Abstract
Objective
From June 2003 through October 2004, the Food and Drug Administration (FDA) released five safety warnings related to antidepressant use and increased suicide risk in children. While researchers have documented a decline in antidepressant use in children over this time period, less is known about whether specific safety information conveyed in individual warnings was reflected in treatment patterns.
Methods
Thomson Marketscan claims data (2001–2005) for a national sample of privately insured children were used to construct treatment episodes (N=23,529). For each new episode of major depressive disorder, it was determined whether children’s treatment followed specific recommendations included in warnings released by the FDA. Treatment recommendations pertained to the use of the antidepressants paroxetine and fluoxetine and to patient monitoring. Treatment patterns were expected to change as the nature of risk information conveyed by the FDA changed over time.
Results
The timing of FDA recommendations was associated with trends in the use of paroxetine and fluoxetine by children with major depressive disorder newly initiating antidepressant treatment. However, no evidence of increases in outpatient visits (i.e., monitoring) among depressed children initiating antidepressants was found.
Conclusions
Release of specific risk and benefit information by the FDA was associated with changes in prescribing, but not outpatient follow-up. These results suggest the FDA plays an important role in communicating information to the public and providers, but while public health safety warnings were associated with changes in some practice patterns, not all recommendations conveyed in warnings were followed.
Introduction
From June 2003 through October 2004, the Food and Drug Administration (FDA) released five separate safety warnings related to antidepressant use and increased suicide risk in children. These FDA actions elicited controversy reflecting the fundamental trade-offs associated with weighing risks and benefits under conditions of scientific uncertainty. Supporters of FDA action argued that the evidence of elevated risks of suicidality linked to use of antidepressants in youth was sufficiently serious to warrant informing providers and consumers. Critics countered that FDA action would significantly reduce the use of an effective treatment for depression (1), thereby producing poorer mental health outcomes (including some upward pressure on the risk of suicide) in an underserved population (2). Researchers documented declines in antidepressant use in children and adolescents of 20 to 30 percent by 2005 (3–8), and there has been a concurrent increase in the national adolescent suicide rate, although no causal association has been documented (9).
Yet, little is still known about whether specific safety information conveyed through these FDA warnings was associated with changes in treatment patterns. This paper differs from prior work in that it examines changes in treatment patterns in the periods after each individual warning, to determine whether specific information contained in these warnings was associated with relevant treatment changes. Understanding how inclusion of specific health information in FDA warnings affects treatment patterns is important because adherence to these safety recommendations could alter the risk-benefit profile of pediatric antidepressant use.
We examine three research questions related to whether specific information contained in FDA safety warnings translated into changes in treatment patterns for children treated for major depressive disorder:
Did the use of paroxetine (either generic or under brand name Paxil) decline after risks in treating children were disclosed in an FDA warning?
Did the use of fluoxetine (either generic or under brand name Prozac) increase after specific benefits in treating children were mentioned in FDA warnings?
Were depressed children who were prescribed antidepressants more likely to have two or more outpatient visits in the first 30 days after initial drug treatment after the recommendation of monitoring was mentioned in FDA warnings?
FDA Public Releases on Suicidality and Antidepressant Use
Over the FDA’s 18-month investigation of pediatric use of antidepressants, the agency issued five separate safety warnings and the content of these public releases shifted as new information surfaced.
The initial safety warning was released on June 19, 2003, shortly after the initial disclosure by GSK of clinical trial evidence of increased risk of suicidality in pediatric patients. This statement focused exclusively on the risks associated with pediatric use of Paxil/paroxetine, recommending that “Paxil not be used in children under the age of 18 for the treatment of major depressive disorder.” In this one-page statement, the FDA described three failed controlled clinical trials on pediatric patients, emphasizing there was “no evidence that Paxil is effective in children or adolescents with major depressive disorder” and that Paxil is “not currently approved for use in children and adolescents.” No risks associated with other medications in the antidepressant class were mentioned.
