Abstract
Immunoglobulin (Ig) administration via the subcutaneous (s.c.) route has become increasingly popular in recent years. The method does not require venous access, is associated with few systemic side effects and has been reported to improve patients' quality of life. One current limitation to its use is the large volumes which need to be administered. Due to the inability of tissue to accept such large volumes, frequent administration at multiple sites is necessary. Most studies conducted to date have investigated the use of subcutaneous immunoglobulin (SCIg) in patients treated previously with the intravenous (i.v.) formulation. New data now support the use of s.c. administration in previously untreated patients with primary immunodeficiencies. SCIg treatment may further be beneficial in the treatment of autoimmune neurological conditions, such as multi-focal motor neuropathy; however, controlled trials directly comparing the s.c. and i.v. routes are still to be performed for this indication. New developments may further improve and facilitate the s.c. administration route. For example, hyaluronidase-facilitated administration increases the bioavailability of SCIg, and may allow for the administration of larger volumes at a single site. Alternatively, more concentrated formulations may reduce the volume required for administration, and a rapid-push technique may allow for shorter administration times. As these developments translate into clinical practice, more physicians and patients may choose the s.c. administration route in the future.
Keywords: hyaluronidase, IgPro20, peripheral neuropathy, primary immunodeficiency, subcutaneous immunoglobulin
Introduction
Immunoglobulin (Ig) replacement has long been used for the treatment of a wide variety of primary and secondary antibody deficiencies and autoimmune disorders. Other current investigational uses include sepsis and neurological diseases such as stroke and even Alzheimer's. IgG may be administered by the intramuscular, subcutaneous (s.c.) or intravenous (i.v.) routes. Intramuscular injection, however, is no longer considered appropriate for routine replacement therapy [1]. The method is painful for most patients, limited in volume and the resulting serum IgG levels in patients with hypogammaglobulinaemia are not comparable to physiological levels [2].
IgG replacement therapy was introduced in 1952 by Colonel Bruton, who used a 16% solution for the treatment of a boy with agammaglobulinaemia [3]. Interestingly, Bruton administered IgG via the s.c. route and demonstrated a beneficial effect. Slow s.c. immunoglobulin (SCIg) infusions using portable syringe drivers were introduced in the United States in 1980, and used subsequently in parts of Europe and New Zealand [4]. Although the slow s.c. infusions were an advance over intramuscular infusions, the infusions were time-consuming and the volume that could be administered in a single infusion was still limited. As a result, the s.c. route did not become popular at that time. In the United States, virtually all primary immunodeficiency (PID) patients have been treated via the i.v. route since intravenous immunoglobulin (IVIg) formulations became available in the early 1980s. In Scandinavia, however, the SCIg administration method has been developed further and was reintroduced in 1991 as rapid SCIg therapy (20 ml/h/pump) [5]. This method has become standard practice in Sweden and Norway [4]. Today, more rapid infusions with rates of up to 35 ml/h/pump are available [6] and make this administration route increasingly popular on both sides of the Atlantic.
Both i.v. and s.c. administration of immunoglobulin at adequate doses increases serum IgG trough levels to physiological concentrations [4,7] and protects PID patients from bacterial infections [8–10]. As expected, the pharmacokinetic (PK) profiles of IgG following i.v. and s.c. administration differ. Administration of IVIg leads to an immediate rise in the serum IgG concentration to extremely high levels, in most cases over 1000 mg/dl, followed by a rapid fall over the next several days, associated with the passage of IgG from the vasculature to the lymph and extracellular fluid compartments. A further slow decline of the serum IgG level is caused mainly by its catabolism [1]. When administered via the s.c. route, IgG is distributed initially in the local subcutaneous tissue, followed by slow diffusion into the vascular and extravascular fluid space [1].
In healthy subjects, IgG has a half-life of 23–25 days [11]. Recent studies in hypogammaglobulinaemic patients receiving IVIg or SCIg have reported half-lives as long as 34–37 days [12,13] and 41 days [14], respectively. Thus, there is no clinically significant difference in the half-life of IgG between the two administration routes. However, s.c. regimens usually involve weekly dosing, compared with i.v. regimens in which a large dose is given every third or fourth week. The use of smaller doses at more frequent intervals with s.c. administration results in stable, higher trough IgG serum concentrations which remain constant between consecutive SCIg infusions [9,10,14,15]. Thus, wear-off effects, reported by i.v. patients towards the end of their 3–4-week interval, are avoided with most SCIg dosing regimens. Because peak Ig levels are lower, adverse effects associated with the very high peaks experienced after large i.v. boluses are much less common. Overall, SCIg is associated with fewer infusion-related events than IVIg, and most patients tolerate SCIg well.
There are a number of advantages of SCIg over IVIg. Venous access is not required and the need for premedication with corticosteroids and anti-histamines is reduced. A programmable pump is usually used to deliver SCIg. The technique is easy to learn and can be performed even by children and elderly patients. IgG can therefore be self-administered by many patients at home, often obviating the need for an infusion nurse. For most patients, self-administration results in improved convenience, better quality of life (QoL) and fewer absences from work [4,7]. Several studies have investigated the switch from hospital-based IVIg therapy to home-based SCIg therapy [4,7]. Increased QoL and treatment satisfaction was reported in a European cohort of 15 children and 32 adults with PID [16]. The adult patients reported increased vitality, mental health and social functioning. All the children and 73% of the adults preferred s.c. over i.v. therapy. In a similar study in North America, patients previously on IVIg reported fewer limitations in daily activities and increased health and vitality after 12 months of SCIg self-infusing at home [17].
