; P = 0·03], while other covariates showed no significant association with the occurrence of adverse events. Using other, less sensitive measures of complement activation (e.g. changes in C3, C4 or C1 inhibitor levels), there was no significant association, which may support the concept that even subtle activation of this inflammatory cascade can trigger clinical signs or symptoms. Intriguingly, premedication, which is used widely to prevent IVIg-related adverse reactions, had no detectable effect in this study. Adverse reactions and complement activation have been linked to the IgG aggregate content of IVIg [7,8]. Upon completion of recruitment into the first part of the study (n = 40), patients will be randomized to receive either standard IVIg or IVIg subjected to a simple IgG-aggregate removal protocol, in order to investigate further whether this reduces the incidence of adverse events in this patient setting.
An analysis of clinical and immunological data from a large cohort of patients with CVID or X-linked agammaglobulinaemia (XLA), collected by the Italian Primary Immunodeficiencies Network (IPINET), was presented by Dr Isabella Quinti. IPINET is a collaborative group of paediatric and adult immunological centres in Italy. Analysis of morbidity and mortality data showed that patients with XLA have a 3-year survival probability of 41% and that chronic lung disease (CLD) and severe respiratory infections were the main causes of morbidity and mortality in this patient population. An IgG trough level of > 600 mg/dl after IVIg or SCIg administration reduced the incidence of pneumonia and acute otitis significantly in XLA patients. In patients with CVID, mortality was due mainly to cancer. In CVID patients with CLD, an inverse correlation was found between the incidence of pneumonia and IgG trough levels. In patients without CLD, the risk of pneumonia correlates with the levels of IgA. At diagnosis, IgG levels in the CVID patients did not differ in patients with or without any of the associated clinical conditions, and IgG trough levels at enrolment and during the prospective study did not correlate with any of the associated clinical conditions. In contrast to patients with XLA, IgG trough levels > 600 mg/dl were not associated with a decreased risk for development of pneumonia in patients with CVID. Dr Quinti concluded that despite immunoglobulin replacement treatment, a progressive increase in the prevalence of patients with chronic diseases was observed in all age groups, including the paediatric population.
Dr Ricardo Sorensen, in collaboration with Dr Lily Leiva, investigated whether the inability of patients with specific antibody deficiency (SAD) to respond to pneumococcal polysaccharide antigens (PPs) was associated with reduced numbers of circulating class-switched memory B cells. A cohort of 53 children with recurrent respiratory infections (20 with SAD and 33 without SAD) and 12 healthy controls were analysed as part of a larger study (Fig. 2). All study subjects had normal immunoglobulin levels and normal anti-protein antibodies. Patients with known immunodeficiencies were excluded from the study. Anti-PPs antibody levels were measured by a standardized enzyme-linked immunosorbent assay (ELISA) test, and expression of CD27, IgD and IgM was analysed on peripheral CD19+ B cells in order to determine the proportions of naive, non-switched memory, class-switched memory and IgM memory B cells. In 15 of 20 patients with SAD, there were reduced relative levels of class-switched memory B cells compared with non-SAD patients and healthy controls (Fig. 2). However, no significant differences in the percentages of IgM memory B cells were found among the three groups. Dr Sorensen concluded that a deficient IgM-to-IgG antibody switch may be associated with a lack of circulating class-switched memory B cells in patients with SAD, which may explain the failure of some patients to respond to PPs. Ongoing research aims to determine the prognostic implications of these differences in patients with specific antibody deficiency.
Fig. 2.
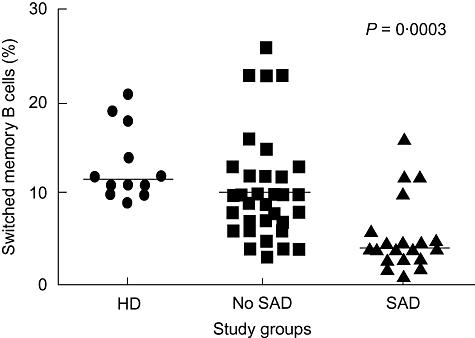
Percentages of switched memory B cells in 12 healthy donors (HD), 33 patients with recurrent respiratory infections without specific antibody deficiency (SAD) (no SAD) and 20 patients with SAD (see text). Peripheral blood mononuclear cells were stained with monoclonal antibodies to CD27, immunoglobulin (Ig)M and IgD and analysis was performed on gated CD19+ B cells. Patients with SAD show markedly lower percentages of switched memory B cells (CD27+ IgD-) compared to healthy donors and to patients without SAD. Bars represent median.