With its second public release on October 27, 2003, a health advisory directed at physicians and other health professionals, the scope of the FDA’s recommendations changed. The FDA alerted clinicians to reports of “suicidality (both suicidal ideation and suicide attempts) in clinical trials of various antidepressant drugs in pediatric patients with major depressive disorder.” The agency noted that it had completed a preliminary review of evidence on 8 antidepressants and determined that additional data and analysis were needed. The FDA stated that the data did not clearly establish an association between the pediatric use of the antidepressants and increased suicidal thoughts or actions, but that it was “not possible at this point to rule out an increased risk of these adverse events in any of these drugs.” The October 2003 warning included two new pieces of information. First, the agency reported that of the drugs evaluated, only fluoxetine showed effectiveness in treating major depressive disorder in children. Second, the advisory noted that “close supervision of high-risk patients should accompany initial drug therapy.”
On March 22, 2004, the FDA issued a third public release. This advisory expanded the focus to 10 antidepressant drugs and required pharmaceutical manufacturers to include a warning statement on their product labeling. Like the prior warning, the March advisory included information on the efficacy of fluoxetine in treating children, and more explicitly emphasized the importance of monitoring.
The fourth public statement by the FDA was released on September 16, 2004 directly following a meeting of the FDA’s Psychopharmacological Drugs and Pediatric Advisory Committees. The committees were presented with results from an FDA-sponsored meta-analysis that found the rate of suicidality among children assigned to receive SSRIs was twice that of the placebo group. This evidence led to a 15 to 8 decision by committee members to recommend a black box warning related to increased risk of suicidality in pediatric patients for all antidepressant drugs. The FDA’s September 16 statement noted that the agency had begun “working expeditiously to adopt new labeling to enhance warnings associated with use of antidepressants.”
On October 15, 2004, the FDA issued a fifth public release. This public health advisory directed manufacturers of all antidepressants to revise product labeling to include a black box warning on the increased risk of suicidality in children and adolescents. This warning applied to 36 drugs including SSRI-class drugs, tricyclic antidepressants and MAOIs. The advisory included recommendations related to the frequency of psychotherapy/medication management visits. The labeling change request explicitly stated, for the first time, that “ideally, such observation would include at least weekly face-to-face contact with patients or their family members or caregivers during the first four weeks of treatment.”
Evidence on Consequences of FDA Actions
Several studies have examined changes in U.S. pediatric antidepressant prescribing rates in response to evidence of suicidality risk. Considering national pharmacy data, two early analyses found 20–22 percent declines in antidepressant use in children and adolescents in 2004, before the issuance of the black box warning (6,7). These first two analyses were not published in the peer-reviewed literature, but rather were conducted by news organizations and published in the lay press. Gibbons et al. examined yearly SSRI prescription rates for children up to age 14, and found overall SSRI use declined approximately 20 percent from 2003 to 2005, while new SSRI prescriptions declined 30 percent over this same time period (8). Libby et al. analyzed treatment episodes for depression using outpatient and prescription drug claims and found rates of diagnosed new episodes of pediatric depression declined sharply (3). Considering the October 2003 FDA advisory as the policy action of interest, this study concluded there was a 58 percent drop in use of SSRIs in children diagnosed with depression from the level to be expected if prescribing trends begun prior to this controversy had continued; the drop for adults was smaller (33 percent) (3,10). They found no increase in use of psychotherapy or provider contact in the first three months of treatment (11). Finally, another study carefully considered the timing of the decline in antidepressant use, and concluded the decline began in February 2004, but leveled off by July 2004, with no further changes in prescribing patterns (4). While these studies suggest that FDA actions led to substantial declines in pediatric antidepressant use, less information is available on whether specific recommendations conveyed in FDA safety warnings were associated with changes in treatment patterns.