Avoiding visits to hospitals or doctors offices and eliminating visiting nurses result in lower long-term costs [4]. One cost analysis performed in Sweden found that the use of s.c. at home instead of i.v. infusions at a hospital would reduce the yearly cost per patient for the health-care sector by $US10 100; however, one driver for this reduction was the difference in price between the SCIg and IVIg preparations [18]. Two more recent pharmacoeconomic evaluations, one from Canada and one from Germany, have shown similarly reduced costs associated with s.c. administration [7,19].
Despite its well-established safety profile, IgG administration via the i.v. route can lead to undesired symptoms, ranging from mild systemic adverse reactions, such as flushing, fever, muscle aches, tiredness, headache and dizziness, to severe reactions, manifesting as chest pain, tachycardia, changes in blood pressure, aseptic meningitis, thrombosis or renal failure [20]. The SCIg administration route has been found to result in very few systemic adverse reactions and may therefore be suitable for patients with previous adverse reactions to IVIg [4]. Local reactions at s.c. injection sites are common but are rarely severe, and are accepted by most patients. In a study of 165 patients who received more than 33 000 SCIg infusions, only 100 mild and six moderate adverse reactions were observed [18], demonstrating that the majority of patients tolerate SCIg well.
Although there are advantages of SCIg over IVIg, potential limitations to its use exist. Although achieving higher trough IgG levels and continuously maintaining more physiological antibody levels, with less drop-off towards the end of the dosing interval, the total area under the curve (AUC) of serum IgG versus time in SCIg treated patients is reduced compared with the AUC achieved with equivalent doses of IVIg in the same patients. While the clinical relevance of AUC differences for the bioavailability of IgG remains unproven, AUCs.c. equivalence to AUCi.v. has become an issue for regulators in the United States. As a consequence, the recommended SCIg dose in the United States is 137% of the IVIg dose in order to achieve an equivalent AUC. In contrast, European regulators do not consider AUC equivalence to be relevant for clinical response, and recommend dosing of SCIg at 100% of the IVIg dose [21].
With the currently available Ig formulations (up to 16%), the inability of tissues to accept large volumes of infusate rapidly may present a limitation to s.c. administration. While IVIg is usually administered every 3–4 weeks, patients receiving IgG via the s.c. route need frequent administration (typically one to two times weekly) of a smaller volume at multiple sites. Some patients and physicians regard the multiple sites and frequent s.c. infusions as burdensome enough to decline or recommend against SCIg therapy.
In the session ‘SCIg: opportunities and outlook’, chaired by Drs Siraj Misbah and Hans Ochs, the presentations focused upon the design of protocols for the use of SCIg in patients with autoimmune neurological diseases and those naive to IgG therapy, and on exploring promising new strategies to improve the ease and efficacy of the s.c. administration route. Professor Mathias Sturzenegger presented data on SCIg use in patients with peripheral neuropathies. Professor Michael Borte reported on his experience with SCIg in previously untreated PID patients. A new rapid manual push administration method without the use of pumps for PID patients was presented by Dr Ralph Shapiro, and Dr Richard Wasserman reported new findings on how locally administered recombinant human hyaluronidase may facilitate the administration, dispersion and bioavailability of s.c.-infused immunoglobulin. Preliminary results from a study using a new highly concentrated immunoglobulin formulation, IgPro20 (SCIg stabilized with proline), were presented by Dr Melvin Berger.
SCIg in the treatment of peripheral neuropathies
High-dose IVIg is an established treatment in acute inflammatory demyelinating polyneuropathy (AIDP, Guillain–Barré syndrome) and immune-mediated inflammatory neuropathies with a chronic course, such as chronic inflammatory demyelinating polyneuropathy (CIDP) and multi-focal motor neuropathy (MMN) [22]. IVIg treatment may also be beneficial in other rare, possibly immune-mediated neuropathies; however, efficacy has not (yet) been established in randomized controlled trials [22].
MMN is defined clinically as progressive asymmetric motor weakness with preserved sensation in the distribution of two or more nerves, and electrophysiologically by conduction blocks affecting only motor fibres [23–25], although definitive diagnostic criteria are still a matter of debate [26,27]. The favourable response to IVIg treatment in up to 80% [28] and the presence of GM1 ganglioside auto-antibodies (anti-GM1) in 30–80% of patients support an immune-mediated pathogenesis [26]. Four randomized, controlled studies with a total of 46 MMN patients have demonstrated that IVIg is an effective treatment, leading to improved muscle strength in two-thirds of patients [29–32]. However, the few studies that have addressed the long-term efficacy of IVIg noted a loss of benefit in some patients, which was attributed to secondary axon loss [33–35]. Nevertheless, IVIg is the only evidence-based treatment available and is recommended as first-line therapy [27].
