Abstract
The vast majority of brain-injured patients with semantic impairment have better comprehension of concrete than abstract words. In contrast, several patients with semantic dementia (SD), who show circumscribed atrophy of the anterior temporal lobes bilaterally, have been reported to show reverse imageability effects, i.e., relative preservation of abstract knowledge. Although these reports largely concern individual patients, some researchers have recently proposed that superior comprehension of abstract concepts is a characteristic feature of SD. This would imply that the anterior temporal lobes are particularly crucial for processing sensory aspects of semantic knowledge, which are associated with concrete not abstract concepts. However, functional neuroimaging studies of healthy participants do not unequivocally predict reverse imageability effects in SD because the temporal poles sometimes show greater activation for more abstract concepts. We examined a case-series of eleven SD patients on a synonym judgement test that orthogonally varied the frequency and imageability of the items. All patients had higher success rates for more imageable as well as more frequent words, suggesting that (a) the anterior temporal lobes underpin semantic knowledge for both concrete and abstract concepts, (b) more imageable items – perhaps due to their richer multimodal representations – are typically more robust in the face of global semantic degradation and (c) reverse imageability effects are not a characteristic feature of SD.
Keywords: semantic dementia, imageability, concreteness, anterior temporal, synonym judgement
Introduction
How do we represent and process the meanings of concrete and abstract words such as COAT and HOPE? Concrete concepts encapsulate the meanings of tangible things that can be experienced through our senses – consequently, we can readily form mental images for concrete words. Abstract concepts, in contrast, do not refer to physical objects and, for the most part, do not readily evoke mental images: instead these concepts refer to ideas or mental states. In behavioural studies, healthy participants often show faster and more accurate processing for imageable words (DeGroot, 1989; James, 1975; Kroll & Merves, 1986; Paivio, 1991). Patients with brain-damage normally show an exaggeration of this effect – for example, people with aphasia and deep dyslexia typically make many more errors for abstract than concrete items (Coltheart, 1980; Goodglass et al., 1969; Jefferies et al., 2007). However, in a small number of neuropsychological cases, reverse imageability effects have been observed; i.e., relative preservation of abstract knowledge (Breedin et al., 1994; Cipolotti & Warrington, 1995; Reilly et al., 2006; Sirigu et al., 1991; Warrington, 1975; Yi et al., 2007). Most of the patients showing this pattern have had damage to the anterior temporal lobes (ATL) bilaterally, typically due to herpes simplex encephalitis or semantic dementia (Marshall et al., 1996, excepted).
This double dissociation suggests that the cognitive and neural organisation of concrete and abstract concepts may be partially distinct. Concrete items have sensory referents, whereas abstract items do not (Paivio, 1986). Visual and other sensory processes may therefore contribute to semantic knowledge for concrete concepts, resulting in more semantic features/richer semantic representations for these items (Paivio, 1986; Plaut & Shallice, 1993). This notion is supported by the fact that people can generate more predicates for imageable words (Jones, 1985). In contrast, abstract concepts might be more dependent on linguistic processes, given that the meaning of these items is strongly affected by sentence context (e.g., Schwanenflugel & Shoben, 1983).
According to these proposals, reverse imageability effects could result from damage to visual (and possibly other sensory) aspects of semantic knowledge. Consequently, the brain regions damaged in semantic dementia (SD) might play a particularly important role in visual/sensory knowledge of objects. Patients with SD have relatively circumscribed bilateral atrophy of the anterior and inferior aspects of the ATL, and the extent of this atrophy correlates with the severity of the semantic impairment (Mummery et al., 2000; Nestor et al., 2006). This pattern of brain damage results in a highly specific impairment of semantic memory: other aspects of cognition and language such as phonology, visual processing and decision-making remain largely intact (Hodges et al., 1992; Snowden et al., 1989). The semantic impairment in SD affects the full range of input and output modalities – including spoken and written words, pictures, real objects, environmental sounds, smells and touch (Bozeat et al., 2000; Coccia et al., 2004; Luzzi et al., 2007). There is also a significant degree of item-specific consistency when the same items are probed using different semantic tasks (Bozeat et al., 2000; Coughlan & Warrington, 1981). These findings indicate that the semantic impairment in SD is amodal and not specific to either verbal or non-verbal information (Rogers et al., 2004). The anterior temporal lobes are a plausible substrate for forming amodal semantic representations as they have extensive connections with cortical areas that represent modality-specific information (see also the theory of "convergence zones"; A. R. Damasio, 1989; H. Damasio et al., 2004; Gloor, 1997). Accordingly, Rogers et al. (2004) implemented a computational model of the ATL semantic system in which semantic representations were formed through the distillation of information required for mappings between different verbal and non-verbal modalities. When the model was damaged, it reproduced the deficits shown by SD patients across different input and output modalities.
Although patients with SD show generalised semantic degradation, there is also evidence to suggest that they have relatively poor knowledge of sensory attributes compared to functional information (though both are markedly impaired). Patients’ definitions of pictures and words contain more associative/functional content than sensory/physical information (Lambon Ralph et al., 1999; 2003; McCarthy & Warrington, 1988). A similar pattern was found for an individual patient studied by Cardebat et al. (1996) who was unable to draw animals and objects from memory despite producing some functional properties. Moreover, SD patients show poorer definition-to-picture matching when given descriptions that contain sensory rather than functional information (Lambon Ralph et al., 2003). The inferior temporal lobes are thought to underpin the ‘ventral visual stream’, which allows object recognition (Ungerleider & Mishkin, 1982). Given that the focus of atrophy in SD is in anterior, inferior temporal lobes, it is possible that the damaged cortex makes a greater contribution to sensory aspects of semantic knowledge than to functional/associative semantic properties.