Research Design and Methods
Thomson’s MarketScan database (2001–2005), which contains claims information for a national sample of more than 2.6 million enrollees, was used to conduct this analysis. Using outpatient claims, we identified all children (age 5–17) diagnosed with major depression from May 2001 through November 2005. To classify major depressive disorder diagnoses, we used ICD-9 codes 300.4 (neurotic depression), 296.2 (major depressive disorder, single episode), 296.3 (major depressive disorder, recurrent episode) and 311 (depression not otherwise specified). For these children, all outpatient drug prescriptions for antidepressants were considered. We used dates on claims and follow prior research (12,13,14) in constructing treatment episodes. We defined an episode of depression as new if a diagnosis is preceded by a period of at least 120 days of no treatment or diagnosis. The start date of the episode is the first day of major depressive disorder diagnosis. We considered care received within the first 30 days of initial diagnosis (for drug treatments) or 30 days of initiating drug treatment (for monitoring). To ensure we witnessed the full ‘wash-out’ period, episodes begun in the first 120 days of 2001 were omitted. We excluded episodes that begin in the last 60 days of 2005 (the last year of our data) to ensure we witnessed all services associated with treatment initiation, including 30 days post drug treatment initiation (we only examined monitoring when the drug was prescribed within the first 30 days of an episode). Approximately 8 percent of individuals experienced more than one episode of care. We included all episodes in analyses, due to concern that omitting later episodes would bias results, and adjust our standard errors to account for the lack of independence between repeat episodes. We also excluded individuals with any diagnosis of bipolar disorder over the time period studied. Using this algorithm, we identified 22,689 new episodes of treatment.
Outcome variables
We created four indicator variables noting whether the treatment episode included: 1) any antidepressant drug within 30 days of diagnosis, 2) a paroxetine prescription, conditional on treatment with an antidepressant within 30 days of diagnosis, 3) a fluoxetine prescription, conditional on treatment with an antidepressant within 30 days of diagnosis, and 4) at least 2 outpatient visits within 30 days of initial drug treatment, conditional on treatment with an antidepressant within 30 days of diagnosis. To determine receipt of specific drugs, we relied on the NDC codes present on pharmacy claims. For outpatient visits, we considered all procedure codes related to either outpatient visits or medication management, because monitoring could occur in either type of visit (CPT codes: 90801, 90802, 90804-90815 and 90862). To ensure the outpatient visit was related to the mental health diagnosis, only visits with mental health diagnoses were considered. In a robustness check, we identified additional evaluation and management visits that may occur in primary care (CPT codes: 99201-99205; 99211-99215), and did not require a mental health diagnosis for these visits.
Statistical methods
We ran logistic regressions, including dummy variables for five distinct time periods: (1) prior to first FDA action on use of the antidepressant paroxetine for pediatric patients (5/01/01-6/18/03), (2) the period between the release of the first and second FDA safety warnings (6/19/03-10/26/03), (3) the period between the second and third FDA safety warnings (10/27/03-3/21/04), (4) the period between the third and fourth FDA safety warnings (3/22/04- 9/13/04), and (5) the period after the fourth FDA safety warnings (9/14/04– 10/30/05). We did not consider the announcement of the BBW in October 2004 as a separate time period, because it was very close in time to the FDA advisory in September 2004. We categorized episodes based on the initial date of diagnosis. We also controlled for age, gender and four regions. For the ‘any antidepressant drug’ outcome, we also controlled for season in which the episode began.
We used regression estimates to determine the adjusted probability of each outcome during each time period. To determine the effects on the original scale, we used the method of recycled predictions, calculating the adjusted probability of the relevant outcome for each individual in our sample, assuming the individual was treated in each of our relevant time periods (15). For each time period, we tested whether there was a significant change in prescribing or treatment from the preceding time period by testing the equality of coefficients using a Wald test. This research was exempt from IRB due to Exemption 45 CFR 46.101(b)(4).