Autoimmunity
IVIg treatment ameliorates successfully the symptoms of a number of autoimmune diseases, such as systemic lupus erythematosus (SLE) and scleroderma, and there are increasing reports on the benefits of immunoglobulin therapy in other conditions caused by immune dysregulation [9,10]. This session, chaired by Drs Abdulgabar Salama and Ivo van Schaik, focused upon new developments in the use of immunoglobulin therapy for the treatment of autoimmune diseases, including alternative modes of administration in both adults and children.
Fetal and neonatal alloimmune thrombocytopenia develops as a result of maternal alloimmunization against fetal platelet antigens inherited from the father [11]. As discussed by Dr James B. Bussel, maternal alloantibodies to fetal platelets can lead in utero to intracranial haemorrhage (ICH). As part of a clinical trial programme started in 1984 in collaboration with Janice McFarland in Wisconsin and Richard Berkowitz, now at Columbia, a series of studies have been published exploring the efficacy of IVIg administered to the mother in the amelioration of fetal thrombocytopenia and the prevention of ICH [12,13]. Three important findings that have emerged are: (i) giving IVIg to the mother at doses of 1–2 g/kg/week with or without prednisone increases the fetal platelet count; (ii) the severity of the thrombocytopenia and presence or absence of ICH in the previously affected sibling determine the risk of ICH in the current affected fetus; and (iii) fetal blood sampling is associated with significant risks to the fetus and should be minimized or avoided. Recently, a case series was analysed comprising 30 pregnant women treated with 1–2 g/kg/week IVIg and prednisone whose previous fetuses had experienced an ICH. Only five of 30 of the subsequent fetuses suffered an ICH: two were premature infants with platelet counts > 100 000; and one had only a grade 1 haemorrhage. In the absence of other treatment options, Dr Bussel concluded that IVIg is unequivocally the treatment of choice for fetal alloimmune thrombocytopenia.
IVIg has been shown previously to be effective in the treatment of the inflammatory myopathies polymyositis and dermatomyositis [14,15]. Dr Maria Giovanna Danieli described her centre's experience with IVIg as an add-on therapy to cyclosporine (CsA) or mycophenolate mofetil (MMF) in patients suffering from polymyositis or dermatomyositis. Thirty-two patients with active, severe disease that was refractory to steroids and immunosuppressants were followed in an open, prospective study, with a mean follow-up time of 58 ± 27 months. Patients were treated with monthly cycles of 2 g/kg IVIg for 6 months, then every other month for three further cycles. CsA was administered at 3 mg/kg/day orally and MMF was titrated to 30 mg/kg/day orally. At the last follow-up, significant differences were observed compared with baseline values in Medical Research Council (MRC) scores, mean serum creatinine kinase levels, modified Rankin score and the daily maintenance prednisone dose (Fig. 3). A positive response to IVIg was observed in approximately 80% of patients with relapsed or refractory disease. This treatment regimen allowed more rapid recovery from disability than treatment with immunosuppressants alone, and the effects were maintained in the long term. No relevant side effects were noted. These clinical benefits need to be balanced against the need for regular infusions and the costs of treatment.
Fig. 3.
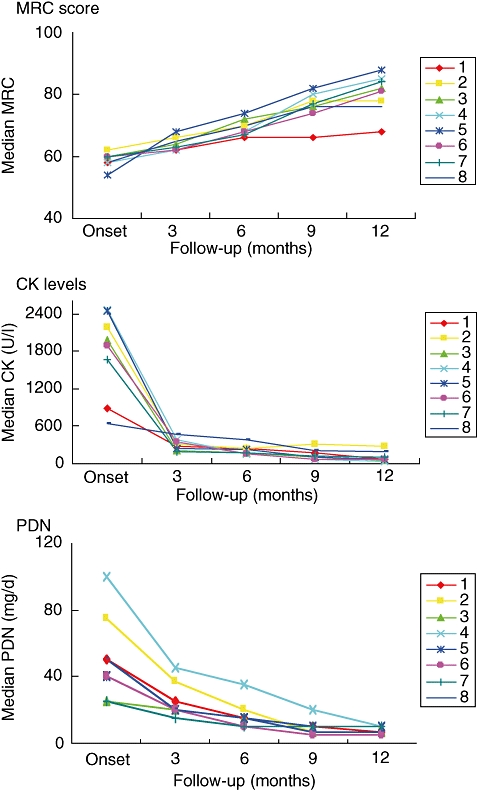
Effect of intravenous immunoglobulin (IVIg) add-on therapy in patients suffering from polymyositis and dermatomyositis. Patients were treated with monthly cycles of 2 g/kg IVIg for 6 months, then every other month for three further cycles. Mycophenolate mofetil (MMF) was titrated to 30 mg/kg/day orally. Patients showed an improvement in muscle strength (Medical Research Council sum scores), decreased serum creatinine (CK) levels and a decrease in the prednisolone maintenance dose.