If visual/sensory properties are especially vulnerable to damage in SD, we might expect these patients to have more pronounced deficits for imageable than abstract concepts. As noted above, some cases with SD have shown precisely this pattern – i.e., reverse imageability effects in semantic tasks (Breedin et al., 1994; Cipolotti & Warrington, 1995; Papagno et al., 2007; Reilly et al., 2007a; 2006; 2007b; Vesely et al., 2007; Warrington, 1975; Yi et al., 2007). A recent review suggested that better comprehension of abstract than concrete concepts is one of the general features of SD (Grossman & Ash, 2004). It is important to emphasise, however, that reverse imageability effects have been reported in a relatively small number of studies, which have largely examined single cases. It is therefore unclear whether reverse imageability effects are the norm in SD, or whether there is a reporting bias. At least some, though not all, of the reports of patients with reverse imageability effects were accompanied by lesion information implicating the ATL – but this does not establish that ATL lesions predictably produce reverse imageability effects. A recent study did find poorer comprehension of motion verbs compared with cognition verbs in a group of twelve patients with SD, although this effect was not found for nouns in the same definition-to-word matching task (Yi et al., 2007) (see also Reilly et al., 2007a). Moreover, a recent study by Pulvermüller et al. (2008) found poorer performance in a lexical decision task for abstract vs. concrete words in eight out of eleven SD patients. Crutch and Warrington (2006) also found that comprehension of abstract concepts was impaired in SD although frequency-matched abstract and concrete concepts were not compared. Therefore, additional research is needed to establish if reverse imageability effects are widespread in SD.
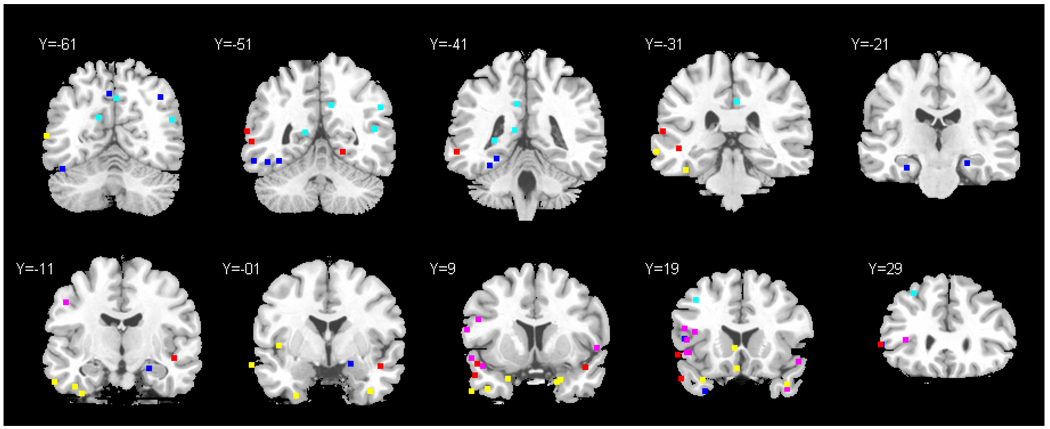
Functional neuroimaging studies of neurologically intact participants also provide relevant evidence about the neural organisation of concrete and abstract concepts. These studies point to considerable overlap in the network representing abstract/imageable words, although some differences have also been observed. Figure 1 shows sites of peak atrophy and hypometabolism in SD (in yellow) together with peak activations from functional neuroimaging studies that directly contrasted concrete (C) and abstract (A) words. This meta-analysis shows that although individual functional neuroimaging studies might be taken as evidence for the importance of the ATL in concrete or abstract concepts, the pattern across studies is inconsistent. Temporal lobe sites showing greater activation for C>A words (in blue/cyan) have almost exclusively been found within occipital, posterior inferotemporal cortex (shown on slices Y=−51 and Y=−41) and medial ATL sites (Y=−21; Y=−11, including one peak in left inferior temporal pole (slice Y=19) (Fiebach & Friederici, 2003; Noppeney & Price, 2002; Sabsevitz et al., 2005; Whatmough et al., 2004; Wise et al., 2000). Meanwhile, sites showing greater activation for A>C words (in red/pink) occurred in more diverse areas linked to language processing, especially left posterior superior temporal areas (including the superior parts of the temporal poles bilaterally; shown on slices Y=9 and Y=19) and left inferior frontal gyrus (Y=9, Y=19) (Binder et al., 2005; Kiehl et al., 1999; Noppeney & Price, 2004; Perani et al., 1999; Sabsevitz et al., 2005; Whatmough et al., 2004). These patterns are broadly consistent with the proposal that concrete concepts are more reliant on occipital-temporal areas that underpin visual object recognition (Ungerleider & Mishkin, 1982), while abstract concepts depend more on brain regions responsible for verbal comprehension (e.g., Scott et al., 2000). However, the functional neuroimaging findings do not unequivocally predict reverse imageability effects in SD. As revealed by Figure 1, SD patients show atrophy and hypometabolism across the ATL, affecting both superior temporal pole areas that might be particularly critical for abstract words and medial ATL regions that might play a greater role in processing concrete words. Moreover, there is considerable overlap in the areas activated by C and A concepts in the ATL. Of the twelve studies reviewed here (see Figure 1 for details), ATL activation (Y>−4) was observed in five studies for A>C and two studies for C>A, suggesting a high level of inconsistency and substantial numbers of null results. From this, we would expect the overwhelming majority of SD patients to show substantial deficits for both concrete and abstract items. Furthermore, given that normal language users tend to reveal a C>A advantage in many tasks, we predict that, as comprehension deteriorates in SD, it will largely maintain this C>A profile. On this account, the reported SD cases showing A>C would be occasional deviations from the typical pattern.
