Abstract
Objective: To compare the prognostic prediction between dichotomized and fractionated evaluations of hormone receptor expressions. Methods: Patients with stages I–III breast cancers, who received adjuvant tamoxifen, were enrolled. The expression of estrogen receptor (ER) and progesterone receptor (PR) was evaluated by immunohistochemistry (IHC). A fractionated score (F score), the percentage of positive-staining nuclei (0=none, 1=1%–10%, 2=11%–30%, 3=31%–50%, 4=51%–70%, and 5=71%–100%), was assigned to each case. The dichotomized scoring method defines an F score >1 as positive. The prognostic values of both scores were compared by multiple Cox’s proportional hazard models of disease-free survival (DFS) and overall survival (OS). Results: Four hundred and sixteen patients with a median follow-up of 78.0 months were included. F scores for ER and PR correlated directly with DFS and OS. Although both the dichotomized and fractionated ER and PR scores were significantly associated with DFS and OS in univariate analyses, only fractionated ER and PR scores remained as independent prognostic factors of DFS and OS in the final multiple Cox’s proportional hazard models. Conclusion: Fractionated IHC hormone receptor expression evaluation enhances the prognostic prediction compared with a dichotomized assessment.
Keywords: Breast cancer, Fractionated evaluation, Estrogen receptor, Progesterone receptor
1. Introduction
In breast cancer patients, both the estrogen receptor (ER) and progesterone receptor (PR) are important prognostic and predictive markers (Ravdin et al., 1992; Elledge et al., 2000; Esteva and Hortobagyi, 2004). Over the past decade, immunohistochemistry (IHC) has replaced the ligand-binding assay (LBA) for measuring ER and PR. Some studies comparing the predictive values of these two methods found that IHC is an equal or a superior method (Reiner et al., 1986; Stierer et al., 1993; Barnes et al., 1996; Harvey et al., 1999; Mohsin et al., 2004; Regan et al., 2006). However, the definitions of ER and PR positivity by IHC were not consistent among the different studies. Using a definition of positivity as the presence of any level of staining by IHC, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG, 2005) showed in a meta-analysis that tamoxifen significantly improved the prognosis of patients with ER positive tumors. However, in clinical practice and in some clinical trials, a cut-off of ≥10% ER and/or PR positive nuclei is often used for determining that a tumor is hormone receptor-positive and should be treated with adjuvant hormonal therapy (Ellis et al., 2001; Jonat et al., 2002; Thurlimann et al., 2005). The commonly applied 10% cut-off was established by Pertschuk et al. (1996) who demonstrated a response to treatment in patients with metastatic breast cancer.
Two recent reports by Viale et al. (2008) and Stendahl et al. (2006) showed that fractionated scoring of ER and PR, defined as assigning scores based on the fraction of cells that were stained positively for ER and PR by IHC, could enhance the prediction of a benefit from adjuvant chemotherapy and/or hormonal therapy. The study by Viale et al. (2008) demonstrated a gradually decreasing benefit to adding chemotherapy to endocrine therapy with increasing ER and PR expression fractions when compared with endocrine therapy alone in patients with node-negative early breast cancer. Stendahl et al. (2006) showed that adjuvant tamoxifen could significantly improve survival in premenopausal patients with tumors showing >75% PR positive nuclei. These two studies highlighted the predictive roles of fractionated evaluation of hormone receptors. In the present study, we assessed whether the hormone receptor status determined by fractionated IHC evaluation could enhance the prognostic significance of the predictive value compared with the traditional dichotomized evaluation.
2. Materials and methods
2.1. Patients and procedures
In this study, women with stage I, II, or III breast cancer who received tamoxifen as adjuvant therapy at the National Taiwan University Hospital (NTUH) between 1992 and 2000 were recruited. Breast cancer staging was conducted according to the American Joint Committee on Cancer (AJCC) criteria (the 6th Ed.). Patients’ demographic information was collected from a genetic epidemiology study on the association between selected genetic polymorphisms and breast cancer in 482 patients, which was carried out at the same hospital (Huang et al., 2008). To avoid bias caused by the uncertain treatment effects of tamoxifen in tumors with weak ER and/or PR expression, 43 patients who did not receive adjuvant tamoxifen were excluded. In addition, 23 patients whose archival tumor tissues were not available for fractionated scoring of ER and PR expression were also excluded. Therefore, a total of 416 patients were enrolled in the present analysis. Clinical data, including staging, type of surgery, adjuvant chemotherapy, and hormone therapy, were extracted from medical charts. Disease status and survival outcome were obtained from medical charts, hospital cancer registry records, and the National Death Certificate Registry system if a patient was lost to follow-up. The data managers of our hospital cancer registry usually contact each patient every 6 months. Additionally, all deaths in Taiwan are registered through the household registration offices. These death certificates are coded by the Department of Health, Executive Yuan, and have been computerized since 1979 (Lu et al., 2000). The survival data in this study were updated to Feb. 5, 2005.
