Abstract
Objective: To explore the feasibility of using regenerated silk fibroin membrane to construct artificial skin substitutes for wound healing, it is necessary to evaluate its cytocompatibility. Methods: The effects of regenerated silk fibroin film on cytotoxicity, adhesion, cell cycle, and apoptosis of L929 cells, growth and vascular endothelial growth factor (VEGF) expression of ECV304 cells, and VEGF, angiopoietin-1 (Ang-1), platelet-derived growth factor (PDGF) and fibroblast growth factor 2 (FGF2) expression of WI-38 cells were assessed by 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT) assay, viable cell counting, flow cytometry (FCM), and enzyme-linked immunosorbant assay (ELISA). Results: We showed that the regenerated silk fibroin film was not cytotoxic to L929 cells and had no adverse influence on their adhesion, cell cycle or apoptosis; it had no adverse influence on the growth and VEGF secretion of ECV304 cells and no effect on the secretion of VEGF, Ang-1, PDGF and FGF2 by WI-38 cells. Conclusion: The regenerated silk fibroin film should be an excellent biomaterial with good cytocompatibility, providing a framework for reparation after trauma in clinical applications.
Keywords: Regenerated silk fibroin film, Cytocompatibility, Cytotoxicity
1. Introduction
Wound healing is defined as the restoration of the continuity of living tissue and is an integrated response of several cell types to injury. It involves platelet aggregation and blood clotting, the formation of fibrin, an inflammatory response, alteration in the ground substance, endothelial and capillary proliferation and surface covering, regeneration of certain cell types, variable contracture and remodeling (Buffoni et al., 1993). Healing will not complete until the disrupted surfaces are firmly knit by collagen. Generally, the use of a skin substitute is necessary to provide an environment conducive to healing (Nangia and Hung, 1990).
Fibroin, the main component of silk protein, was reported to be a perfect substrate for the proliferation and adhesion of a large variety of cells. Silk fibroin has found diverse applications in the biomedical field, which can be attributed to its high tensile strength, controllable biodegradability, haemostatic properties, non-cytotoxicity, low antigenicity and non-inflammatory characteristics (Mori and Tsukada, 2000; Horan et al., 2005; Meinel et al., 2005; Mauney et al., 2007). Findings of a recent study indicated that silk fibroin can form a good basic component for development of new biomedical materials, especially in the tissue-engineering field (Altman et al., 2003). Regenerated silk fibroin membrane is made from silk fibroin which is a kind of natural polymer biomaterial extracted from worm silk with good phy-chemical characteristics, especially biocompatibility (Ning et al., 2000). Therefore, silk fibroin has long been viewed as a potential biological matrix for wound healing (Halsted, 1892). For example, most commonly used surgical threads are made of natural silk fiber (Vepari and Kaplan, 2007; Wang et al., 2006a). To develop an ideal skin substitute, the performance of regenerated silk fibroin membrane made from silk fibroin was examined. Studies evaluated the biocompatibility of the membrane with fibroblasts and endothelial cells, which are the most important tissue repairing cells in healing. It was found that regenerated silk fibroin membrane did not have toxicity (Sheng et al., 2005) or genotoxicity (Miao et al., 2007; Gong et al., 2005), so it can be made into biomaterials such as wound dressings, tissue repair material, and artificial skin. To explore the feasibility of using regenerated silk fibroin membrane to construct artificial skin substitutes, it is necessary to evaluate its biocompatibility with peripheral skin tissues and cells. Since the most abundant cell types that contribute to wound healing are fibroblasts and endothelial cells, in our study murine fibroblast cell line L929, human umbilical vein endothelial cells (ECV304) and normal human fetal lung fibroblast (WI-38) cells were used to test the cell biocompatibility with regenerated silk fibroin. Cytotoxicity and cell proliferation assays by 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT) and flow cytometric analysis confirmed the ability of the regenerated silk fibroin membrane to support cell growth and proliferation comparable to that of a cell culture plate. The adhesion of L929 murine fibroblasts was monitored for cytocompatibility evaluation. Proliferation and vascular endothelial growth factor (VEGF) secretion of ECV304 on the silk fibroin were observed to evaluate molecular cytocompatibility. The effects of the regenerated silk fibroin membrane on the expression of VEGF, angiopoietin-1 (Ang-1), platelet-derived growth factor (PDGF) and fibroblast growth factor 2 (FGF2) by WI-38 cells were also studied.