Figure 1. Meta-analysis of functional neuroimaging studies directly comparing concrete and abstract words.
Note: Twelve functional neuroimaging studies supplied the peaks (Binder et al., 2005; Fiebach & Friederici, 2003; Giesbrecht et al., 2004; Grossman et al., 2002; Jessen et al., 2000; Kiehl et al., 1999; Noppeney & Price, 2002; 2004; Perani et al., 1999; Sabsevitz et al., 2005; Whatmough et al., 2004; Wise et al., 2000). Red/Pink = sites showing greater activation for abstract stimuli (pink = lexical decision; red = other tasks, primarily semantic judgements). Blue/cyan = sites showing greater activation for concrete stimuli (cyan = lexical decision; blue = other tasks, primarily semantic judgements). Yellow = sites of peak atrophy (Mummery et al., 2000) and hypometabolism (Nestor et al., 2006) in SD.
The current study examined this issue by assessing the comprehension of concrete and abstract words in a case-series of eleven patients. Our sample should be unaffected by the “reporting bias” discussed above (i.e., the tendency to selectively publish case reports that show reverse concreteness effects), because the patients were selected only on the basis that they had a diagnosis of SD and were available for testing. The patients ranged in severity from mild to severe, allowing an investigation of the relationship between the degree of semantic impairment and the size of any difference between abstract and concrete words. More severely impaired cases might be less likely to reveal a difference in either direction because the atrophy in SD spreads as the disease progresses. We used a synonym judgement task that orthogonally varied the frequency and imageability of the items. Word frequency was manipulated as well as imageability for two reasons. First, this allowed the test to be sensitive to imageability effects in both mild and severely impaired cases (avoiding floor and ceiling effects). Secondly, the frequency findings are of interest in their own right. Although the meanings of frequently encountered words/pictures are reported to be better preserved than less frequent stimuli in SD, this work is limited to picture naming (Lambon Ralph et al., 1998), regression analyses of comprehension tasks (Bozeat et al., 2000) and single case-studies (Funnell, 1995).
As well as addressing these theoretical considerations, this paper also has a practical motivation: we publish a new synonym test which has some advantages over the existing alternatives. (1) The test examines the influence of imageability and frequency at the same time. These factors are varied orthogonally so that interactions between them can be investigated. (2) The test includes substantial variation of both of these variables. (3) Frequency and imageability are manipulated for the response choices as well as for the probe words, increasing the sensitivity of the test to these effects. (4) There are three rather than the usual two response choices per trial, reducing the chance rate from 0.5 to 0.33.
Method
Test construction
There were 96 trials split evenly between two frequency bands (mean frequency of probe words (with standard deviations in parentheses) = 128 (102) and 4.6 (4.5) counts per million in the Celex database; Baayen et al., 1993) and three imageability bands (mean imageability of probe words = 275 (17.3), 452 (26.0) and 622 (14.0) respectively, on a scale of 100–700, from the MRC Psycholinguistic Database; Coltheart, 1981). The frequency ranges of the high/low frequency sets did not overlap, and similarly, the high, medium and low imageability words had non-overlapping imageabilities. Frequency and imageability were varied orthogonally; there were sixteen trials in each of the six frequency-by-imageability conditions. Frequency was matched in triplets across the high, medium and low imageability words, and imageability was matched pairwise for the high and low frequency sets. Target words (i.e., the intended correct choice) were presented alongside two unrelated distracters. Both the targets and distracters were matched to the probe word for frequency and imageability. As a consequence, imageability and frequency were varied in the trial as a whole. The conditions were not matched for word length (average = 5.6, 6.5 and 7.7 letters per word for the high, medium and low imageability conditions respectively). Simultaneous auditory and visual presentation was used and patients indicated their choice by pointing. The test was not timed. The items are provided in supplemental material published online.
Participants
The synonym judgement task was administered to eleven patients with a clinical diagnosis of SD, recruited from Cambridge, Bath or Liverpool, UK. IRB approval was provided by a Multi-Centre Research Ethics Committee (covering patients in Bath and Liverpool) and the Cambridgeshire Research Ethics Committee. The patients fulfilled all of the published criteria for SD (e.g., Hodges et al., 1992): they had word-finding difficulties in the context of fluent speech and showed impaired semantic knowledge and single word comprehension; in contrast, phonology, syntax, visual-spatial abilities and day-to-day memory (assessed informally in conversation) were relatively well preserved. Table 1 provides demographic details and background neuropsychological scores on tasks administered periodically as part of our standard battery of assessments. All of the scores were obtained within a year of the synonym judgement task. MRI revealed focal atrophy of the anterior temporal lobes bilaterally in every case.
Table 1.