Histological slides were reviewed by one pathologist, Dr. H.C. Lien, who was blinded to the patient information. Histological grade was categorized as grades I, II, and III according to the Nottingham modification of the Scarff-Bloom-Richardson criteria (Elston and Ellis, 1991), except for invasive lobular and mucinous carcinomas. The status of ER and PR in tumors was determined by IHC performed on formalin-fixed (fixation time 6–8 h), paraffin-embedded tissue sections (thickness 4 μm) in the Central Pathology Laboratory at NTUH. Ventana Benchmark system (Ventana Medical Systems Inc., Tucson, AZ, USA) and prediluted antibodies (anti-ER Clone 6F11 and anti-PgR Clone 16) were used. The reviewing pathologist assigned a six-point fractionated score called “F score” to each case after estimating the percentage of positive-staining tumor cells (0=none, 1=1%–10%, 2=11%–30%, 3=31%–50%, 4=51%–70%, and 5=71%–100%). A percentage of 10% (i.e., F score=1) was chosen as the cut-off value to dichotomize the assessments of ER and PR into positive versus negative.
2.2. Statistical analysis
Statistical analyses were performed with SAS software (version 9.1.3, SAS Institute Inc., Cary, NC, USA) and S-PLUS software (version 7.0.4, Insightful Corporation, Seattle, WA, USA). A two-sided P≤0.05 was considered statistically significant. The dichotomized ER and PR scores are binary variables, whereas the fractionated ER and PR scores (i.e., F scores) are ordinal variables. The recruited subjects were followed from the date of diagnosis to the one of death or last contact. The disease-free survival (DFS) time was defined as the duration from diagnosis to either the confirmation of disease recurrence, including local, regional, and distant recurrences, or death from breast cancer (if the time of recurrence was missed), except for contralateral breast cancer. The overall survival (OS) time was defined as the duration between diagnosis and death from any cause. The dependent variables evaluated were age, histological type, histological grade, tumor size, axillary lymph node status, AJCC stage, dichotomized ER, dichotomized PR, fractionated ER, fractionated PR, and neoadjuvant and/or adjuvant chemotherapy. Survival curves were estimated with the Kaplan-Meier method. The association between each of the categorical variables and survival was analyzed by log-rank test. Multivariate analysis was performed using Cox’s proportional hazard models to predict the hazard rates of DFS and OS, respectively. The time lag from the time of recurrence to each time at which a death from any cause occurred was computed for each patient and was included in the Cox’s proportional hazard model of OS as a time-dependent covariate to falsify the hypothesis that earlier recurrence leads to earlier death. For patients whose breast cancer had not recurred at the time of death, this time lag was set to zero.
Basic model-fitting techniques for (1) variable selection, (2) assessment of goodness-of-fit (GOF), and (3) regression diagnostics were used in our regression analysis to assure the quality of the analysis results. In the stepwise variable selection procedure, all the significant univariates and non-significant covariates were considered and both the significance levels for entry (SLE) and for stay (SLS) were set to 0.15 or larger. The adjusted generalized R 2 and the Grønnesby-Borgan GOF test were examined to assess the GOF of the fitted Cox’s proportional hazard model. However, the value of the adjusted generalized R 2 for Cox’s proportional hazard model is usually low. Larger P values in the Grønnesby-Borgan GOF test indicate better fit. Regression diagnostics, including verification of proportional hazard assumption, residual analysis, detection of influential cases, and check for multi-collinearity, were performed. Based on the fitted final Cox’s regression models for DFS and OS, estimated covariate-adjusted survival curves were plotted for personalized prediction of the patient’s prognosis.