2. Materials and methods
2.1. Reagents, cells and equipments
The ECV304, L929 and WI-38 cells preserved in our laboratory were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, Shanghai, China) supplemented with 10% (v/v) fetal calf serum (FCS) (Hyclone, Logan, UT). The MTT was purchased from Sigma (Shanghai, China) for the in vitro cytotoxicity experiment. All VEGF, PDGF and FGF2 enzyme-linked immunosorbant assay (ELISA) detection kits were obtained from Jingmei (Shanghai, China) and the ELISA kit for angiogenin-1 was purchased from the Wuhan Boster Bioengineering Co. Ltd. of China. The regenerated silk fibroin film was provided by Dr. Ming-zhong LI, Soochow University (Jiangsu, China). The polyvinyl chloride (PVC) plastic films and polyethylene (PE) plastic films were provided by the Suzhou Plastic Corp. (Jiangsu, China).
2.2. Preparation for regenerated silk fibroin films and extraction
The domestic silkworm silk fiber was degummed using Na2CO3 solution, dissolved in CaCl2-CH3CH2OH-H2O (1:2:8 in mole ratio), dialysed, filtered, and dried at 60 °C. In this way, purified regenerated silk fibroin films were obtained (Li et al., 2001; Lu et al., 2000). After 60Co-irradiation, sterile regenerated silk fibroin (SF) films, PVC plastic films and PE plastic films were lixiviated in sterile DMEM medium (samples’ surface area/medium volume=3 cm2/ml) at 37 °C for 24 h, and then cells of each experimental group were cultivated in the extractions containing 10% FCS. The cells of the control group were cultivated only in complete medium DMEM with 10% FCS.
2.3. Cytotoxicity experiment
MTT assay was applied to determine the cytotoxicity of regenerated silk fibroin film on mouse fibroblast line L929. Briefly, L929 cells were dispensed in 96-well culture plates at a density of 1×104 cells per well and incubated at 37 °C. After 24 h, the culture medium was replaced by the above prepared extractions. The medium containing only 10% FCS was used as a control. Each group was analyzed in triplicate. After 72 h, 10 μl MTT (5 mg/ml) was added to the medium which was then incubated for another 4 h at 37 °C. The formazan crystals in the cells were solubilized with stop solution (100 μl/well). The optical density (OD) value was then measured at 570 nm using a Microplate Reader Model 550 (Bio-Rad, Shanghai, China). The relative growth rate (RGR) of cells was calculated using the formula: RGR=(OD value of samples)/(OD value of negative control), and cytotoxicity was assessed according to cytotoxicity grading criteria (Table 1).
Table 1.
Cytotoxicity grading criteria
| RGR (%) | Cytotoxicity grading criteria |
| 100 | 0 (non-poisonous, qualification) |
| 75–99 | 1 (light poisonous, qualification) |
| 50–74 | 2 (moderate poisonous, disqualification) |
| 25–49 | 3 (severe poisonous, disqualification) |
| 1–24 | 4 (disqualification) |
| 0 | 5 (disqualification) |
2.4. Cell adhesion experiment
L929 cells were treated with SF, PVC, or PE as described above in 6-well culture plates. At 1, 2, 3, and 4 h, the liquid was withdrawn, and phosphate buffered saline (PBS) liquid was added to wash the cells in each group lightly three times. The cells were then treated for 10 min with 0.25% (w/v) trypsin and counted. The cell adherence ratio (AR) was calculated by the formula: AR=(the number of cells adhering to films)/(the number of total cells)×100%.