Biographical details and background neuropsychological scores
| AN | LS | SJ | WM | EK | ATe | GE | KI | GT | PD | MK | No. items |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 64 | 60 | 59 | 54 | 59 | 66 | 50 | 65 | 70 | 72 | 67 | |
| Sex | M | M | F | F | F | M | M | M | M | F | F | |
| Education (leaving age) | 14 | 18 | 16 | 21 | 15 | 24 | 16 | 14 | 14 | 14 | 17 | |
| Composite semantic score | 1.7 | 1.2 | 0.8 | 0.6 | 0 | −0.2 | −0.4 | −0.5 | −0.5 | −1.4 | −1.4 | |
| Word-picture match | 63 | 63 | 59 | 52 | 46 | 58 | 32 | 36 | 32 | 17 | 11 | 64 |
| Picture naming | 53 | 43 | 29 | 26 | 17 | 10 | 13 | 15 | 11 | 4 | 2 | 64 |
| PPT: Pictures | NT | 49 | 48 | 44 | 35 | 47 | 34 | 31 | 37 | 26 | 33 | 52 |
| PPT: Words | NT | 49 | 42 | 39 | 36 | 44 | 28 | 35 | 32 | 26 | 26 | 52 |
| CCT: Pictures | 49 | 53 | 51 | 52 | 33 | 40 | 32 | 20 | 27 | 17 | 26 | 64 |
| CCT: Words | 55 | 54 | 47 | 30 | 26 | 45 | 27 | 33 | 28 | 24 | NT | 64 |
| Fluency: 8 categories | 60 | 32 | 31 | 30 | 31 | 6 | 22 | 27 | 24 | 7 | 1 | - |
| Fluency: 3 letters | 32 | 7 | 23 | NT | 29 | 8 | 19 | 17 | 24 | 22 | 2 | - |
| Raven's Matrices | 35 | 31 | 34 | 35 | 33 | 32 | 33 | 21 | 35 | 25 | 22 | 36 |
| Digit span forwards | 8 | 8 | 5 | 8 | 6 | 7 | 7 | 8 | 6 | 7 | 5 | - |
| Digit span backward | 6 | 7 | 3 | 5 | 7 | 4 | 4 | 5 | 4 | 5 | 4 | - |
| Rey Figure copy | 36 | 21 | 33 | 36 | 34 | 36 | 35 | 34 | 34 | 36 | 30 | 36 |
| VOSP: Screening | NT | 16 | 20 | NT | 20 | 19 | 20 | 20 | 20 | 19 | 17 | 20 |
| VOSP: Dot counting | NT | 10 | 10 | NT | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| VOSP: Position discrimin | 20 | 20 | 20 | NT | 20 | 20 | 20 | 19 | 20 | 16 | 17 | 20 |
| VOSP: Number location | 10 | 10 | 10 | NT | 10 | 8 | 9 | 10 | 10 | 9 | 6 | 10 |
Patients are arranged in order of composite semantic score, derived from word-picture matching, picture naming, picture CCT and category fluency. PPT = Pyramids and Palm Trees test (Howard & Patterson, 1992). CCT = Camel and Cactus Test, a test of semantic associations for words and pictures, similar to the PPT test but with four choices per trial (Bozeat et al., 2000). Fluency = total number of words produced in one minute, from eight semantic categories and three letters combined. Raven’s Matrices = Coloured Progressive Matrices Test, tapping non-verbal reasoning (Raven, 1962). Digit span from Wechsler Memory Scale (Wechsler, 1987). Rey Figure copy involved copying a complex geometrical figure. VOSP = Visual Object and Space Perception battery (Warrington & James, 1991).
Eleven healthy participants matched in age to the SD group (age range 56 to 65) also completed the synonym judgement task. They had an average of 15.3 years of education. There was no relationship between educational level and synonym judgement performance (for either the controls or the patients). All of the participants (patients and controls) provided written consent.
Results
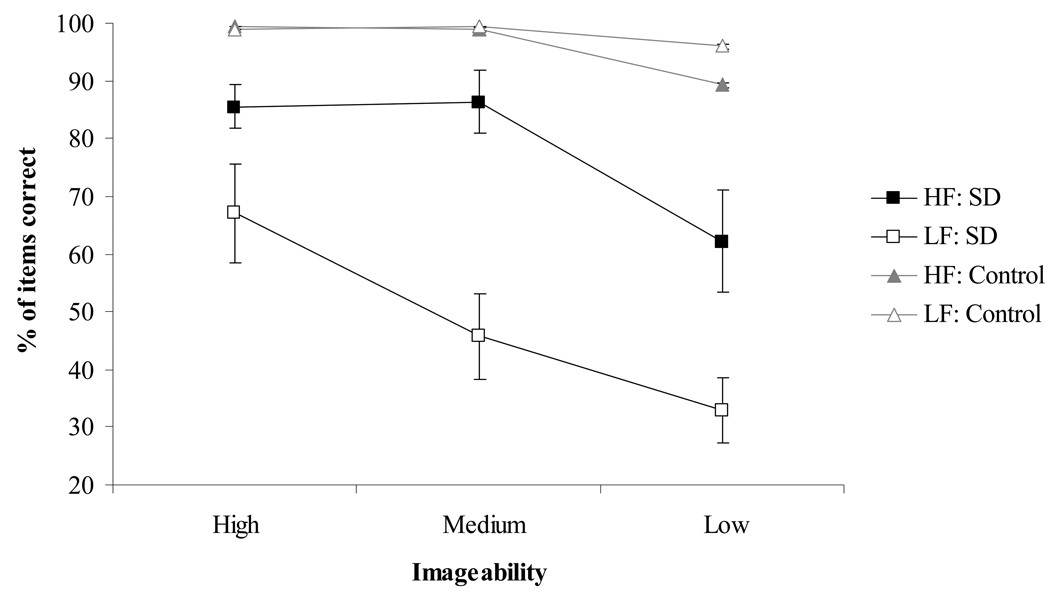
The results of the synonym judgement test are shown in Figure 2. The SD patients performed substantially more poorly than controls in every condition (t(20) = 9.6–2.3, p < .04; Cohen’s d = 1.2–4.2). The control group showed a positive effect of higher imageability (F(2,20) = 7.2, p = .004; partial Eta squared (ηp 2) = .42) although performance was near ceiling on all conditions (there was no main effect of frequency and no interaction). The SD patients’ comprehension showed strong positive effects of both higher imageability (F(2,20) = 25.3, p < .0001; ηp 2 = .72) and higher frequency (F(1,10) = 62.6, p < .0001; ηp 2 = .86). The interaction between these factors approached significance (F(2,20) = 2.9, p = .08; ηp 2 = .22). For high frequency items, medium imageability words were understood better than low imageability words (Bonferroni t(10) = 4.7, p = .004) but there was no advantage for high over medium imageability words (t(10) < 1). For low frequency items, accuracy was significantly greater for high vs. medium imageability words (Bonferroni t(10) = 4.0, p = .01) but the difference between medium and low imageability words did not reach significance (t(10) = 1.9, n.s.). These patterns of significance can be interpreted as follows: (1) Frequent words are understood comparatively well by patients with SD so their performance was only affected by the other variable – imageability – when it reached its lowest value. (2) SD patients are so poor at comprehending low-frequency words that their performance had already dropped to a low level for medium-imageability words; it declined further (essentially to chance) for the lowest value of imageability, but there was no room for this decrease to reach statistical significance.