For patients with multi-focal motor neuropathy (MMN), IVIg should be considered as first-line therapy when disability is sufficiently severe to warrant treatment. If initial treatment is effective, repeated IVIg treatment should be considered [16]. The frequency of IVIg maintenance therapy should be guided by the individual response; typical regimens are 1 g/kg every 2–4 weeks or 2 g/kg every 4–8 weeks [16]. Dr Ivo van Schaik presented data from a single-centre, open-label pilot study on whether SCIg treatment is safe and effective in maintaining muscle strength in MMN patients. Ten patients who had been treated previously with IVIg were switched to weekly SCIg treatment. The first five patients were treated with SCIg at a dose equivalent to 50% of the IVIg maintenance dose. One patient withdrew informed consent due to local adverse events, and the other four patients deteriorated. These patients were therefore given a loading dose of IVIg and the SCIg dose was doubled. The second group of five patients was therefore treated with SCIg at a dose equivalent to the IVIg maintenance dose. Four of the five patients maintained muscle strength (as measured using the MRC sum score) during the 6-month follow-up period (Fig. 4). Local adverse events were observed frequently in both groups (especially during the first 4 weeks of treatment), but SCIg was generally well tolerated. Nine mild systemic adverse events were reported in three patients, seven of which occurred in the first week of treatment. Dr van Schaik concluded that SCIg may be a viable alternative to IVIg as maintenance therapy in selected patients with MMN, and recommended careful monitoring of patients while switching to SCIg.
Fig. 4.
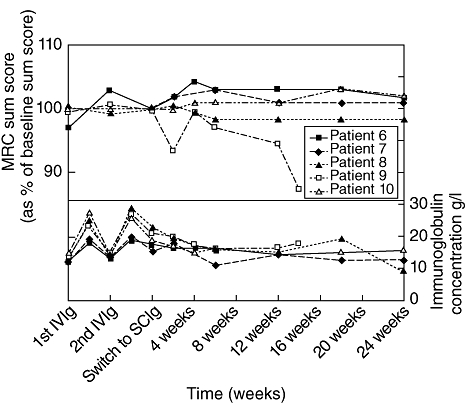
Medical Research Council sum scores and immunoglobulin concentrations in five multi-focal motor neuropathy (MMN) patients switched from intravenous immunoglobulin (IVIg) to subcutaneous immunoglobulin (SCIg). Patients were treated with an IVIg loading dose and subsequently SCIg at 100% of the previous monthly IVIg maintenance dose. Four of the five patients treated with this regimen maintained muscle strength.
In women with relapsing–remitting multiple sclerosis (RRMS), pregnancy modulates disease activity. There is an initial decline in relapse rate, especially during the third trimester, but a higher risk of exacerbation during the postpartum period [17,18]. Dr Alexander Winkelmann and colleagues studied the effect of IVIg treatment on the rate of relapse during pregnancy and the postpartum period in 54 consecutive pregnant RRMS patients. One group of patients (n = 28) was treated with IVIg (0·4 g/kg/day) before gestation for 5 consecutive days, followed by doses once every 4 weeks until the second trimester of pregnancy. Therapy was restarted after delivery and continued throughout the lactation period following the same dosing schedule. A second group of 21 patients was treated only during the lactation period. Five patients in group III were not treated. Analysis of the data revealed the typical decrease in annualized relapse rate (ARR) during pregnancy (from 1·1 to 0·39 in group I, and from 0·7 to 0·15 in group II) and a lower relapse rate in the postpartum period of IVIg-treated patients compared with either group III or the rates reported previously by Confavreux and colleagues. Dr Winkelmann concluded that IVIg treatment of RRMS patients during and/or after delivery is an effective option to reduce the incidence of pregnancy- and postpartum-related relapses.
Basic research
Drs Srini Kaveri and Nobuhiro Yuki chaired a session highlighting the latest scientific research developments in the field of immunoglobulins.