CIDP is an acquired, most probably immune-mediated polyneuropathy that follows a chronic progressive or relapsing course, with symmetrical weakness mainly in distal muscles, impaired sensation and absent or diminished deep tendon reflexes. Nerve-conduction studies indicating diffuse demyelinating nerve damage are essential for diagnosis [36]. Therapeutically, corticosteroids and IVIg are equally effective, with response rates of 70–80%. In patients not responding to these treatments, plasma exchange should be considered. IVIg efficacy has been established in six randomized controlled studies involving 170 patients [37,38].
Many aspects of IVIg treatment, such as dose or optimal time interval between infusions, have not been evaluated systematically for these neuropathies. Establishment of an IVIg dose–response curve would require a large study with stratified treatment groups. Different dose responses might exist in different patient subpopulations, which would require careful investigation. In this disease setting, it is unknown whether pulsed treatment with high peaks (and relatively large intervals between peak and trough levels) might be clinically more effective, or if fractionated dosing maintaining stable serum IgG levels might prevent recurrent weakness at the end of the dosing interval. Of further interest may be strategies to reduce the necessary cumulative dose and thus the related costs.
Although efficacy results derived from PID patients may not be translated directly to neuropathy patients, there are few theoretical arguments against the use of SCIg in patients with peripheral neuropathies responsive to IVIg. It is not known which of the multiple effects exerted by high-dose IgG administration might be necessary and/or responsible for the efficacy of this treatment in different immune-mediated neuropathies with established responsiveness to IVIg. Furthermore, the PK parameters responsible for optimal efficacy in neuropathy patients are not known and there are only few data regarding optimal dosing regimens [39].
SCIg administration to CIDP patients has been documented in only three case reports [40,41]. All three patients were switched successfully from effective IVIg therapy to SCIg, which was well tolerated and resulted in a stabilization or even improvement of the disease course. SCIg use in MMN has been reported in a small single-blind, cross-over Danish study [42], which included 10 patients responsive to IVIg and two case reports [40]. In the Danish study SCIg was given two to three times per week, whereas IVIg was infused once every 3–8 weeks, with the total monthly IgG dose being the same for both administration routes. After three IVIg treatments with mean intervals of 42 days, patients were crossed-over to SCIg treatment. SCIg was not effective in one patient who withdrew from the study, but efficacy was equivalent to that of the previous IVIg treatment in the remaining nine patients. Compared with IVIg, no end-of-dose-interval weakening was observed. There was no difference in the evolution of muscle strength between the administration routes, and patients did not experience an improvement of their quality of life or show a preference towards either administration route. In the two case reports, patients were switched from IVIg to SCIg with high satisfaction and tolerability [42].
A multi-centre study of SCIg use in MMN patients has been completed recently. The study included eight MMN patients who were previously on IVIg treatment with a stable clinical course for at least 3 months. Patients were switched to SCIg at the same total monthly IgG dose as when on IVIg treatment. SCIg treatment duration was 25 weeks. End-points considered included muscle strength, Guy's Neurological Disability Scale, QoL and adverse events. While efficacy results were similar to the Danish study, the results regarding patient satisfaction with the s.c. administration seemed to be far more promising, with all patients completing the study expressing their preference to stay on s.c. treatment (unpublished data).
Overall, SCIg appears to be an option for improving tolerability and patient comfort and reducing long-term costs of high-dose immunoglobulin treatment in patients with neurological disorders. However, it is important to demonstrate that SCIg is as efficacious as IVIg for each indication in long-term clinical studies.
Use of SCIg in previously untreated PID patients
The overall efficacy of immunoglobulin replacement therapy in PID patients with predominant antibody deficiency is well established [43]. The usual approach is to start with a loading dose of IVIg in newly diagnosed PID patients. After normalization of serum IgG levels, IVIg supplementation is continued at a specific dose at regular intervals or it is possible to switch the patient from IVIg to SCIg. Follow-up studies have shown equal efficacy of the SCIg treatment and equal or improved tolerability when compared to IVIg treatment [9,10].
An alternative approach would be to begin treatment of PID patients with SCIg. However, so far there have been only few reports on the administration of SCIg in previously untreated patients (PUPs). Gardulf [4] reported results with weekly SCIg infusions of 100 mg/kg in previously untreated adult patients suffering from common variable immunodeficiency (CVID) or X-linked agammaglobulinaemia (XLA). This procedure increased the serum IgG concentration from a mean of 107 mg/dl pre-infusion to 640 mg/dl after 6 months.
A recent open-label, multi-centre, single-arm, prospective study investigated the use of SCIg in PUPs with XLA or CVID. Each patient underwent a loading and training phase in an out-patient setting at the hospital, receiving 100 mg/kg Vivaglobin® on 5 consecutive days, followed by a maintenance phase at home with a weekly infusion of 100 mg/kg body weight Vivaglobin®. The primary efficacy end-point of the study was the increase of the IgG trough level to ≥ 500 mg/dl on day 12 and the proportion of patients achieving IgG serum levels ≥ 500 mg/dl on day 12. Secondary end-points included IgG increase (change from baseline) on day 12, total serum IgG trough levels, health-related QoL, overall rate of infections and the use of antibiotics for infection prophylaxis and treatment. The study protocol included the possibility of increasing the dose at day 12 to either 150 or 200 mg/kg per week if the IgG trough level was below 500 mg/dl. Patients not achieving IgG trough levels of 500 mg/dl by day 26 were classified as non-responders and were withdrawn from the study.