Figure 2. Performance on the synonym judgement task.
Error bars show standard error of mean
Every individual SD patient showed better comprehension of high than low frequency words (Fisher exact one-tailed p = .06 to < .0001; Cramer’s V = .18–.64; see Table 2). The majority of the SD group also demonstrated significantly better comprehension of the more imageable words (9/11 patients; Fisher exact two-tailed p = .05 to < .0001; Cramer’s V = .24–.47). Two cases, GE and KI, who did not show significant positive effects of imageability were investigated in more detail. GE’s performance was at ceiling for high frequency words and at floor for low frequency words, regardless of imageability. He was therefore tested on an additional set of medium frequency items to avoid floor and ceiling effects. On these items there was a significant positive effect of imageability (high imageability = 15/16 correct; medium imageability = 11/16; low imageability = 7/16; Fisher’s exact two-tailed p = .01; Cramer’s V = .44). The other exception was patient KI, who showed only a non-significant trend towards better performance for more imageable items on the original test. When KI was retested approximately a month later, his performance revealed a clear imageability advantage (high imageability = 27/32 correct; medium imageability = 19/32; low imageability = 14/32 collapsing across frequency; Fisher’s exact twotailed p = .003). The imageability effect remained significant when the data from the two test sessions were combined (p = .02; Cramer’s V = .20). We can therefore conclude that all eleven patients showed better comprehension of more imageable words. Reverse imageability effects were absent from the group.
Table 2.
Synonym judgement results for individual SD patients
| AN | LS | SJ | WM | EK | ATe | GE | KI | GT | PD | MK | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HI, HF | 93.8 | 62.5 | 100 | 100 | 93.8 | 75.0 | 93.8 | 90.6 | 81.3 | 75.0 | 75.0 |
| HI, LF | 100 | 87.5 | 100 | 93.8 | 87.5 | 18.8 | 43.8 | 56.3 | 62.5 | 56.3 | 31.3 |
| MI, HF | 100 | 93.8 | 100 | 100 | 100 | 50.0 | 100 | 75.0 | 87.5 | 87.5 | 56.3 |
| MI, LF | 56.3 | 62.5 | 87.5 | 62.5 | 43.8 | 12.5 | 37.5 | 34.4 | 43.8 | 62.5 | 0 |
| LI, HF | 93.8 | 68.8 | 81.3 | 93.8 | 62.5 | 6.3 | 93.8 | 65.6 | 43.8 | 56.3 | 18.8 |
| LI, LF | 62.5 | 25.0 | 43.8 | 56.3 | 37.5 | 0 | 25.0 | 37.5 | 37.5 | 31.3 | 6.3 |
| HF average | 95.8 | 75.0 | 93.8 | 97.9 | 85.4 | 43.8 | 95.8 | 77.1 | 70.8 | 72.9 | 50.0 |
| LF average | 72.9 | 58.3 | 77.1 | 70.8 | 56.3 | 10.4 | 35.4 | 42.7 | 47.9 | 50.0 | 12.5 |
| HI average | 96.9 | 75.0 | 100 | 96.9 | 90.6 | 46.9 | 68.8 | 73.4 | 71.9 | 65.6 | 53.1 |
| MI average | 78.1 | 78.1 | 93.8 | 81.3 | 71.9 | 31.3 | 68.8 | 54.7 | 65.6 | 75.0 | 28.1 |
| LI average | 78.1 | 46.9 | 62.5 | 75.0 | 50.0 | 3.1 | 59.4 | 51.6 | 40.6 | 43.8 | 12.5 |
| Total correct | 84.4 | 66.7 | 85.4 | 84.4 | 70.8 | 27.1 | 65.6 | 59.9 | 59.4 | 61.5 | 31.3 |
Table shows percentage of items that were correct in each condition. Patients are arranged in order of composite semantic score. HI = high imageability; MI = medium imageability; LI = low imageability; HF = high frequency; LF = low frequency.
To examine whether comprehension differences between abstract and concrete words varied with the severity of SD, a composite semantic score was derived from the background semantic tests available for all cases (picture naming, word-picture matching, Pyramids and Palm Trees test for pictures and category fluency; see Table 1). This showed a significant positive correlation with overall synonym judgement performance (r = .66, one-tailed p = .01) and with five of the six individual frequency/imageability conditions (r = .54 to .76, one-tailed p < .05); the one exception was the high frequency, high imageability condition which was prone to ceiling effects. There was no relationship between the severity of the semantic impairment and the size of frequency/imageability effects (as measured by the difference between these conditions). In addition, there was no correlation between educational level and any aspect of synonym judgement performance in SD – including accuracy in each condition and the magnitude of frequency/imageability effects.