Dr Jagadeesh Bayry presented his group's findings on the role of interaction of DC-SIGN, a molecule expressed on human dendritic cells (DCs) and α2,6-sialylated IgG Fc on the anti-inflammatory activity of IVIg. The investigations were based on previous data from experimental mouse models, which demonstrated that anti-inflammatory activity of IVIg is mediated mainly by antibodies that contain terminal α2,6-sialic acid linkages that recognize SIGN-R1. SIGN-R1 is a mouse orthologue of DC-SIGN. Furthermore, in experiments with human cells, sialylated IgG Fc proteins were shown to bind to DC-SIGN. In order to determine whether the sialylated Fc region is responsible for the immunomodulatory effect of IVIg, immature human DCs were cultured in the presence of F(ab′)2 IVIg fragments (which lack the Fc region and thus the terminal α2,6-sialic acid linkages) or in the presence of intact IVIg. The DCs were then stimulated with a Toll-like receptor (TLR)-4 agonist, lipopolysaccharide (LPS). Activation of DCs was measured by assessing the phosphorylation of intracellular signalling molecules responsible for inflammatory responses [extracellular regulated kinase (ERK)1/2] and the expression of maturation-associated markers (CD83, CD95 and CD40) (Fig. 5). Both intact IVIg and F(ab′)2 fragments were found to inhibit the phosphorylation of ERK1/2 and the expression of maturation-associated markers on DCs (Fig. 5). Thus, the team concluded that the DC-SIGN/α2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human DCs, in contrast to what has been shown in the mouse models [19]. The results illustrate that caution should be used when translating results from murine models to patients.
Fig. 5.
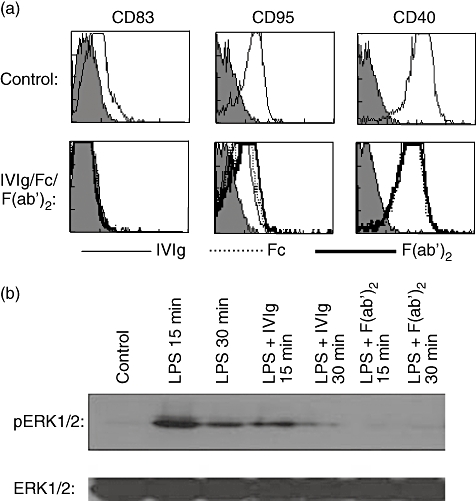
Dendritic cell (DC)-SIGN and α2,6-sialylated immunoglobulin (Ig)G Fc interaction is dispensable for the anti-inflammatory activity of intravenous immunoglobulin (IVIg) on human DCs. (a) Immature monocyte-derived human DCs were incubated in the presence of equimolar concentrations (0·15 mM) of IVIg (thin lines) or F(ab′)2 fragments of IVIg (thick lines) or Fc fragments (dashed lines) for 48 h. The surface markers were analysed by flow cytometry. The upper panel shows DCs cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 alone. This research was originally published by Bayry et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood 2003; 101: 758–765 [21]. © the American Society of Hematology. (b) Immature DCs were cultured in the presence of 0·15 mM IVIg or F(ab′)2 fragments of IVIg for 12 h. The cells were then exposed to lipopolysaccharide (LPS) (1 µg) for 15 and 30 min. The activation of extracellular regulated kinase (ERK)1/2 was monitored by immunoblotting with antibody to phospho-ERK1/2 (pERK1/2). An equal amount of protein loaded in each lane was confirmed with immunoblotting by using antibody to the non-phosphorylated form of ERK1/2 (ERK1/2).
On a related topic, Dr Fabian Käsermann presented his group's findings on the importance of sialylation on the effectiveness of IVIg immunomodulation. Their approach involved separating sialic acid-enriched (+SA IVIg) and -depleted (–SA IVIg) IgG from a new liquid polyclonal IVIg product (Privigen®) using sialic acid-specific Sambucus nigra (SNA) lectin affinity chromatography. Analysis of the different fractions revealed that several specific antibodies were distributed differently into the +SA and –SA IVIg fractions. No differences were observed in the overall binding of +SA versus–SA IVIg to Fc gamma receptors. However, the +SA IVIg fraction was more potent than –SA IVIg in down-regulating the activation of leucocytes in response to phytohaemagglutinin stimulation. The contribution of Fab versus Fc sialylation on the lectin-based separation was addressed by characterization of the +SA and –SA fractions produced from the IVIg F(ab′)2 and Fc fragments. Further studies will show whether the extent of IgG sialylation in human plasma products is important for their activity.