An interim analysis of this study of 18 patients showed promising results, with patients generally achieving normalized IgG trough levels and improved QoL. The mean serum IgG level of all 18 untreated patients at screening was 356 mg/dl. The mean IgG levels on day 12 had increased to well above 500 mg/dl. No dose adjustment was needed. One can emphasize the positive training effect due to the close patient follow-up (daily visits) in the first week of treatment, which increases the safety for each patient. To our knowledge, these are the first data from a tightly monitored study, suggesting that SCIg may be used to initiate the treatment of PID in children and adults and may increase the overall QoL in this patient population.
SCIg administration by rapid push
When IgG replacement is given s.c. to PID patients, administration occurs typically once a week using a programmable infusion pump. Because mechanical devices such as infusion pumps may be difficult for some patients to use, and the costs of one or more pumps may add to the total cost of the treatment regimen, simpler devices and/or direct manual push from a syringe could potentially increase the acceptance of s.c. administration. At one clinic in the United States, patients were given a choice between a frequent ‘rapid push’ administration method without a pump and standard weekly administration with a programmable pump. Results on which method was preferred by patients were collected and presented as a retrospective review.
Rapid push is a simple method using a syringe and a 23–25-gauge butterfly needle to push SCIg under the skin as fast as the patient is comfortable with (usually 1–2 cc/min). This push method generally takes between 5 and 20 min. The time of administration may vary within the same patient for consecutive administrations, depending upon the comfort level. Using the rapid push method, 3–20 ml of SCIg (Vivaglobin®, 16% solution) is administered typically into a single site as often as every day. Some patients may, however, prefer administration at two sites simultaneously, taking their infusions less frequently, such as three times a week.
Patients who received at least one complete course of SCIg therapy were included in the analysis. For the majority of patients, data were collected for at least 2 years with four or more visits at intervals of 5–6 months. Patients were eligible for analysis if they were naive to IVIg treatment or had switched voluntarily from IVIg to SCIg. A switch between the two administration methods was possible at all times. Initially, both rapid push and pump administration of SCIg was described and demonstrated, and the patients encouraged to indicate their preference. After being trained on the chosen method in the clinic, each patient self-administered SCIg at home. Demographic information and data on dose, infusion frequency, duration and volume, number of sites, adverse events, serum IgG levels and previous IVIg regimen were recorded on standardized case report forms.
Charts were reviewed from 104 patients [43% male, 57% female; mean age 21·1 years (range 0·5–67·6)]. Seventy-four patients (72%) initially chose the rapid push method. Rapid push was chosen preferably by parents for use in infants under the age of 2 years, who require only small volumes. Rapid push was chosen less often for use in children 2–10 years of age, but was the preferred method in teenagers and adults. The mean SCIg dose in these patients was 32·1 g/month (range 1·92–89·6) divided and administered an average of 3·11 times per week, most frequently (88%) at one site. More patients switched from the pump administration to the push method (45%) compared with push to pump (12%). Some of the patients (7%) who chose the pump method initially used a combination of both methods.
Mean serum IgG levels did not differ significantly between administration methods: pump 1153·1 mg/dl [±240·8 mg/dl, standard deviation (s.d.)]; rapid push 1225·8 mg/dl (±299·8 mg/dl, s.d.) (Fig. 1). These IgG trough levels were 20–40% higher than those achieved by IVIg administration, despite the fact that SCIg was administered at 100% or less of the previous monthly IVIg dose. The most common adverse event was local infusion-site reaction, occurring in one-third of patients in each administration group. There was no difference in the rates of AEs between administration groups (Table 1), and only two patients discontinued therapy due to an AE.
Fig. 1.
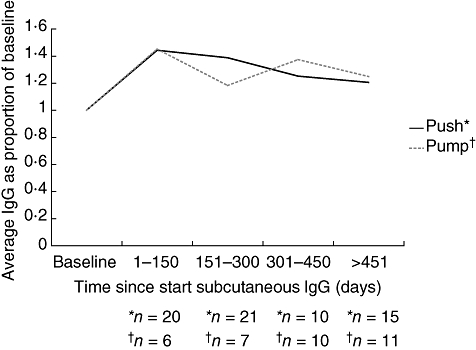
Mean serum immunoglobulin (Ig)G levels over time in patients self-administering subcutaneous immunoglobulin (SCIg) by a pump or the rapid push method. The graph represents data from those patients who switched from IVIg to SCIg. Levels as expressed as proportions of the baseline (i.e. start of study) through IgG levels while on IVIg.
Table 1.
Adverse events following subcutaneous immunoglobulin (SCIg) administration by pump or rapid push reported at three visits.
| Visit number | Rapid push | Pump |
|---|---|---|
| Visit 1 | n = 72 | n = 29 |
| Patients with ≥ 1 adverse event | 22 (31%) | 9 (31%) |
| Local reaction | 20 (28%) | 8 (28%) |
| Headache | 1 (1%) | 0 |
| Gastrointestinal (nausea, vomiting, diarrhoea) | 1 (1%) | 0 |
| Fever | 1 (1%) | 0 |
| Rash | 0 | 1 (3%) |
| Other | 2 (3%) | 0 |
| Visit 2 | n = 68 | n = 23 |
| Patients with ≥ 1 adverse event | 15 (22%) | 5 (22%) |
| Local reaction | 15 (22%) | 4 (17%) |
| Headache | 0 | 1 (4%) |
| Rash | 0 | 1 (4%) |
| Other | 0 | 1 (4%) |
| Visit 3 | n = 53 | n = 21 |
| Patients with ≥ 1 adverse event | 8 (15%) | 3 (14%) |
| Local reaction | 7 (13% | 3 (14%) |
| Rash | 0 | 1 (5%) |
These results suggest that rapid push is an effective administration method of SCIg delivery in PID patients and presents a valid alternative to pump administration. In this study, this method was preferred by many of the patients over the pump administration method.