2.5. Flow cytometric analysis
L929 cells were dispensed in 6-well culture plates containing regenerated silk fibroin films (SF group), PVC plastic films (PVC group), or PE plastic films (PE group) at a density of 4×105 cells per well and incubated at 37 °C. Cells which were not cultured on any film, were used as a control group. After 72 h, cells were harvested and washed with cold PBS (pH 7.4). The cell pellets were fixed in 70% (v/v) cold alcohol for more than 24 h at 4 °C, washed with cold PBS, and stained with propidium iodide (PI) solution at 4 °C in the dark for 30 min. The cell cycle phases were analyzed by flow cytometry.
2.6. Growth morph and curve of ECV304 cells on SF
The effect of regenerated silk fibroin film on growth of ECV304 cells was determined by MTT assay. ECV304 cells were dispensed in 96-well culture plates containing regenerated silk fibroin films (SF group), PVC plastic films (PVC group), or PE plastic films (PE group) at a density of 1×104 cells per well and incubated at 37 °C for the indicated time periods (1–8 d). Cells which were not cultured on any film, were used as a control group. Each group was analyzed in triplicate. After different time periods of treatment, the cell growth morphology was observed and the growth of ECV304 cells was also assessed by MTT assay as described above. The growth curve of ECV304 cells in the different cultures was then plotted with the time (day) on the X-axis and OD value on the Y-axis.
2.7. Determination of level of VEGF secretion by ECV304 cells on SF using ELISA
The effect of SF on VEGF secretion by ECV304 cells was determined. The ECV304 cells were treated with SF, PVC, or PE as described above. After incubation for 72 h, the VEGF in the supernatants was assessed using ELISA.
2.8. Determination of VEGF, Ang-1, PDGF and FGF2 expression in WI-38 cells on SF using ELISA
The effect of SF on VEGF, Ang-1, PDGF and FGF2 expression in WI-38 cells was determined by ELISA. Briefly, WI-38 cells were dispensed in 24-well culture plates containing regenerated silk fibroin films (SF group) and incubated at 37 °C. Plates without SF were used as the cell control (negative control). After incubation for 72 h, the concentrations of VEGF, Ang-1, PDGF and FGF2 in the supernatants were assessed using ELISA.
2.9. Data analysis
Data are presented as mean±standard deviation (SD). Statistical significance was evaluated using one-way repeated measure analysis of variance (ANOVA).
3. Results
3.1. Cytotoxicity experiment
The RGR of L929 cells on SF and PE films was 106% and 103%, respectively, and the cytotoxicity grades of the SF and PE films were both 0, indicating that these films are non-poisonous. The RGR of L929 cells on PVC films was 93% and its cytotoxicity was grade 1, indicating that the PVC film is ‘light poisonous’. According to the cytotoxicity grading criteria, SF film qualifies as a non-cytotoxic biomaterial.
3.2. Cell adhesion experiment
The AR of L929 cells on the SF film was close to that of those cultured on plates (control group) (P>0.05), and most L929 cells adhered to SF film within 3 h, indicating that SF film exerts no adverse effect on adhesion of L929 cells (Table 2). However, the AR of L929 cells on PVC and PE films was significantly lower than that on SF film or that of the control group (P<0.05).
Table 2.