Discussion
This study examined the impact of word frequency and imageability on synonym judgement in a case-series of eleven patients with semantic dementia (SD). Every case showed significantly better comprehension of high than low imageability words, along with more intact understanding of high compared with low frequency words. We did not observe a single instance of the reverse imageability effect reported previously for a few individual SD patients. In addition, there was no relationship between the degree of semantic impairment and the size of the imageability effect. Although the majority of patients showing relative preservation of abstract concepts to date have had damage to the ATL bilaterally, either in the context of SD or herpes simplex encephalitis, these studies have mostly examined single cases that were presumably selected because of the interesting nature of their semantic impairment. Investigations of single cases cannot resolve the question of whether ATL lesions consistently produce reverse imageability effects. Our case-series study of eleven patients indicates that, contrary to the suggestion that reverse imageability effects may be the norm in SD (Grossman & Ash, 2004), the typical pattern in SD is an advantage for concrete or high-imageability concepts. The impression of an association between SD and reverse imageability effects in the literature is likely to result from a reporting bias.
The marked frequency effect observed here fits with all known research on SD; it is mainly noteworthy because it is perhaps the clearest demonstration so far of the impact of this variable on performance in a receptive task, rather than the expressive tasks (such as object naming, reading, past-tense verb generation, etc) in which frequency effects have been amply documented in SD (e.g., Funnell, 1995; Lambon Ralph et al., 1998; Patterson et al., 2006; Woollams et al., 2007). More frequently encountered items are thought to form stronger semantic representations than less frequent ones, making them less vulnerable to degradation in SD (Rogers & McClelland, 2004). Other factors might also contribute to this effect, however; frequent concepts are typically acquired at an earlier age and continue to be encountered regularly as the semantic system degrades. This continued exposure may also afford them some protection from degradation in SD (see Lambon Ralph et al., 1998).
The marked positive imageability effect is more newsworthy because it clearly contradicts previous suggestions that reverse effects of imageability are the norm in this group. Our findings are inconsistent with the idea that the ATL is strongly specialised for visual aspects of knowledge. Instead, this brain region appears to underpin a single semantic store that is critical for understanding all types of stimuli, both concrete and abstract. Abstract words might have fewer semantic features and/or more impoverished semantic representations than imageable words (Paivio, 1986; Plaut & Shallice, 1993). Healthy participants are able to generate more predicates for imageable words, suggesting that these items have richer semantic representations (Jones, 1985). Even the control participants in this study revealed some benefit in choosing synonyms for high-imageability words. The outcome for the SD patients was therefore just an extension of the normal pattern. If the idea of richer, more detailed representations for concrete concepts is plausible, then it is also plausible that – as semantic memory deteriorates – the amount of information necessary to perform the forced-choice synonym judgement task will drop below ‘threshold’ sooner for abstract than for concrete concepts.
Our finding that SD patients were impaired at both concrete and abstract concepts is broadly consistent with neuroimaging studies that have found overlapping activation for these items within the ATL. It is important to note, however, that patient and neuroimaging studies provide rather different information about the neural basis of conceptual knowledge. The current neuropsychological investigation suggests that the ATL plays a critical role in abstract as well as concrete knowledge; however, we cannot rule out the possibility of functionally dissociable regions within this region (e.g., medial vs. superior ATL for concrete and abstract concepts respectively). Moreover, functional neuroimaging studies show widespread and partially distinct areas of brain activation for concrete and abstract items beyond the ATL, indicating that the wider neural networks that support these functions may be different. Transcranial magnetic stimulation (TMS) in healthy volunteers may provide a means of establishing which specific areas (a) within ATL and (b) beyond ATL are critical for understanding abstract and concrete concepts (Pobric et al., submitted).
Given our findings, how is one to understand the published reports of an abstractness advantage in a few patients with ATL lesions? There are at least two possibilities. First, whilst the temporal pole forms amodal representations of concepts by interacting with modality-specific areas devoted to sights, sounds, words, smells, touch etc., there might be some specialisation in areas of the temporal lobe as these inputs come together. The meta-analysis of neuroimaging studies shown in Figure 1 partially supports this view. There are peak activations for the abstract > concrete contrast all the way along the superior aspects of the temporal lobes. Previous research has shown that superior temporal cortex underpins speech comprehension, with more anterior areas responding only to intelligible speech and posterior areas uninfluenced by intelligibility (Crinion et al., 2003; Davis & Johnsrude, 2003; Narain et al., 2003; Scott et al., 2000). Superior temporal cortex might show greater activation for abstract words, at least in some studies, because these stimuli are highly reliant on this verbal comprehension pathway. In contrast, temporal lobe peaks for the concrete > abstract comparison fall within inferior and medial temporal cortex, amongst other areas. Similar regions are activated by visual object recognition (Kellenbach et al., 2005; Stewart et al., 2001), mental imagery (D'Esposito et al., 1997) and picture-based semantic tasks (Adams & Janata, 2002; Bright et al., 2004; Vandenberghe et al., 1996). One possibility, therefore, is that reverse concreteness effects occur in patients with an unusual distribution of ATL atrophy – for example, in cases with relative sparing of superior aspects of the ATL despite pronounced damage to medial temporal structures, or following the spread of atrophy to more posterior areas of inferior temporal cortex (especially in cases with only a mild degree of ATL atrophy). Further comparative studies of patients with different distributions of temporal lobe damage are required to test this atrophy-distribution hypothesis.