SCIg administration with recombinant human hyaluronidase
As mentioned previously, a potential drawback of the SCIg administration method is the limited fluid volume that can be administered into a single site in a single infusion, with the consequence that most patients use multiple sites and/or frequent infusions. The reason for the limitation in infusion volume lies in the architecture of the subcutaneous space, which comprises a collagen matrix filled with hyaluronan, a very high molecular weight gel-like co-polymer of glucuronic acid and N-acetyl glucosamine. One approach to facilitating s.c. infusion is to modify the subcutaneous space by using hyaluronidase [44,45]. Hyaluronidase cleaves the hyaluronan in the subcutaneous tissue, facilitating dispersion of solutions and thereby enhancing the delivery of drugs and fluids through the extracellular matrix and into the circulation [45]. Sheep and bovine hyaluronidase preparations have been used for decades to facilitate s.c. infusions of local anaesthetics and fluids. Due to the animal origin of the preparations and potential contamination with other proteins, they are not suitable for chronic use in humans.
rHuPH20 is a recombinant human hyaluronidase, expressed as a 61 kDa glycoprotein. In a study of 100 healthy human volunteers who received rHuPH20 there were no positive immediate hypersensitivity skin tests (data on file, Halozyme Therapeutics, Inc., San Diego, CA, USA). Following subcutaneous injection of rHuPH20, the structure of the gel-like hyaluronan is restored within 24–48 h with no discernable adverse effects [46] Animal and human studies have shown that rHuPH20 facilitates absorption and dispersion of fluids and small molecules [47]. Animal studies have shown improved bioavailability of pegylated interferon and monoclonal IgG administered with rHuPH20 [46].
A pilot study was conducted in 11 PID patients to determine the amount of rHuPH20 required to enable s.c. infusion of a monthly dose of a 10%IgG solution (10%Ig) at a single site at rates equivalent to i.v. infusions. rHuPH20 was supplied as a 150 U/ml or 1500 U/ml hyaluronidase solution. rHuPH20 was given s.c. using an infusion pump similar to that used for IVIg administration, followed by 10%Ig through the same catheter. This initial dose-ranging study showed that a minimum dose of 50 U rHuPH20/g 10%Ig was effective at allowing rapid infusion of several hundred ml of 10%IgG, but 25 U/g was not. Doses of rHuPH20 up to 300 U/g were studied, but conferred no advantage in terms of tolerability over the 50 U/g dose.
The study also evaluated the effect of rHuPH20 on the bioavailability of 10%Ig given s.c. compared with i.v. Patients were infused, at appropriate intervals, with varying amounts of rHuPH20, followed by 25%, 50%, 75% and 100% of the their 4-week dose of 10%Ig. Ten patients completed the study. One patient withdrew from the study because of moderate infusion site discomfort with the 1-week (25% of the monthly dose) treatment.
PK analyses were performed using data from seven patients. The mean AUC for rHuPH20-facilitated 10% Ig was 92% (range 75·8–102·7%) of the AUC with the same 10% IgG preparation using the i.v. route. As with SCIg administration without hyaluronidase, peak IgG levels are lower compared with IVIg administration. However, unlike s.c. regimens using weekly dosing, trough levels continue to drop by the time the next infusion is due, as is seen with every three or four week i.v. infusions. Examples of the IgG versus time profiles from two patients are shown in Fig. 2. Fig. 2b compares the AUCs achieved using 50 and 200 U/g rHuPH20, respectively, in the same patient. As noted above, increasing the dose of rHuPH20 beyond 50 U/g did not increase the bioavailability.
Fig. 2.
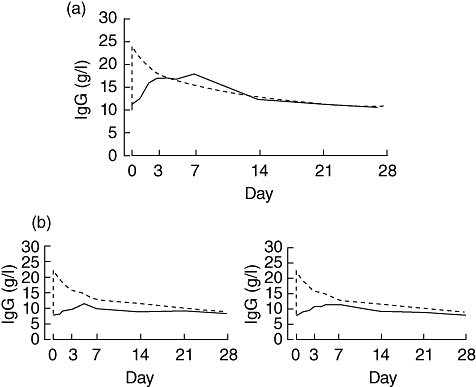
Serum immunoglobulin (Ig)G concentrations over time following intravenous (i.v.) (---) or subcutaneous (s.c.) (—) infusion of 10%Ig facilitated by rHuPH20. (a) Serum IgG concentrations in one patient following s.c. infusion using rHuPH20 at a concentration of 50 U/g 10%Ig. (b) Serum IgG concentrations in a second patient following s.c. infusion using rHuPH20 at a concentration of either 50 U/g 10%Ig (left panel) or 200 U/g 10%Ig (right panel). Area under the curve (AUC) was 75·83% and 77·83% of the AUC following i.v. infusion of the same IgG quantity, respectively.