3. Results
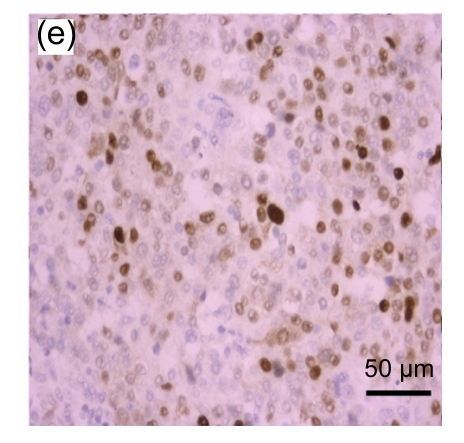
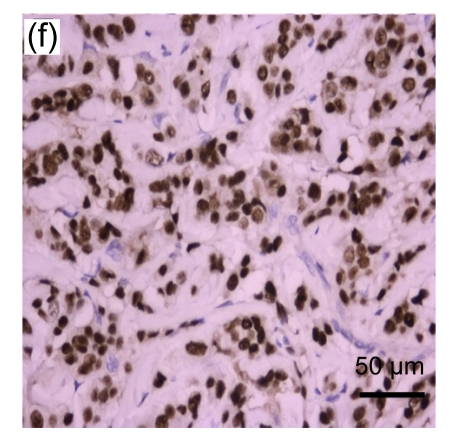
A total of 416 patients with a median age of 49 (range: 25–81) years were included. The representative immunohistochemical image of each F score of ER is shown in Fig. 1. The distribution of each F score of ER and PR expression is shown in Table 1. Using the dichotomized definition of an F score >1 as positive, 216 (63%) and 257 (62%) cases were ER+ and PR+, respectively. The clinical and pathological characteristics of the patients are summarized in Table 2 by dichotomized ER and PR status. The tumor histology consisted of infiltrating ductal carcinoma (95%), infiltrating lobular carcinoma (2%), mucinous carcinoma (3%), and others (1%) including 2 medullary carcinomas and 2 invasive papillary carcinomas. The frequency of ER+ and/or PR+ status was heterogeneous among tumors of different sizes, axillary lymph node statuses, histologic types, and grades. Specifically, ER+ was associated with a higher probability of a tumor size ≤2 cm and PR+ was associated with higher probability of absence of axillary lymph node involvement. All of the lobular carcinomas and mucinous carcinomas were ER+. Tumors with histologic grades I and II were more likely to be ER+ or PR+, whereas tumors with histologic grade III were more likely to be ER− or PR−. The expression of ER and PR was not significantly associated with the use of neoadjuvant and/or adjuvant chemotherapy.
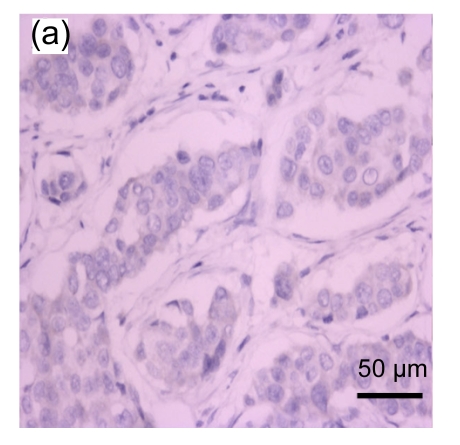
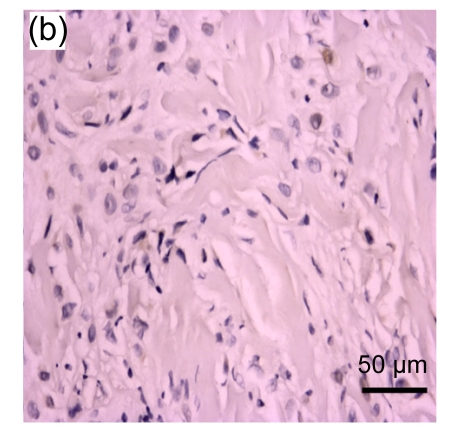
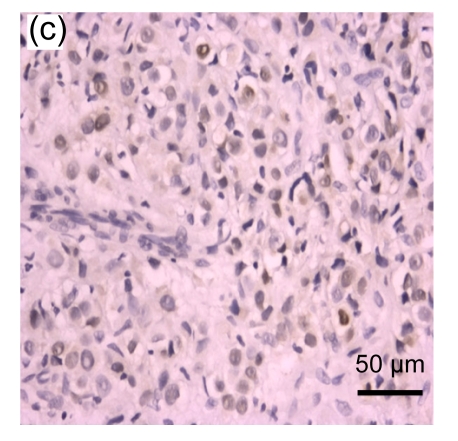
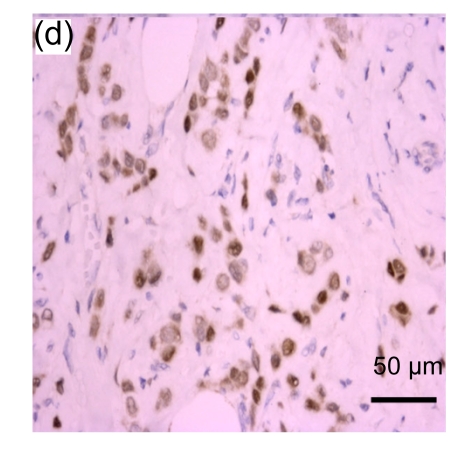
Fig. 1.
Representative immunohistochemical image of each fractionated score (F score) of estrogen receptor (ER). F scores 0 (a), 1 (b), 2 (c), 3 (d), 4 (e), and 5 (f) were shown
Table 1.