Results
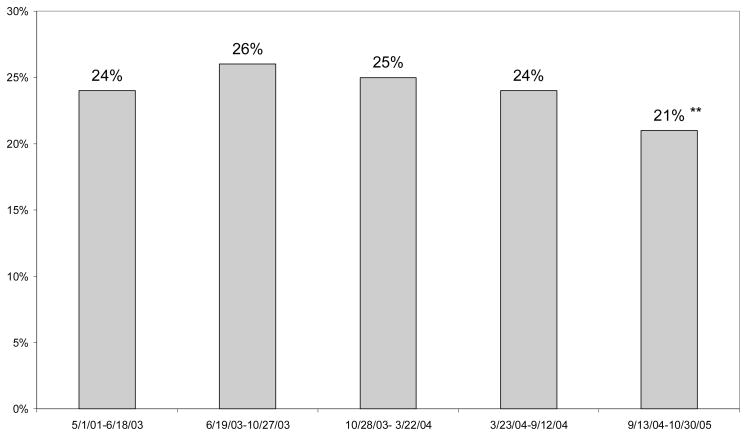
We found significant declines in use of antidepressant drugs after the September 2004 Committee meeting (Figure 1). For children diagnosed with major depression, antidepressant use peaked in the period beginning October 2003, and we observed a 24 percent decline from this peak (26 versus 21 percent). This is in the range of the decline found in other studies.
Figure 1. Receipt of Antidepressant within 30 Days of Diagnois Among Children Diagnosed with Major Depressive Disorder.
Note: The first FDA warning was issued June 18, 2003.
Significance tests indicate whether probability is different from the preceding time period.
***p<.01; **p<.05;
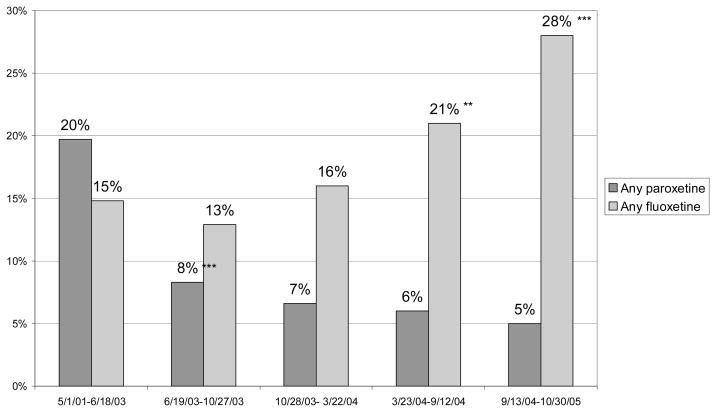
We next examined receipt of paroxetine. Because the first FDA safety warning only noted a suicide risk associated with use of paroxetine (and not other antidepressants), we expected providers to be more likely to choose an antidepressant other than paroxetine for children newly diagnosed with depression after this warning. In the period after this first warning, paroxetine use declined dramatically from 20 to 8 percent (p<.01) (Figure 2). Rates of paroxetine use then remained low, although approximately five percent of new episodes of childhood depression were treated with paroxetine through 2005.
Figure 2. Receipt of Paroxetine or Fluoxetine within 30 days of Diagnosis Among Children Diagnosed with Major Depressive Disorder Treated with Antidepressant Drugs.
Significance tests indicate whether probability is different from use of the same drug in the preceding time period.
***p<.01; **p<.05;
Next, we examined changes in the share of children receiving fluoxetine. Conditional on being treated with an antidepressant, we found that use of fluoxetine increased after the October 2003 warning, which first noted the benefits of fluoxetine, from 13 percent to 16 percent, although this change was not statistically significant (Figure 2). The use of fluoxetine continued to increase, with a statistically significant increase to 21 percent of episodes treated with fluoxetine in the following period, and 28 percent of children being treated with fluoxetine by the last period studied.