The 10 patients who were evaluable achieved monthly doses of 25·5–61·2 g (255–612 ml of 10%Ig) at a single site, at rates of 120–300 ml/h. The maximum rate was determined by pump characteristics, not patient tolerability. The mean duration of infusion was 2·9 h (±0·8 h), comparing favourably to the infusion time for monthly i.v. treatment and for weekly s.c. infusion at one or two sites. Eight of the 10 patients achieved the maximum tested rate of 300 ml/h. A minimum of 50 U rHuPH20/g 10%Ig was required to achieve these rates. The 10 patients completing the study experienced only mild local reactions, such as swelling and redness. No drug-related allergic reactions occurred.
In conclusion, these results suggest that rHuPH20 enables single-site s.c. administration of a monthly dose of 10% IgG solution of more than 400 mg/kg with an infusion time comparable to that for i.v. infusions and may enhance the bioavailability of SCIg. Experience with this hyaluronidase-facilitated administration is, however, limited and studies on long-term safety are required.
Pharmacokinetics, safety and efficacy of s.c.-administered 20% IgG (IgPro20)
One approach to shorten the SCIg infusion time is to reduce the amount of volume needed per infusion through the development of more concentrated IgG solutions. IgPro20 was developed specifically for s.c. administration, and has an IgG concentration of 20%. IgPro20 is stabilized with proline, which allows for storage at room temperature. The higher concentration results in a lower infusion volume and therefore a shorter infusion time and/or fewer sites per infusion.
Clinical development of IgPro20 included a phase I study in healthy volunteers that investigated the local tolerability of the s.c. administered product and the systemic tolerability of a low dose for inadvertent i.v. administration (CSL Behring, data on file). All participants completed the study and no treatment-related adverse events occurred. Local infusion reactions were common and consistent with the known pattern of local reactions after s.c. infusion of 16% IgG preparations. Most of the local reactions resolved within 3 days after the end of infusions and overall the infusions were well tolerated, as assessed by the subject as well as the investigator.
Phase III studies include two pivotal trials, one in the United States and one in Europe. In the US study, 49 subjects with PID on stable IVIg regimens were enrolled. A subgroup of patients consented to detailed PK assessments; 18 patients completed the PK substudy. Subjects began treatment with SC IgPro20 1 week after their last i.v. infusion, at an initial dose equal to 130% of their previous i.v. dose, based on Food and Drug Administration (FDA) requirements. After a 12-week wash-in/wash-out phase, the dose was adjusted based on the results for the PK subset. Patients then entered the 52-week efficacy phase (Fig. 3).
Fig. 3.
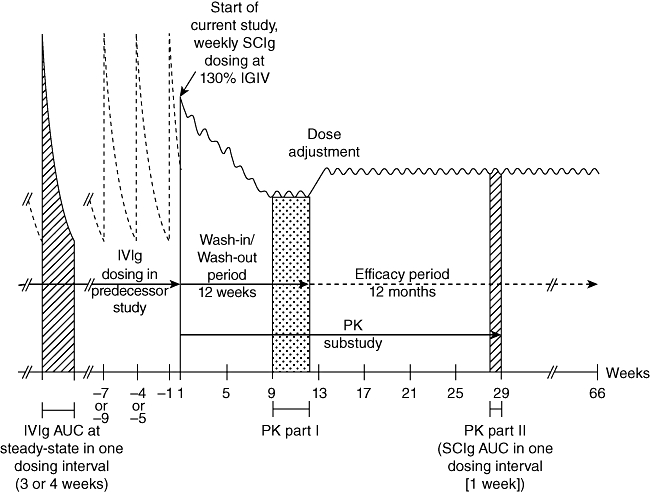
Study design of the US IgPro20 phase III study. The study consisted of a 12-week wash-in/wash-out period and a 12-month efficacy period.
The preliminary results of this study show that IgPro20 is safe, well tolerated and effective for use in antibody replacement therapy for PID patients (unpublished data). The availability of a 20% IgG preparation may facilitate the attainment of sustained high IgG levels in patients with PID, and may be preferable for studies of high-dose SCIg treatment for neuromuscular and autoimmune diseases.
Summary
IgG administration by the s.c. route is becoming increasingly popular. The potential benefits of s.c. self-administration at home, such as increased QoL for the patients, appear to outweigh the potential disadvantages for many patients. A number of recent developments, such as the use of rapid push methods, hyaluronidase-facilitated SCIg administration and/or the availability of highly concentrated IgG solutions, may further improve and facilitate this administration route. Results from new studies indicate that SCIg can be used in previously untreated PID patients and in specific disease areas requiring higher doses than PID, such as peripheral neuropathies. As these developments translate into clinical practice, more physicians and patients may choose the s.c. administration route in the future.
Acknowledgments
Dr Wasserman would like to thank the following researchers for their contributions: Isaac R. Melamed; Mark R. Stein; Richard C. Yocum; Richard I. Schiff. The authors thank nspm ltd for providing medical writing services, with financial support through an unrestricted educational grant from CSL Behring.