Distribution of ER and PR fractionated scores
| ER score | PR score |
Total | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| 0 | 77 | 15 | 6 | 4 | 5 | 21 | 128 |
| 1 | 10 | 15 | 2 | 0 | 0 | 0 | 27 |
| 2 | 0 | 0 | 6 | 1 | 5 | 6 | 18 |
| 3 | 7 | 1 | 4 | 3 | 2 | 4 | 21 |
| 4 | 8 | 1 | 0 | 4 | 10 | 5 | 28 |
| 5 | 22 | 3 | 10 | 11 | 20 | 128 | 194 |
| Total | 124 | 35 | 28 | 23 | 42 | 164 | 416 |
Table 2.
Patients’ clinical and pathological characteristics by dichotomized ER and PR expression
| All cases (n=416) | ER, No. (%) |
PR, No. (%) |
|||||
| Positive (n=261) | Negative (n=155) | P-value | Positive (n=257) | Negative (n=159) | P-value | ||
| Age | 0.0922 | 0.0926 | |||||
| <36 years | 26 (6%) | 15 (6%) | 15 (10%) | 14 (6%) | 16 (10%) | ||
| 36–50 years | 204 (49%) | 135 (52%) | 65 (42%) | 132 (51%) | 68 (43%) | ||
| >50 years | 186 (45%) | 111 (43%) | 75 (48%) | 111 (43%) | 75 (47%) | ||
| Tumor size | 0.0057 | 0.1190 | |||||
| ≤2 cm | 167 (40%) | 120 (46%) | 47 (30%) | 111 (43%) | 56 (35%) | ||
| 3–5 cm | 216 (52%) | 124 (48%) | 92 (59%) | 130 (51%) | 86 (54%) | ||
| >5 cm | 33 (8%) | 17 (7%) | 16 (10%) | 16 (6%) | 17 (11%) | ||
| Axillary lymph node | 0.9399 | 0.0271 | |||||
| None | 232 (56%) | 148 (57%) | 84 (54%) | 158 (61%) | 74 (47%) | ||
| 1–3 | 96 (23%) | 59 (23%) | 37 (24%) | 53 (21%) | 43 (27%) | ||
| 4–9 | 54 (13%) | 34 (13%) | 20 (13%) | 29 (11%) | 25 (16%) | ||
| ≥10 | 34 (8%) | 20 (8%) | 14 (9%) | 17 (7%) | 17 (11%) | ||
| AJCC stage | 0.0445 | 0.0198 | |||||
| I | 114 (27%) | 82 (31%) | 32 (21%) | 82 (32%) | 32 (20%) | ||
| II | 209 (50%) | 121 (46%) | 88 (57%) | 125 (49%) | 84 (53%) | ||
| III | 93 (22%) | 58 (22%) | 35 (23%) | 50 (19%) | 43 (27%) | ||
| Histologic subtype | 0.0006 | 0.3523 | |||||
| Ductal | 391 (94%) | 237 (91%) | 154 (99%) | 238 (93%) | 153 (96%) | ||
| Lobular | 8 (2%) | 8 (3%) | 0 (0%) | 5 (2%) | 3 (2%) | ||
| Mucinous | 13 (3%) | 13 (5%) | 0 (0%) | 11 (4%) | 2 (1%) | ||
| Others | 4 (1%) | 3 (1%) | 1 (1%) | 3 (1%) | 1 (1%) | ||
| Histologic grade | <0.0001 | <0.0001 | |||||
| I | 103 (25%) | 78 (30%) | 25 (16%) | 79 (31%) | 24 (15%) | ||
| II | 164 (39%) | 115 (44%) | 49 (32%) | 113 (44%) | 51 (32%) | ||
| III | 98 (24%) | 33 (13%) | 65 (42%) | 37 (14%) | 61 (38%) | ||
| Not available | 51 (12%) | 35 (13%) | 16 (10%) | 28 (11%) | 23 (15%) | ||
| Neoadjuvant or adjuvant chemotherapy | 0.3577 | 0.3098 | |||||
| Yes | 262 (63%) | 160 (61%) | 102 (66%) | 157 (61%) | 105 (66%) | ||
| No | 154 (37%) | 101 (39%) | 53 (34%) | 100 (39%) | 54 (34%) | ||
3.1. Univariate survival analyses of prognostic factors
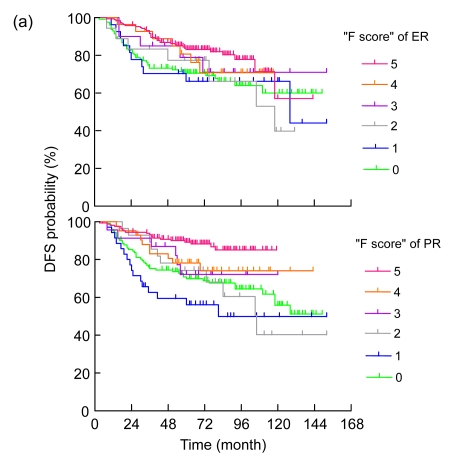
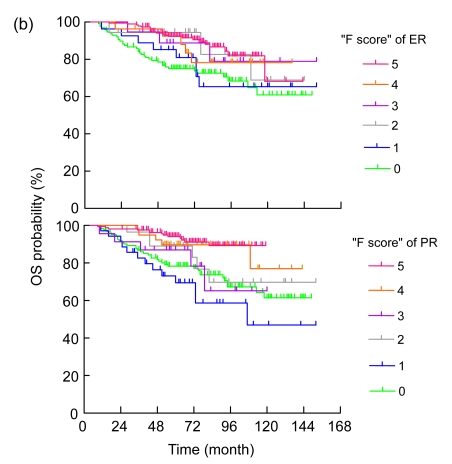