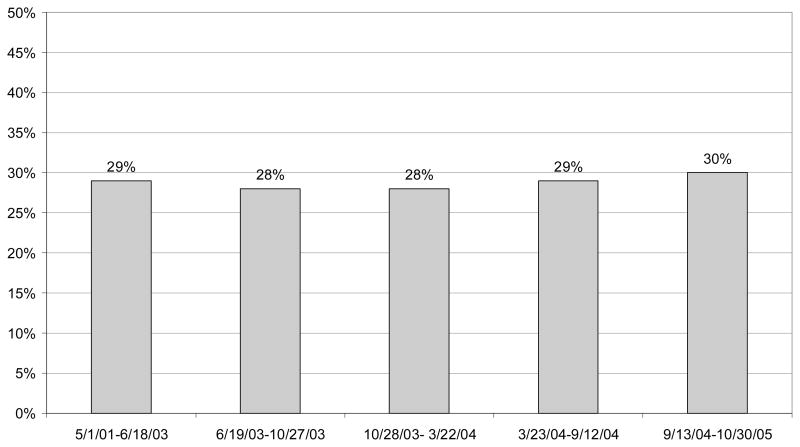
Finally, we found no significant change in the use of monitoring, conditional on filling a prescription for an antidepressant (Figure 3). Because some monitoring may occur in primary care, we used evaluation and management CPT codes to identify additional opportunities for monitoring. This outcome measure also indicated no significant change in the use of monitoring.
Figure 3. Receipt of at Least Two Outpatient Visits within 30 days of Initial Drug Prescription Among Children Diagnosed with Major Depressive Disorder Treated with Antidepressant Drugs.
Significance tests indicate whether probability is different from the preceding time period.
***p<.01; **p<.05;
Discussion
Consistent with prior research, we found significant declines in use of antidepressants. We found a substantial decline in the use of paroxetine and an increase in the use of fluoxetine at time periods consistent with the release of information by the FDA on the dangers of paroxetine (June 2003) and the benefits of fluoxetine (October 2003). Using pharmacy data, Olfson and colleagues identified declines in prescribing rates for paroxetine in June 2003; however, it was not clear from pharmacy data how these declines translated into changing treatment patterns (5). Our results suggest that treatment initiation with paroxetine for children newly diagnosed with major depressive disorder was relatively rare following the initial information about its risks conveyed in the June 2003 FDA warning. We found that the total decline in overall antidepressant use was greater than the increase in fluoxetine use, indicating this was not simply providers switching from other antidepressants to fluoxetine. This suggests that at least in some cases, providers did not believe the proven benefits of fluoxetine treatment outweighed the potential risks of pediatric SSRI use. We found no effects of the FDA warnings encouraging increased use of monitoring when prescribing antidepressants.
There are several important implications of this research. First, the FDA was widely criticized for making the decision to issue a black box warning for pediatric antidepressant use. Yet, the black box warning may not have been the primary factor contributing to the observed changes in treatment patterns. Confirming earlier studies, we found that the decline in antidepressant use began before the announcement of the black box warning. It is difficult to know whether pediatric antidepressant use would have rebounded if the FDA had not issued this black box warning, but there is no evidence to suggest this would have occurred.
Second, we found that both risk and benefit information provided by the FDA led to changes in treatment patterns. There is concern that only potential risks would be communicated, due to media reporting which emphasized risks rather than benefits (16). However, we found that physicians increased their use of fluoxetine, the only SSRI approved for pediatric use during this time period. This increase in fluoxetine use was gradual, and not significant until March 2004, approximately five months after the proven benefit of fluoxetine was noted in FDA warnings. It is interesting to note that the strong evidence base for the efficacy of fluoxetine in treating children had been previously available, but did not lead to high rates of use until this evidence was highlighted by the FDA.
Third, it is interesting to note that while we did see changes in prescribing patterns, the FDA’s recommendation regarding increased physician monitoring appears to have been largely ignored. This may be due to the relatively high cost of office visits, a shortage of providers (particularly child psychiatrists) or the high cost sharing associated with mental health visits in many health plans. This finding highlights the limited powers of the FDA to directly affect the care received by patients. The FDA is “responsible for protecting the public health by assuring the safety, efficacy, and security of human and veterinary drugs…” Yet, the agency possesses few direct methods for addressing safety risks beyond regulating specific marketing practices, requiring manufacturers to include warning labels on products and, in extreme situations, pulling products from the market. In the case of pediatric antidepressant use, providers appear to have heeded some FDA health and safety recommendations while ignoring others.