Cell adhesion ratio of each group at different time periods
| Group | Adhesion rate (%) |
|||
| 1 h | 2 h | 3 h | 4 h | |
| SF | 4.13 | 19.10 | 84.50 | 90.30 |
| PVC | 3.05 | 4.53* | 5.33* | 6.13* |
| PE | 2.00 | 2.32* | 3.05* | 3.15* |
| Control | 5.18 | 28.70 | 95.50 | 97.80 |
P<0.05 when the PVC or PE group was compared with the SF or control group at 2, 3 or 4 h
3.3. Cell cycle analysis
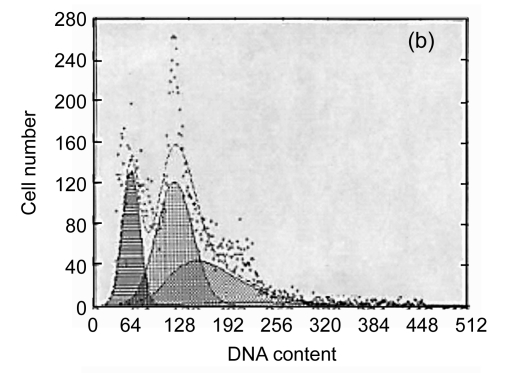
The apoptotic rates of the SF, PVC, PE and control groups were 1.7%, 25.2%, 2.9% and 2.1%, and the corresponding G2/G1 phases were 1.957, 1.773, 1.946 and 1.965, respectively (Fig. 1). There were significant differences between the PVC group and the other three groups (P<0.05). These data indicated that SF did not have an adverse influence on the cell cycle or apoptosis of L929 cells. It is a safe biomaterial.
Fig. 1.
The results of flow cytometric analysis
(a) SF; (b) PVC; (c) PE; (d) Control
3.4. Morphology and growth curve of ECV304 cells on SF
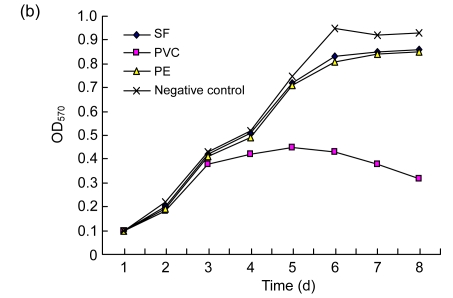
ECV304 cells adhered and grew well with a fusiform shape on SF and PE, showing no significant difference compared with the negative control group (Fig. 2a). The shape of ECV304 cells on SF was not very clear because of the rough texture and poor clarity of SF. Cells on the PVC did not grow well, and most of them were round or elliptical in shape and showed rarefaction. The ECV304 growth curve on SF was similar to those of cells on the PE and negative control (P>0.05), but the cells on the PVC showed poor growth (P<0.05) (Fig. 2b). These data indicate that SF had no adverse influence on the growth of the ECV304 cells.
Fig. 2.
The morphologies (×200) (a) and the growth curves (b) of ECV304 cells on different materials
3.5. Secretion of VEGF by ECV304 cells on SF
The amount of VEGF secreted by ECV304 cells cultured on either SF or PE was not significantly different from that of the negative control group (P>0.05), but secretion by the PVC group was significantly lower (P<0.05) (Fig. 3). Thus, regenerated silk fibroin film had no adverse influence on VEGF secretion of ECV304 cells.
Fig. 3.
The levels of VEGF secreted by ECV304 cells on different materials
3.6. Expression of VEGF, Ang-1, PDGF and FGF2 by WI-38 cells on SF
To address the effect of SF on the expression of angiogenesis-related factors, we analyzed VEGF, Ang-1, PDGF and FGF2 expression in WI-38 cells cultured on SF by ELISA. The amounts of VEGF, Ang-1, PDGF and FGF2 in WI-38 cells were similar between the SF group and the control group (P>0.05) (Fig. 4), indicating that regenerated silk fibroin film had no adverse influence on secretion of these factors in WI-38 cells. The results suggest that SF can help WI-38 cells to express stably VEGF, Ang-1, FGF2, and PDGF factors involved in angiogenesis and wound healing.
Fig. 4.