The median follow-up time was 78.0 (range: 74.7–81.3) months. As listed in Table 3, the univariate analysis showed that age, tumor size, axillary lymph node status, AJCC stage, dichotomized ER and PR, histologic grade, and use of neoadjuvant or adjuvant chemotherapy were significantly associated with DFS and OS. Specifically, age <36 years, larger tumor size, higher AJCC stage, negative ER, negative PR, more axillary lymph node involvement, higher tumor grade, and use of neoadjuvant and/or adjuvant chemotherapy were prognostic factors for a poor outcome (Table 3). The estimated DFS and OS curves according to the F scores of hormone receptors for all patients are shown in Figs. 2a and 2b), respectively. Univariate linear trend analysis also revealed that higher ER and PR IHC scores were significantly associated with favorable DFS (P=0.003 and P<0.001, respectively) and OS (P<0.001 and P<0.001, respectively).
Table 3.
Univariate analyses of clinical and pathological variables in patients with stages I–III breast cancers
| N (%) | DFS |
OS |
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age | 0.0129 | 0.0191 | |||||
| <36 years | 26 (6%) | 1.00 | 1.00 | ||||
| 36–50 years | 204 (49%) | 0.41 | 0.23–0.76 | 0.40 | 0.20–0.77 | ||
| >50 years | 186 (45%) | 0.50 | 0.28–0.91 | 0.48 | 0.25–0.92 | ||
| Tumor size | <0.0001 | <0.0001 | |||||
| ≤2 cm | 167 (40%) | 1.00 | 1.00 | ||||
| 3–5 cm | 216 (52%) | 1.86 | 1.18–2.92 | 2.27 | 1.28–4.02 | ||
| >5 cm | 33 (8%) | 5.43 | 3.02–9.79 | 7.99 | 4.04–15.84 | ||
| Axillary lymph nodes | <0.0001 | <0.0001 | |||||
| None | 232 (56%) | 1.00 | 1.00 | ||||
| 1–3 | 96 (23%) | 1.98 | 1.17–3.35 | 1.92 | 0.99–3.70 | ||
| 4–9 | 54 (13%) | 3.81 | 2.22–6.53 | 4.67 | 2.49–8.75 | ||
| ≥10 | 34 (8%) | 12.08 | 7.19–20.30 | 11.81 | 6.47–21.55 | ||
| AJCC stage | <0.0001 | <0.0001 | |||||
| I | 114 (27%) | 1.00 | 1.00 | ||||
| II | 209 (50%) | 3.23 | 1.53–6.84 | 4.10 | 1.45–11.62 | ||
| III | 93 (22%) | 11.11 | 5.27–23.40 | 16.43 | 5.90–45.80 | ||
| ER expression | 0.0090 | 0.0001 | |||||
| Positive | 261 (63%) | 1.00 | 1.00 | ||||
| Negative | 155 (37%) | 1.65 | 1.13–2.42 | 2.35 | 1.50–3.69 | ||
| PR expression | <0.0001 | <0.0001 | |||||
| Positive | 257 (62%) | 1.00 | 1.00 | ||||
| Negative | 159 (38%) | 2.12 | 1.44–3.13 | 2.67 | 1.68–4.22 | ||
| Histologic subtype | 0.7717 | 0.8421 | |||||
| Ductal | 391 (94%) | 1.00 | 1.00 | ||||
| Lobular | 8 (2%) | 1.09 | 0.27–4.44 | 0.75 | 0.10–5.40 | ||
| Mucinous | 13 (3%) | 1.03 | 0.33–3.23 | 0.87 | 0.21–3.55 | ||
| Others | 4 (1%) | NA | NA | NA | NA | ||
| Histologic grade | 0.0072 | 0.0010 | |||||
| I | 103 (25%) | 1.00 | 1.00 | ||||
| II | 164 (39%) | 2.07 | 1.14–3.77 | 1.88 | 0.89–3.99 | ||
| III | 98 (24%) | 2.88 | 1.55–5.36 | 3.75 | 1.78–7.88 | ||
| Not available | 51 (12%) | 1.84 | 0.85–3.98 | 1.98 | 0.79–5.00 | ||
| Neoadjuvant or adjuvant chemotherapy | 0.0010 | 0.0013 | |||||
| Yes | 262 (63%) | 1.00 | 1.00 | ||||
| No | 154 (37%) | 0.49 | 0.31–0.75 | 0.43 | 0.25–0.73 | ||
HR: hazard ratio; CI: confidence interval; NA: not available
Fig. 2.