A number of limitations with this study are worth noting. First, we have no information on the compensatory use of uncovered psychosocial therapies (i.e., school counselors, clergy). Also important, we only have information on privately insured individuals and results may differ for a publicly insured population. Finally, since we do not consider a control group (since no appropriate control group was available), changes may be due, in part, to secular changes in treatment patterns that would have occurred in the absence of any FDA warnings. Young adults are not an appropriate control group, because we expect there to be spillover effects of the FDA warnings to young adults, even though they were not included in the original warnings (17).
Conclusion
Reductions in pediatric antidepressant use raise important public health concerns. In the 1980s and 1990s, the introduction of SSRI-class antidepressants led to a substantial increase in depression treatment in children and adolescents (18,19,20). Because depression is an under-treated disease (21) with the potential for long term negative consequences, these increases were viewed at the time by many as important reductions in unmet need. Our findings suggest the FDA plays an important role in communicating information to the public and providers, and that FDA actions (i.e., public statements, public health advisories) were associated with some significant changes in practice patterns. Yet, this role can be fraught with challenges in the context of an issue such as this one which involves substantial scientific uncertainty. And, as our findings indicate, the FDA’s ability to directly protect the public’s health is limited in many cases, and treatment choice relies on physician judgment in weighing risks and benefits.
Table 1.
Logistic regression results
| Antidepressant prescription filled within 30 days of initial diagnosist | Paroxetine within 30 days of diagnosis, conditional on AD receipt | Fluoxetine within 30 days of diagnosis, conditional on AD receipt | At least two outpatient visits within 30 days of AD initiation, conditional on AD receipt | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | Robust standard error | Beta | Robust standard error | Beta | Robust standard error | Beta | Robust standard error | |||||
| Age | 0.139 | 0.007 | *** | 0.017 | 0.021 | −0.038 | 0.015 | ** | 0.011 | 0.013 | ||
| Male | −0.227 | 0.033 | *** | 0.033 | 0.092 | −0.279 | 0.073 | −0.078 | 0.062 | |||
| Region: | ||||||||||||
| North east | omitted | omitted | omitted | omitted | ||||||||
| North central | 0.137 | 0.060 | ** | −0.209 | 0.160 | −0.157 | 0.125 | 0.120 | 0.110 | |||
| South | 0.234 | 0.060 | *** | −0.337 | 0.161 | ** | −0.250 | 0.126 | * | −0.112 | 0.111 | |
| West | −0.167 | 0.069 | ** | −0.223 | 0.193 | 0.063 | 0.145 | −0.177 | 0.133 | |||
| Episode Start: | ||||||||||||
| 5/1/01-6/18/03 | omitted | omitted | omitted | omitted | ||||||||
| 6/19/03-10/27/03 | 0.084 | 0.056 | −1.003 | 0.154 | *** | −0.168 | 0.134 | −0.027 | 0.101 | |||
| 10/28/03-3/22/04 | 0.055 | 0.064 | −1.277 | 0.193 | *** | 0.092 | 0.140 | −0.051 | 0.113 | |||
| 3/23/04-9/12/04 | −0.030 | 0.054 | −1.626 | 0.195 | *** | 0.447 | 0.114 | *** | 0.023 | 0.099 | ||
| 9/13/04-10/30/05 | −0.172 | 0.039 | *** | −1.560 | 0.125 | *** | 0.807 | 0.083 | *** | 0.061 | 0.073 | |
| Constant | −3.099 | 0.127 | *** | −1.437 | 0.357 | *** | −0.932 | 0.266 | *** | −1.013 | 0.234 | *** |
NOTE: Significance levels on episode start date coefficients noted above indicate whether there are significant differences compared to omitted category. Significance levels noted in figures indicate whether there are significant differences from preceding time period using Wald test of equality of coefficients.
Regression predicting antidepressant prescription filled includes dummy variables to control for seasonal effects.
p<.10;
p<.05;
p<.01
Acknowledgments
We would like to acknowledge funding from NIMH (R01 MH080883).