Disclosures
MB is a salaried employee of CSL Behring and owns stock in the company. HO is a consultant for CSL Behring. SM has received honoraria for lectures and funding for research presented in this paper. RSS has received an unrestricted grant from CSL Behring which was used to conduct this retrospective review. RW has acted as a paid consultant to Baxter Healthcare and has received funding for research carried out in this work. All other authors have declared that they have no conflicts of interest.
References
- 1.Bonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol Allergy Clin North Am. 2008;28:803–19. doi: 10.1016/j.iac.2008.06.006. ix. [DOI] [PubMed] [Google Scholar]
- 2.Weiler CR. Immunoglobulin therapy: history, indications, and routes of administration. Int J Dermatol. 2004;43:163–6. doi: 10.1111/j.1365-4632.2004.02236.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–8. [PubMed] [Google Scholar]
- 4.Gardulf A. Immunoglobulin treatment for primary antibody deficiencies: advantages of the subcutaneous route. BioDrugs. 2007;21:105–16. doi: 10.2165/00063030-200721020-00005. [DOI] [PubMed] [Google Scholar]
- 5.Gardulf A, Hammarström L, Smith CI. Home treatment of hypogammaglobulinaemia with subcutaneous gammaglobulin by rapid infusion. Lancet. 1991;338:162–6. doi: 10.1016/0140-6736(91)90147-h. [DOI] [PubMed] [Google Scholar]
- 6.Hansen S, Gustafson R, Smith CI, Gardulf A. Express subcutaneous IgG infusions: decreased time of delivery with maintained safety. Clin Immunol. 2002;104:237–41. doi: 10.1006/clim.2002.5215. [DOI] [PubMed] [Google Scholar]
- 7.Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;28:779–802. doi: 10.1016/j.iac.2008.07.002. viii. [DOI] [PubMed] [Google Scholar]
- 8.Chapel HM, Spickett GP, Ericson D, et al. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. doi: 10.1023/a:1006678312925. [DOI] [PubMed] [Google Scholar]
- 9.Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – a prospective, multi-national study. J Clin Immunol. 2006;26:177–85. doi: 10.1007/s10875-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 10.Ochs HD, Gupta S, Kiessling P, et al. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 11.Knapp MJ, Colburn PA. Clinical uses of intravenous immune globulin. Clin Pharm. 1990;9:909–12. [PubMed] [Google Scholar]
- 12.Ballow M, Berger M, Bonilla FA, et al. Pharmacokinetics and tolerability of a new intravenous immunoglobulin preparation, IGIV-C, 10% (Gamunex (TM), 10%) Vox Sang. 2003;84:202–10. doi: 10.1046/j.1423-0410.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman RL, Church JA, Peter HH, et al. Pharmacokinetics of a new 10% intravenous immunoglobulin in patients receiving replacement therapy for primary immunodeficiency. Eur J Pharm Sci. 2009;37:272–8. doi: 10.1016/j.ejps.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson R, Gardulf A, Hansen S, et al. Rapid subcutaneous immunoglobulin administration every second week results in high and stable serum immunoglobulin G levels in patients with primary antibody deficiencies. Clin Exp Immunol. 2008;152:274–9. doi: 10.1111/j.1365-2249.2008.03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waniewski J, Gardulf A, Hammarström L. Bioavailability of gammaglobulin after subcutaneous infusions in patients with common variable immunodeficiency. J Clin Immunol. 1994;14:90–7. doi: 10.1007/BF01541341. [DOI] [PubMed] [Google Scholar]
- 16.Gardulf A, Nicolay U, Math D, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114:936–42. doi: 10.1016/j.jaci.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 17.Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26:65–72. doi: 10.1007/s10875-006-8905-x. [DOI] [PubMed] [Google Scholar]
- 18.Gardulf A, Andersen V, Björkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345:365–9. doi: 10.1016/s0140-6736(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 19.Högy B, Keinecke HO, Borte M. Pharmaco-economic evaluation of immunoglobulin treatment in patients with antibody deficiencies from the perspective of the German statutory health insurance. Eur J Health Econom. 2005;50:24–9. doi: 10.1007/s10198-004-0250-5. [DOI] [PubMed] [Google Scholar]
- 20.Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238–9. doi: 10.1016/j.jaci.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Evaluation Agency (EMEA) Committee for Proprietary Medicinal Products (CPMP) Note for guidance on the clinical investigation of human normal immunoglobulin for subcutaneous and intramuscular use. CPMP/BPWG/283/00. 2002. Available at: http://www.emea.europa.eu/pdfs/human/bpwg/028300en.pdf (accessed 18 May 2009.