Disease-free (a) and overall (b) survival curves (unadjusted analysis) by estrogen receptor (ER) and progesterone receptor (PR) IHC score
3.2. Multivariate survival analyses of prognostic factors
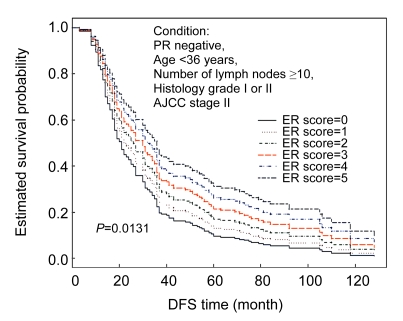
The results of multivariate analyses by fitting Cox’s proportional hazard models for DFS and OS are listed in Table 4. Fractionated ER, age <36 years, ≥10 positive axillary lymph nodes, histologic grade III, and AJCC stage were independent prognostic factors for DFS. Fractionated PR, 1–9 positive axillary lymph nodes, ≥10 positive axillary lymph nodes, tumor size, histologic grade III, and the time lag between recurrence and the time of death from any cause were independent prognostic factors for OS. Longer lag time implied an earlier recurrence. Neither dichotomized ER nor dichotomized PR was an independent prognostic factor for DFS or OS at the completion of the stepwise variable selection procedure. Moreover, we could draw covariate-adjusted survival curves for the prognostic prediction of patient’s DFS or OS under any given condition. As an illustration, the estimated covariate-adjusted DFS curves by six-point fractionated ER scores for a young woman (aged <36 years, AJCC stage II breast cancer, histologic grade I or II, fractionated PR score 0, and ≥10 involved axillary lymph nodes) are shown in Fig. 3.
Table 4.
Multivariate analyses of disease-free survival and overall survival using Cox’s proportional hazard models
| Covariate | Estimate | Standard error | Wald chi-square | Hazard ratio | P value |
| Disease-free survival (month)1 | |||||
| Fractionated ER | −0.13960 | 0.05628 | 6.1530 | 0.870 | 0.0131 |
| Fractionated PR | −0.10107 | 0.05869 | 2.9655 | 0.904 | 0.0851 |
| Age <36 years | 0.67577 | 0.29191 | 5.3592 | 1.966 | 0.0206 |
| Lymph node ≥10 | 1.35863 | 0.27997 | 23.5496 | 3.891 | <0.0001 |
| Histology grade III | 1.32154 | 0.61828 | 4.5687 | 3.749 | 0.0326 |
| AJCC stage | 0.91674 | 0.18495 | 24.5675 | 2.501 | <0.0001 |
| Overall survival (month)2 | |||||
| Fractionated PR | −0.21830 | 0.06066 | 12.9512 | 0.804 | 0.0003 |
| Lymph node 1–9 | 0.81272 | 0.30782 | 6.9707 | 2.254 | 0.0083 |
| Lymph node ≥10 | 1.56278 | 0.33739 | 21.4549 | 4.772 | <0.0001 |
| Histology grade III | 0.68063 | 0.25213 | 7.2875 | 1.975 | 0.0069 |
| Tumor size (cm) | 0.32670 | 0.16657 | 3.8469 | 1.386 | 0.0498 |
| Neoadjuvant or adjuvant chemotherapy | 0.61693 | 0.35181 | 3.0750 | 1.853 | 0.0795 |
| Time lag since recurrence | 0.07444 | 0.00775 | 92.2451 | 1.077 | <0.0001 |
Cox’s proportional hazard model: n=416, adjusted generalized R 2=0.2486
Cox’s proportional hazard model: n=416, adjusted generalized R 2=0.3971
Fig. 3.
Covariate-adjusted survival curves for disease-free survival (DFS) time based on a fitted multiple Cox’s proportional hazard model (n=416)
4. Discussion
In this study, we found that fractionated ER and PR were predictors of DFS and OS, respectively, both independently and after competing with the dichotomized ER and dichotomized PR in a stepwise variable selection procedure. We concluded that implementing a fractionated evaluation of ER and PR enhances the prognostic correlation when compared with the dichotomized assessment.
All of the patients included in this analysis received adjuvant tamoxifen, including a substantial proportion (26%) of patients with ER−/PR− breast cancer. This provided us with a suitable platform to examine the effect of ER and PR expression on survival. By examining the Kaplan-Meier estimates of DFS curves stratified by fractionated ER and PR scores (Fig. 2a), the best cut-off points of dichotomized evaluation of ER and PR positivity were more than 10% positive nuclei (F score>1). Dichotomized ER and PR expression was also highly associated with favorable DFS and OS in the present study, which provides evidence to support the criteria commonly used in clinical practice and some clinical trials in the adjuvant setting (Ellis et al., 2001; Jonat et al., 2002; Thurlimann et al., 2005).