Contributor Information
Susan H. Busch, Yale University School of Medicine
Richard G. Frank, Harvard Medical School
Doug Leslie, Medical University of South Carolina
Andres Martin, Yale University School of Medicine
Erika Martin, Yale University School of Medicine
Robert Rosenheck, Yale University School of Medicine
Colleen L. Barry, Yale University School of Medicine
Endnotes
- 1.Treatment for Adolescents with Depression Study Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression. Journal of the American Medical Association. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 2.Brent DA. Antidepressants and pediatric depression - The risk of doing nothing. New England Journal of Medicine. 2004;351:1598–1599. doi: 10.1056/NEJMp048228. [DOI] [PubMed] [Google Scholar]
- 3.Libby AM, Brent DA, Morrato EH, et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. American Journal of Psychiatry. 2007;164:884–891. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- 4.Nemeroff CB, Kalali A, Keller MB, et al. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Archives of General Psychiatry. 2007;64:466–472. doi: 10.1001/archpsyc.64.4.466. [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration warnings on antidepressant use in a national sample. Archives of General Psychiatry. 2008;65:94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- 6.Rosack J. New data show declines in antidepressant prescribing. Psychiatric News. 2005;40 [Google Scholar]
- 7.Harris G. Study finds less youth antidepressant use. New York Times. 2004 September 21; [Google Scholar]
- 8.Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. American Journal of Psychiatry. 2007;164:1356–1363. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- 9.Bridge JA, Greenhouse JB, Weldon AH. Suicide trends among youths aged 10 to 19 years in the United States, 1996–2005. Journal of the American Medical Association. 2008;300:1025–1026. doi: 10.1001/jama.300.9.1025. [DOI] [PubMed] [Google Scholar]
- 10.Valuck RJ, Libby AM, Orton HD, et al. Spillover effects on treatment of adult depression in primary care after FDA advisory on risk of pediatric suicidality with SSRIs. American Journal of Psychiatry. 2007;164:1198–1205. doi: 10.1176/appi.ajp.2007.07010007. [DOI] [PubMed] [Google Scholar]
- 11.Morrato EH, Libby AM, Orton HD, et al. Frequency of provider contact after FDA advisory on risk of pediatric suicidality with SSRIs. American Journal of Psychiatry. 2008;165:42–50. doi: 10.1176/appi.ajp.2007.07010205. [DOI] [PubMed] [Google Scholar]
- 12.Wingert TD, Kralewski JE, Lindquist TJ, et al. Constructing episodes of care from encounter and claims data: some methodological issues. Inquiry. 1995;32:430–443. [PubMed] [Google Scholar]
- 13.Keeler EB, Wells KB, Manning WG, et al. The Demand for Episodes of Mental Health Services. Santa Monica, CA: RAND Report R-3432-NIMH; 1986. [DOI] [PubMed] [Google Scholar]
- 14.Wells KB, Roland S, Sherbourne CD, et al. Caring for Depression: A RAND Study. Cambridge: Harvard University Press; 1996. [Google Scholar]
- 15.Kleinman LC, Norton EC. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Services Research. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry CL, Busch SH. News media coverage of FDA warnings on pediatric antidepressant use and suicidality, working paper. 2009 doi: 10.1542/peds.2009-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valuck RJ, Libby AM, Orton HD, et al. American Journal of Psychiatry. Vol. 164. 2007. Spillover effects on treatment of adult depression in primary care after FDA advisory on risk of pediatric suicidality with SSRIs; pp. 1198–1205. [DOI] [PubMed] [Google Scholar]
- 18.Olfson M, Marcus S, Weissman MM, et al. National trends in the use of psychotropic medications by children. Journal of American Academy of Child and Adolescent Psychiatry. 2002;41:514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Zito JM, Safer JD, DosReis S, et al. Psychotropic practice patterns for youth: A 10-year perspective. Archives of Pediatric and Adolescent Medicine. 2003;157:17–25. doi: 10.1001/archpedi.157.1.17. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Lee KV, Stafford R. Depression treatment during outpatient visits by U.S. children and adolescents. Journal of Adolescent Health. 2005;37:434–442. doi: 10.1016/j.jadohealth.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Demler O, Frank RG, et al. Prevalence and treatment of mental disorders, 1990–2003. New England Journal of Medicine. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]