- 22.European Federation of Neurologocal Societires (EFNS) EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008;15:893–908. doi: 10.1111/j.1468-1331.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- 23.Bouche P, Moulonguet A, Younes-Chennoufi AB, et al. Multifocal motor neuropathy with conduction block: a study of 24 patients. J Neurol Neurosurg Psychiatry. 1995;59:38–44. doi: 10.1136/jnnp.59.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olney RK, Lewis RA, Putnam TD, et al. Consensus criteria for the diagnosis of multifocal motor neuropathy. Muscle Nerve. 2003;27:117–21. doi: 10.1002/mus.10317. [DOI] [PubMed] [Google Scholar]
- 25.Parry GJ, Clarke S. Multifocal acquired demyelinating neuropathy masquerading as motor neuron disease. Muscle Nerve. 1988;11:103–7. doi: 10.1002/mus.880110203. [DOI] [PubMed] [Google Scholar]
- 26.Nobile-Orazio E, Cappellari A, Priori A. Multifocal motor neuropathy: current concepts and controversies. Muscle Nerve. 2005;31:663–80. doi: 10.1002/mus.20296. [DOI] [PubMed] [Google Scholar]
- 27.Van Schaik IN, Bouche P, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Eur J Neurol. 2006;13:802–8. doi: 10.1111/j.1468-1331.2006.01466.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Schaik IN, van den Berg LH, de Haan R, Vermeulen M. Intravenous immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst Rev. 2005;2:CD004429. doi: 10.1002/14651858.CD004429.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Azulay JP, Blin O, Pouget J, et al. Intravenous immunoglobulin treatment in patients with motor neuron syndromes associated with anti-GM1 antibodies: a double-blind, placebo-controlled study. Neurology. 1994;44:429–32. doi: 10.1212/wnl.44.3_part_1.429. [DOI] [PubMed] [Google Scholar]
- 30.Federico P, Zochodne DW, Hahn AF, et al. Multifocal motor neuropathy improved by IVIg: randomized, double-blind, placebo-controlled study. Neurology. 2000;55:1256–62. doi: 10.1212/wnl.55.9.1256. [DOI] [PubMed] [Google Scholar]
- 31.Leger JM, Chassande B, Musset L, et al. Intravenous immunoglobulin therapy in multifocal motor neuropathy: a double-blind, placebo-controlled study. Brain. 2001;124:145–53. doi: 10.1093/brain/124.1.145. [DOI] [PubMed] [Google Scholar]
- 32.Van den Berg LH, Kerkhoff H, Oey PL, et al. Treatment of multifocal motor neuropathy with high dose intravenous immunoglobulins: a double blind, placebo controlled study. J Neurol Neurosurg Psychiatry. 1995;59:248–52. doi: 10.1136/jnnp.59.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terenghi F, Cappellari A, Bersano A, et al. How long is IVIg effective in multifocal motor neuropathy? Neurology. 2004;62:666–8. doi: 10.1212/01.wnl.0000110185.23464.a1. [DOI] [PubMed] [Google Scholar]
- 34.Van den Berg-Vos RM, Franssen H, Wokke JH, Van den Berg LH. Multifocal motor neuropathy: long-term clinical and electrophysiological assessment of intravenous immunoglobulin maintenance treatment. Brain. 2002;125:1875–86. doi: 10.1093/brain/awf193. [DOI] [PubMed] [Google Scholar]
- 35.Delmont E, Azulay JP, Uzenot D, et al. Long-term follow-up of multifocal motor neuropathy with conduction block under intravenous immunoglobulin. Rev Neurol. 2007;163:82–8. doi: 10.1016/s0035-3787(07)90358-6. [DOI] [PubMed] [Google Scholar]
- 36.Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–56. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 37.Van Schaik IN, Winer JB, De Haan R, Vermeulen M. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2002;2:CD001797. doi: 10.1002/14651858.CD001797. [DOI] [PubMed] [Google Scholar]
- 38.Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate chromatography purified for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7:136–44. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- 39.Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;28:779–802. doi: 10.1016/j.iac.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Köller H, Schroeter M, Feischen H, et al. Subcutaneous self-infusions of immunoglobulins as a potential therapeutic regimen in immune-mediated neuropathies. J Neurol. 2006;253:1505–6. doi: 10.1007/s00415-006-0258-0. [DOI] [PubMed] [Google Scholar]
- 41.Lee DH, Linker RA, Paulus W, et al. Subcutaneous immunoglobulin infusion: a new therapeutic option in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2008;37:406–9. doi: 10.1002/mus.20909. [DOI] [PubMed] [Google Scholar]
- 42.Harbo T, Andersen H, Hess A, et al. Subcutaneous versus intravenous immunoglobulin treatment for MMN patients: a randomized, single-blinded, cross-over study. Eur J Neurol. 2009;16:631–8. doi: 10.1111/j.1468-1331.2009.02568.x. [DOI] [PubMed] [Google Scholar]
- 43.Toubi E, Etzioni A. Intravenous immunoglobulin in immunodeficiency states: state of the art. Clin Rev Allergy Immunol. 2005;29:167–72. doi: 10.1385/CRIAI:29:3:167. [DOI] [PubMed] [Google Scholar]
- 44.Thomas JR, Yocum RC, Haller MF, et al. Assessing the role of human recombinant hyaluronidase in gravity-driven subcutaneous hydration: the INFUSE-LR study. J Palliat Med. 2007;10:1312–20. doi: 10.1089/jpm.2007.0126. [DOI] [PubMed] [Google Scholar]
- 45.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Exp Opin Drug Deliv. 2007;4:427–40. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 46.Bookbinder LH, Hofer A, Haller MF, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114:230–41. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Olsson O, Lofgren O. Hyaluronidase as a factor hastening the spread and absorption of water-soluble radiopaque substances deposited intracutaneously, subcutaneously, or intramuscularly. Acta Radiol. 1949;31:250–6. doi: 10.3109/00016924909176961. [DOI] [PubMed] [Google Scholar]