A second possibility is that individual differences in education, interests and experiences may substantially change the relative frequency with which concrete and abstract words are encountered and produced by patients premorbidly and/or during the course of the disease. At least some of the SD cases who have shown reverse concreteness effects have been highly educated; for example, patient DM studied by Breedin et al. (1994) was a professional with a master’s degree and patient AB examined by Warrington (1975) was a high-ranking civil servant. These individuals might have had greater familiarity with less frequent abstract words, protecting these concepts to some degree from the effects of semantic degradation. Against this hypothesis, there was no clear relationship between educational level and the size of the imageability effect in the current study. We have demonstrated that high levels of education are not always accompanied by reverse concreteness effects (one of the patients showing the standard pattern here had obtained a PhD). Nevertheless, the number of years spent in education is at best a crude measure of individual differences in premorbid exposure to abstract and concrete vocabulary. In addition, continued use of abstract and concrete words later in life might be a more critical factor. Very little is currently known about the fate of general vs. specialised, expert knowledge in SD – therefore an interesting question for further research is the extent to individual differences in education or ongoing experiences affect the profile of semantic degradation across different categories of knowledge.
The main contribution of the current study is to show that these individual cases with reverse imageability effects are not representative of SD more generally. Instead, every patient in our case-series study showed poorer comprehension of abstract than concrete words, suggesting that the ATL semantic system underpins the meanings of both imageable and abstract concepts.
Supplementary Material
Acknowledgements
We are indebted to the patients and their carers for their generous assistance with this study and to John Hodges and Mark Doran for referring some of the patients to us. We would also like to thank Tim Rogers and Paul Hoffman for useful discussions about these data. The work was supported by a grant from the NIMH (MH64445), an RCUK fellowship awarded to E. Jefferies and an MRC programme grant (G0501632).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
References
- Adams RB, Janata P. A comparison of neural circuits underlying auditory and visual object categorization. NeuroImage. 2002;16:361–377. doi: 10.1006/nimg.2002.1088. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX Lexical Database [CD-ROM] Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1993. [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of the concreteness effect in a patient with semantic dementia. Cognitive Neuropsychology. 1994;11:617–660. [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs. multiple semantics: PET studies of word and picture processing. Brain and Language. 2004;89:417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Cardebat D, Demonet JF, Celsis P, Puel M. Living/nonliving dissociation in a case of semantic dementia: A SPECT activation study. Neuropsychologia. 1996;34:1175–1179. doi: 10.1016/0028-3932(96)00040-1. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Warrington EK. Semantic memory and reading abilities: A case report. Journal of the International Neuropsychological Society. 1995;1:104–110. doi: 10.1017/s1355617700000163. [DOI] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA. Semantic memory is an amodal, dynamic system: Evidence from the interaction of naming and object use in semantic dementia. Cognitive Neuropsychology. 2004;21:513–527. doi: 10.1080/02643290342000113. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Deep dyslexia: A review of the syndrome. In: Coltheart M, Patterson K, Marshall JC, editors. Deep Dyslexia. London: Routledge and Kegan Paul; 1980. [Google Scholar]
- Coltheart M. The MRC Psycholinguistic Database. Quarterly Journal of Experimental Psychology. 1981;33A:497–505. [Google Scholar]
- Coughlan AK, Warrington EK. The impairment of verbal semantic memory: A single case study. Journal of Neurology, Neurosurgery and Psychiatry. 1981;44:1079–1083. doi: 10.1136/jnnp.44.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Lambon Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. Partial knowledge of abstract words in patients with cortical degenerative conditions. Neuropsychology. 2006;20:482–489. doi: 10.1037/0894-4105.20.4.482. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippett LJ, Farah MJ. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Computation. 1989;1:123–132. [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. Journal of Neuroscience. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot AMB. Representational aspects of word imageability and word frequency as assessed through word association. Journal of Experimental Psychology: Learning, Memory and Cognition. 1989;15:824–845. [Google Scholar]
- Fiebach CJ, Friederici AD. Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia. 2003;42:62–70. doi: 10.1016/s0028-3932(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Funnell E. Objects and properties: A study of the breakdown of semantic memory. Memory. 1995;3:497–518. doi: 10.1080/09658219508253162. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Camblin CC, Swaab TY. Separable effects of semantic priming and imageability on word processing in human cortex. Cerebral Cortex. 2004;14:521–529. doi: 10.1093/cercor/bhh014. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and the Limbic System. Oxford: Oxford University Press; 1997. [Google Scholar]
- Goodglass H, Hyde MR, Blumstein S. Frequency, picturability and availability of nouns in aphasia. Cortex. 1969;5:104–119. doi: 10.1016/s0010-9452(69)80022-5. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary Progressive Aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. The neural basis for category-specific knowledge: An fMRI study. NeuroImage. 2002;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic Dementia: Progressive fluent aphasia with temporal-lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and Palm Trees: A test of semantic access from pictures and words. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1992. [Google Scholar]
- James CT. The role of semantic information in lexical decisions. Journal of Experimental Psychology. 1975;104:130–136. [Google Scholar]
- Jefferies E, Baker SS, Doran M, Lambon Ralph MA. Refractory effects in stroke aphasia: A consequence of poor semantic control. Neuropsychologia. 2007;45:1065–1079. doi: 10.1016/j.neuropsychologia.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Jessen F, Heun R, Erb M, Granath D-O, Klose U, Papassotiropoulos A, Grodd W. The concreteness effect: Evidence for dual coding and context availability. Brain and Language. 2000;74:103–112. doi: 10.1006/brln.2000.2340. [DOI] [PubMed] [Google Scholar]