Dr Peter Späth and his colleagues have examined variations in anti-measles virus (MeV) antibody levels in IVIg pools over decades, and have assessed the impact of these changes on the protection of primary immunodeficiency (PID) patients against measles. Long-term variations in anti-MeV levels were measured by ELISA in samples from 4356 US plasma donors born between 1947 and 1989. While in plasma of donors born between 1947 and 1962 mean anti-MeV levels consistently exceeded 1·5 IU/ml, a drop to levels below 0·5 IU/ml was observed in individuals born between 1963 and 1968. The estimated mean anti-MeV level of plasma donors in 2007 was found to be 1·99 IU/ml. Statistical analyses suggested that these mean levels in US-sourced plasma may drop by approximately 28% to 1·45 IU/ml in the next 10 years, as the number of donors with high anti-MeV titres (those born before 1963) declines. In order to investigate whether anti-MeV levels in donor plasma correlate with the levels in IVIg preparations, immunoglobulin lots prepared between 1986 and 2008 were examined. Irrespective of whether the lots were prepared from plasma of US or EU origin, anti-MeV levels have decreased. When assessing lots produced in 1 calendar year, significant lot-to-lot variability in anti-MeV levels in the same product (Sandoglobulin®) was found over decades, independent from the origin of the plasma and independent from plasma pool size (plasma pooled from 8000 up to 60 000 donors). The clinical significance of these differences remains unclear. Analysis of serum samples from 20 PID patients treated with SCIg (Vivaglobin®) between 2001 and 2003 assessed at trough levels of total IgG (median 9·1 g/l; range 5·7–19·2 g/l) revealed a mean anti-MeV titre of 3·17 IU/ml (range 1·3–6·2 IU/ml) when assessed by virus neutralization test. These levels are five- to 20-fold higher than the titre considered protective for MeV (0·26 IU/ml) and therefore seem sufficient to protect PID patients from MeV infection.
The surveillance of prions, which are responsible for transmissible spongiform encephalopathies (TSEs) such as variant Creutzfeldt–Jakob disease (vCJD), is a potential safety consideration when using IVIg, as was discussed by Dr Matthew Helbert. In the 1990s, four individuals were infected with prions after receiving transfusions of non-leucodepleted blood [20], showing that infection through blood products is possible. Since then, leucocytes are being removed from all blood used for transfusion in the United Kingdom. Furthermore, a haemophiliac patient, exposed to UK-sourced Factor VIII, has shown evidence of abnormal prion infection at post mortem. The theoretical risk of prion infection in patients exposed to UK-sourced immunoglobulin is no greater than 1%, and is considered lower than for recipients of coagulation plasma factors and blood transfusions. A prospective study was funded by the National Health Service National Institute for Health Research to establish a resource for collecting DNA, tissue and blood samples from recipients of UK-sourced immunoglobulin (Fig. 6). Several difficulties arose when conducting the study. Clinicians were reluctant to register for the study and patients were reluctant to provide consent (often giving only partial consent). Furthermore, data on immunoglobulin batch usage was often incomplete or difficult to trace. The results indicated that prion infection is unlikely to be present in more than 5% of patients. Importantly, although there is currently no evidence of prion infection in recipients of UK-sourced immunoglobulin, experience has highlighted practical implications for the management of patients undergoing immunoglobulin replacement or treatment. Dr Helbert suggested that measures such as the continued voluntary registration of immunoglobulin recipients and the creation of a bank of blood and tissue samples from all patients undergoing exposure to immunoglobulin, may help to better track the incidence of prion infection from immunoglobulin products.
Fig. 6.
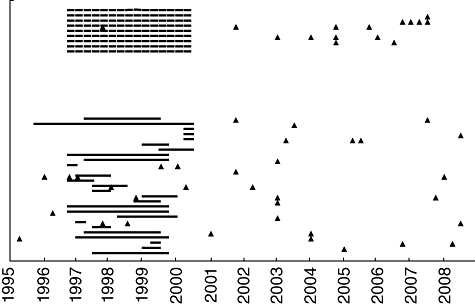
Surveillance of primary immunodeficiency (PID) patients having received blood donations from the United Kingdom or Scotland by an individual risk assessment programme. The unbroken horizontal lines indicate the years exposure to UK-sourced batches of immunoglobulin. The broken horizontal lines indicate estimated years of exposure when exact batch documentation was not available. The triangles indicate the date of a tissue sample.