In addition to the prognostic factors identified by our univariate analyses for DFS and OS, which include age, tumor size, number of axillary lymph nodes, and AJCC stage, dichotomized ER and PR are well-recognized prognostic factors for breast cancer survival (Cianfrocca and Goldstein, 2004). The present study provides empirical evidence to show that earlier recurrence leads to an earlier death in breast cancer patients. Although this result is biologically sound, most of the previous studies analyzed time to recurrence (DFS) and death (OS) separately. The causal relationship between recurrence and death should be pursued further in the future, because it may shed light on improvements of the prognostic predictions in such patients. Moreover, we found that fractionated ER was an independent prognostic factor of DFS, and fractionated PR was an independent prognostic factor of OS after adjusting for the effect of time lag since recurrence. Since time to recurrence was also a prognostic factor for death, both fractionated ER and fractionated PR could predict OS, but fractionated PR had a direct prognostic effect on OS. The finding that PR added significant prognostic value beyond that obtained with ER alone was consistent with recent studies (Banerjee et al., 2004; Arpino et al., 2005; Liu et al., 2010). Fractionated ER and PR remained in the final multiple Cox’s proportional hazard models after competing with the dichotomized ER and PR in the stepwise variable selection procedure. Thus, a fractionated evaluation of ER and PR enhanced the prognostic prediction when compared with the dichotomized assessment.
ER analysis using LBA (as fmol/mg of protein) revealed a broad range of values for ER, so there has been expectation that immunohistochemical assay for ER analysis should result in a similar distribution. However, both fractionated ER and PR in the present study were shown to display a bimodal distribution, with substantial proportions at F scores of 0 (ER: 30.8%; PR: 29.8%) and 5 (ER: 46.6%; PR: 39.4%) (Table 1). The distribution was consistent with several previous studies of ER and PR expression measured by immunohistochemical assay (Collins et al., 2005; Nadji et al., 2005; Stendahl et al., 2006; Badve et al., 2008; Viale et al., 2008). Despite the bimodal distribution of ER and PR expression, the present study clearly demonstrates that fractionated ER and PR scores predict a patient’s prognosis more accurately than a dichotomized assessment.
The Allred, Quick and H scores, which include the evaluation of both the proportion and staining intensity of positive cells, have been used to assess IHC staining of breast tumors (Huang et al., 1996; Harvey et al., 1999; Ferrero-Pous et al., 2001). The Allred scores for both of ER and PR expression were reported to correlate highly with patient’s survival outcome, and the predictive value of Allred score was superior to the LBA (Harvey et al., 1999; Mohsin et al., 2004). The Quick and H scores for ER and/or PR expression were shown to correlate with LBA (Huang et al., 1996; Ferrero-Pous et al., 2001) and possess similar predictive values as LBA for response to hormonal therapy (Elston and Ellis, 1998). Nevertheless, many clinical practices simply adopt a proportion score rather than the semi-quantitative scoring systems for predictive and prognostic evaluations, because the intensity interpretation is relatively subjective and some tumors have heterogeneous intensity (Horii et al., 2007).
The superiority of prognostic value of fractionated assessment to dichotomized assessment may have important clinical implications. For example, the information could be used to improve risk estimation by Adjuvant! Online (https://www.adjuvantonline.com/breastnew.jsp), which is one of the most popular and ambitious tools to estimate the risk of breast cancer relapse and death. One of the limitations inherent in the current Adjuvant! Online version is that only dichotomized ER is used to estimate the risk of relapse and mortality. As illustrated in Fig. 3, the covariate-adjusted survival curves may provide some fine tuning according to the level of ER positivity and PR status to adjust the risk estimation. Of course, further population-based studies with larger sample sizes and a comparison between pure fractionated (F score) and intensity incorporated (Allred, Quick or H score) evaluations are necessary to prove that the “F score” is a simple and powerful method for risk adjustment.
5. Acknowledgement
We thank the members of the Office of Medical Records at National Taiwan University Hospital for their help in assessing the prospectively collected data.