- Jones GV. Deep dyslexia, imageability, and ease of predication. Brain and Language. 1985;24:1–19. doi: 10.1016/0093-934x(85)90094-x. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Hovius M, Patterson K. A PET study of visual and semantic knowledge about objects. Cortex. 2005;41:121–132. doi: 10.1016/s0010-9452(08)70887-6. [DOI] [PubMed] [Google Scholar]
- Kiehl K, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD. Neural pathways involved in the processing of concrete and abstract words. Human Brain Mapping. 1999;7:225–233. doi: 10.1002/(SICI)1097-0193(1999)7:4<225::AID-HBM1>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Merves JS. Lexical access for concrete and abstract words. Journal of Experimental Psychology. 1986;12:92–107. [Google Scholar]
- Lambon Ralph MA, Graham KS, Ellis AW, Hodges JR. Naming in semantic dementia - what matters? Neuropsychologia. 1998;36:775–784. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Patterson K, Hodges JR. Is a picture worth a thousand words? Evidence from concept definitions by patients with semantic dementia. Brain and Language. 1999;70:309–335. doi: 10.1006/brln.1999.2143. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Semantic dementia with category specificity: A comparative case-series study. Cognitive Neuropsychology. 2003;20:307–326. doi: 10.1080/02643290244000301. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Ralph MAL. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Marshall J, Pring T, Chiat S, Robson J. Calling a salad a federation: An investigation of semantic jargon. Part 1: Nouns. Journal of Neurolinguistics. 1996;9:237–250. [Google Scholar]
- McCarthy RA, Warrington EK. Evidence for modality-specific meaning in the brain. Nature. 1988;334:428–430. doi: 10.1038/334428a0. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Narain C, Scott SK, Wise RJS, Rosen S, Leff A, Iversen SD, Matthews PM. Defining a left-lateralized response specific to intelligible speech using fMRI. Cerebral Cortex. 2003;13:1362–1368. doi: 10.1093/cercor/bhg083. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer's disease and semantic dementia. NeuroImage. 2006;30:1010–1020. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of visual, auditory and abstract semantics. NeuroImage. 2002;15:917–926. doi: 10.1006/nimg.2001.1016. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of abstract semantics. NeuroImage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Paivio A. Mental Representations: A Dual Coding Approach. Oxford: Oxford University Press; 1986. [Google Scholar]
- Paivio A. Dual coding theory: Retrospect and current status. Canadian Journal of Psychology. 1991;45:255–287. [Google Scholar]
- Papagno C, Capasso R, Zerboni H, Miceli G. A reverse concreteness effect in a subject with semantic dementia. Brain and Language. 2007;103:90–91. [Google Scholar]
- Patterson K, Lambon Ralph MA, Jefferies E, Woolams A, Jones R, Hodges J, Rogers TT. ‘Pre-semantic’ cognition in Semantic Dementia: Six deficits in search of an explanation. Journal of Cognitive Neuroscience. 2006;18:169–183. doi: 10.1162/089892906775783714. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti C, Gorno-Tempini M, Cappa SF, Fazio F. Word and picture matching: a PET study of semantic category effects. Neuropsychologia. 1999;37:293–306. doi: 10.1016/s0028-3932(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Shallice T. Deep dyslexia: A case-study of connectionist neuropsychology. Cognitive Neuropsychology. 1993;10:377–500. [Google Scholar]
- Pobric G, Lambon Ralph MA, Jefferies E. The role of the anterior temporal lobes in the comprehension of concrete and abstract words: rTMS evidence. doi: 10.1016/j.cortex.2009.02.006. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Pye E, Dine C, Hauk O, Nestor P, Patterson K. Word category deficits in semantic dementia; Paper presented at the Cognitive Neuroscience Society 2008 Annual Meeting.2008. [Google Scholar]
- Raven JC. Coloured progressive matrices sets A, AB, B. London: H. K. Lewis; 1962. [Google Scholar]
- Reilly J, Cross K, Troiani V, Grossman M. Single-word semantic judgements in semantic dementia: Do phonology and grammatical class count? Aphasiology. 2007a;21:558–569. [Google Scholar]
- Reilly J, Grossman M, McCawley MC. Concreteness effects in lexical processing of semantic dementia. Brain and Language. 2006;99:157–158. [Google Scholar]
- Reilly J, Peelle JE, Grossman M. A unitary semantics account of reverse concreteness effects in semantic dementia. Brain and Language. 2007b;103:86–87. [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. The structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Semantic cognition: A parallel distributed processing approach. Cambridge Massachusetts: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. NeuroImage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Schwanenflugel PJ, Shoben EJ. Differential context effects in the comprehension of abstract and concrete verbal materials. Journal of Experimental Psychology: Learning, Memory and Cognition. 1983;9:82–102. [Google Scholar]
- Scott SK, Blank SC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Poncet M. The role of sensorimotor experience in object recognition: A case of multimodal agnosia. Brain. 1991;114:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2:167–182. [Google Scholar]
- Stewart L, Meyer BU, Frith U, Rothwell U. Left posterior BA37 is involved in object recognition: a TMS study. Neuropsychologia. 2001;39:1–6. doi: 10.1016/s0028-3932(00)00084-1. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behaviour. Cambridge, MA: MIT Press; 1982. [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Vesely L, Bonner MF, Reilly J, Grossman M. Free association in semantic dementia: The importance of being abstract. Brain and Language. 2007;103:154–155. [Google Scholar]
- Warrington EK. Selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception battery. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised (WMS-R) New York: Psychological Corporation; 1987. [Google Scholar]
- Whatmough C, Verret L, Fung D, Chertkow H. Common and contrasting areas of activation for abstract and concrete concepts: An H215O PET study. Journal of Cognitive Neuroscience. 2004;16:1211–1226. doi: 10.1162/0898929041920540. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Howard D, Mummery CJ, Fletcher P, Leff A, Buchel C, Scott SK. Noun imageability and the temporal lobes. Neuropsychologia. 2000;38:985–994. doi: 10.1016/s0028-3932(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Lambon Ralph MA, Plaut DC, Patterson K. SD-squared: On the association between semantic dementia and surface dyslexia. Psychological Review. 2007;114:316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- Yi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in patients with semantic dementia. Neuropsychology. 2007;21:9–19. doi: 10.1037/0894-4105.21.1.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.