Summary
The oral poster presentations covered diverse current topics in immunoglobulin therapy and illustrated the breadth of immunoglobulin therapy usage. These presentations highlighted the progress in diverse areas of basic and clinical research, extending our understanding of the mechanisms of immunoglobulin action and contributing to improved patient care.
Acknowledgments
The authors thank nspm ltd for providing medical writing services, with financial support through an unrestricted educational grant from CSL Behring.
Disclosures
JBB receives clinical research support from the following companies: Amgen, Cangene, GlaxoSmithKline, Genzyme, Immunomedica, Ligand, Eisai Inc, and Sysmex. JBB also participates for Scienta in their speaker's bureau program. JBB's family owns stock in Amgen, GlaxoSmithKline, Ligand and Shionogi.
PS has acted and is acting as a paid consultant for CSL Behring.
IVS has received an unrestricted grant for research carried out in this work of Sanquin Blood Supply Foundation.
FK is employed by CSL Behring.
AW received travel grants and lecture fees from the following companies: Schering, Bayer Healthcare, Octapharma AG, Merck Serono, CSL Behring.
All other authors have declared that they have no conflicts of interest.
References
- 1.Ballow M. Safety of IGIV therapy and infusion-related adverse events. Immunol Res. 2007;38:122–32. doi: 10.1007/s12026-007-0003-5. [DOI] [PubMed] [Google Scholar]
- 2.Durandy A, Wahn V, Petteway S, et al. Immunoglobulin replacement therapy in primary antibody deficiency diseases – maximizing success. Int Arch Allergy Immunol. 2005;136:217–29. doi: 10.1159/000083948. [DOI] [PubMed] [Google Scholar]
- 3.Péron S, Metin A, Gardès P, et al. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J Exp Med. 2008;205:2465–72. doi: 10.1084/jem.20080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai K, Slupphaug G, Lee WI, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–8. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 5.Kadyrov FA, Dzantiev L, Constantin N, et al. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999;21:171–85. doi: 10.2165/00002018-199921030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Barandun S, Kistler P, Jeunet F, et al. Intravenous administration of human g-globulin. Vox Sang. 1962;7:157–64. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238–9. doi: 10.1016/j.jaci.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 9.Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28:413–37. doi: 10.1016/j.iac.2008.01.008. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Looney RJ, Huggins J. Use of intravenous immunoglobulin G (IVIG) Best Pract Res Clin Haematol. 2006;19:3–25. doi: 10.1016/j.beha.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Arnold DM, Smith JW, Kelton JG. Diagnosis and management of neonatal alloimmune thrombocytopenia. Transfus Med Rev. 2008;22:255–67. doi: 10.1016/j.tmrv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Berkowitz RL, Bussel JB, McFarland JG. Alloimmune thrombocytopenia: state of the art 2006. Am J Obstet Gynecol. 2006;195:907–13. doi: 10.1016/j.ajog.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Berkowitz RL, Kolb EA, McFarland JG, et al. Parallel randomized trials of risk-based therapy for fetal alloimmune thrombocytopenia. Obstet Gynecol. 2006;107:91–6. doi: 10.1097/01.AOG.0000192404.25780.68. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 15.Danieli MG, Calcabrini L, Marchetti A, et al. Intravenous immunoglobulin treatment in autoimmune diseases. Recenti Prog Med. 2007;98:322–6. in Italian. [PubMed] [Google Scholar]
- 16.European Federation of Neurological Societies, Peripheral Nerve Society. van Schaik IN, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Eur J Neurol. 2006;13:802–8. doi: 10.1111/j.1468-1331.2006.01466.x. [DOI] [PubMed] [Google Scholar]
- 17.Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 18.Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127:1353–60. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- 19.Bayry J, Bansal K, Kazatchkine MD, Kaveri SV. DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc Natl Acad Sci. 2009;106:E24. doi: 10.1073/pnas.0900016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner ML, Ludlam CA. An update on the assessment and management of the risk of transmission of variant Creutzfeldt–Jakob disease by blood and plasma products. Br J Haematol. 2009;144:14–23. doi: 10.1111/j.1365-2141.2008.07376.x. [DOI] [PubMed] [Google Scholar]
- 21.Bayry J, Lacroix-Desmazes S, Carbonneil C, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–65. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]