Footnotes
Project supported by the National Center of Excellence for Clinical Trial and Research of National Taiwan University Hospital (No. DOH97-TD-B-111-001), Taiwan, Taipei
References
- 1.Arpino G, Weiss H, Lee AV, Schiff R, de Placido S, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 2.Badve SS, Baehner FL, Gray RP, Childs BH, Maddala T, Liu ML, Rowley SC, Shak S, Perez EA, Shulman LJ, et al. Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26(15):2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee M, George J, Song EY, Roy A, Hryniuk W. Tree-based model for breast cancer prognostication. J Clin Oncol. 2004;22(13):2567–2575. doi: 10.1200/JCO.2004.11.141. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996;74(9):1445–1451. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 6.Collins LC, Botero ML, Schnitt SJ. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. Am J Clin Pathol. 2005;123(1):16–20. doi: 10.1309/HCF035N9WK40ETJ0. [DOI] [PubMed] [Google Scholar]
- 7.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 8.Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immunohistochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89(2):111–117. doi: 10.1002/(SICI)1097-0215(20000320)89:2<111::AID-IJC2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Janicke F, Miller WR, Evans DB, Dugan M, Brady C, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19(18):3808–3816. doi: 10.1200/JCO.2001.19.18.3808. [DOI] [PubMed] [Google Scholar]
- 10.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO. The Breast. Edinburgh: Churchill Livingston; 1998. p. 673. [Google Scholar]
- 12.Esteva FJ, Hortobagyi GN. Prognostic molecular markers in early breast cancer. Breast Cancer Res. 2004;6(3):109–118. doi: 10.1186/bcr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero-Pous M, Trassard M, Le Doussal V, Hacene K, Tubiana-Hulin M, Spyratos F. Comparison of enzyme immunoassay and immunohistochemical measurements of estrogen and progesterone receptors in breast cancer patients. Appl Immunohistochem Mol Morphol. 2001;9(3):267–275. doi: 10.1097/00022744-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 15.Horii R, Akiyama F, Ito Y, Iwase T. Assessment of hormone receptor status in breast cancer. Pathol Int. 2007;57(12):784–790. doi: 10.1111/j.1440-1827.2007.02174.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang A, Pettigrew NM, Watson PH. Immunohistochemical assay for oestrogen receptors in paraffin wax sections of breast carcinoma using a new monoclonal antibody. J Pathol. 1996;180(2):223–237. doi: 10.1002/(SICI)1096-9896(199610)180:2<223::AID-PATH635>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Huang CS, Kuo SH, Lien HC, Yang SY, You SL, Shen CY, Lin CH, Lu YS, Chang KJ. The CYP19 TTTA repeat polymorphism is related to the prognosis of premenopausal stage I–II and operable stage III breast cancers. Oncologist. 2008;13(7):751–760. doi: 10.1634/theoncologist.2007-0246. [DOI] [PubMed] [Google Scholar]
- 18.Jonat W, Kaufmann M, Sauerbrei W, Blamey R, Cuzick J, Namer M, Fogelman I, de Haes JC, de Matteis A, Stewart A, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20(24):4628–4635. doi: 10.1200/JCO.2002.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, Nielsen TO. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat. 2010;119(1):53–61. doi: 10.1007/s10549-009-0318-0. [DOI] [PubMed] [Google Scholar]
- 20.Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000;29(2):336–343. doi: 10.1093/ije/29.2.336. [DOI] [PubMed] [Google Scholar]
- 21.Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, Roanhle D, To TV, Qian Z, Love RR, Allred DC. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol. 2004;17(12):1545–1554. doi: 10.1038/modpathol.3800229. [DOI] [PubMed] [Google Scholar]
- 22.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5993 breast cancers. Am J Clin Pathol. 2005;123(1):21–27. doi: 10.1309/4WV79N2GHJ3X1841. [DOI] [PubMed] [Google Scholar]
- 23.Pertschuk LP, Feldman JG, Kim YD, Braithwaite L, Schneider F, Braverman AS, Axiotis C. Estrogen receptor immunocytochemistry in paraffin embedded tissues with ER1D5 predicts breast cancer endocrine response more accurately than H222Sp gamma in frozen sections or cytosol-based ligand-binding assays. Cancer. 1996;77(12):2514–2519. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2514::AID-CNCR14>3.3.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 24.Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- 25.Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, Brown B, Suurkula M, Langman G, Mazzucchelli L, et al. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98(21):1571–1581. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 26.Reiner A, Spona J, Reiner G, Schemper M, Kolb R, Kwasny W, Fugger R, Jakesz R, Holzner JH. Estrogen receptor analysis on biopsies and fine-needle aspirates from human breast carcinoma. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Am J Pathol. 1986;125(3):443–449. [PMC free article] [PubMed] [Google Scholar]
- 27.Stendahl M, Ryden L, Nordenskjold B, Jonsson PE, Landberg G, Jirstrom K. High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res. 2006;12(15):4614–4618. doi: 10.1158/1078-0432.CCR-06-0248. [DOI] [PubMed] [Google Scholar]
- 28.Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tuchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 1993;218(1):13–21. doi: 10.1097/00000658-199307000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 30.Viale G, Regan MM, Maiorano E, Mastropasqua MG, Golouh R, Perin T, Brown RW, Kovacs A, Pillay K, Ohlschlegel C, et al. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors–International Breast Cancer Study Group. J Clin Oncol. 2008;26(9):1404–1410. doi: 10.1200/JCO.2007.10.6393. [DOI] [PubMed] [Google Scholar]