The secretion levels of VEGF, Ang-1, FGF2 and PDGF by WI-38 cells on SF
4. Discussion
Silk fibroin (SF), as a natural fibrous protein with a wide range of molecular structures, remarkable mechanical properties, controllable morphology, versatile processing ability and surface modification options, has become a promising polymeric biomaterial for tissue engineering (Altman et al., 2003; Wang et al., 2006b). By blending with other natural or synthetic polymers, such as cellulose (Freddi et al., 1995) or chitosan (Chen et al., 1997), silk fibroin can be processed into films or fiber silk fibroin scaffolds for a number of tissue engineering applications in skeletal tissues like bone and ligaments, and in connective tissues like skin. Moreover, SF films, fibers and gels have been investigated in vitro with different cell types including fibroblasts, epithelial cells, endothelial cells, glial cells, keratinocytes, hepatocytes, osteoblasts and marrow stromal cells. Promising results support the usage of SF as an implant biomaterial (Unger et al., 2004). Silk fibroin material is a novel medical biomaterial with good biocompatibility. Our study showed that the regenerated silk fibroin membrane supported cell growth and proliferation to a standard comparable to that of a cell culture plate.
The body repairing mechanism observes the following process: microvessels are formed, granulation tissue is shaped, repairing cells and nutritional ingredients are transferred to the wounded area, and finally the wound is healed. The recovery from trauma should go through three major stages step by step: inflammation, tissue formation and reparation. The most important phase is the formation of the granulation tissue full of capillaries, and a new granulo-like basement. VEGF, Ang-1, FGF2 and PDGF are cytokines involved in the late inflammatory and proliferation stages of the wound healing process and play an important role in granulation tissue formation, collagen synthesis and angiogenesis. VEGF is known to be a mitogen specific to vascular endothelium (Pettersson et al., 2000). In the process of angiogenesis, VEGF and its receptors on vascular endothelial cells are considered to be the key regulatory factors with the strongest action and the highest specificity (Neufelda et al., 1999). The hypoxial microenvironment of trauma-injured tissues and cells can up-regulate VEGF gene expression (Leung et al., 1989). VEGF can promote endothelial cell growth in vitro and induce angiogenesis in vivo (Olofsson et al., 1999). VEGF participates in the initial processes of primitive blood vessel formation, while Ang-1 promotes subsequent vascular remodeling and helps to form a mature vascular network with spatial structure (Augustin and Breier, 2003). FGF2 facilitates the regeneration of fibroblasts in the injured tissue, contributes to neovascularization, promotes the proliferation of vascular smooth muscle cells and endothelial cells, and participates in inflammatory reaction and injury repair. Nissen et al. (1998) collected post-operative wound effusion and found that FGF2 had the chemotaxis of endothelial cells and the capability to promote angiogenesis, indicating that FGF2 is also a breakaway medium of angiogenesis. PDGF can promote the proliferation of fibroblasts and vascular endothelial cells, leading to collagen aggregation, capillary angiogenesis and granulation tissue production, all of which have strong chemotactic effects on inflammatory cells. Our study found that regenerated silk fibroin film could maintain stable expression of FGF2 and PDGF by fibroblasts thus showing great potential for promoting angiopoiesis and accelerating wound healing.
An effective artificial skin should have the ability to induce endothelium differentiation and angiogenic activity, and the wound dressing should not inhibit the secretion of angiogenesis growth factors by the adhering cells. So it is important to check the effects of candidate biomaterials on the secretion of angiogenesis related factors. This research showed that SF did not have an adverse influence on the expression of VEGF, Ang-1, FGF2 and PDGF. Dermagraft is a cryopreserved human fibroblast-derived dermal substitute composed of fibroblasts, extracellular matrix, and a bioabsorbable scaffold. It is manufactured from human fibroblast cells derived from newborn foreskin tissue. The research on Dermagraft (advanced tissue sciences) showed that Dermagraft functions to promote vascularization (Jiang and Harding, 1998). SF has not yet proved to be a useful artificial skin, and can be used only as a wound dressing. Further research is needed to transform silk fibroin into an engineering biomaterial suitable for a wide range of applications. Regenerated silk fibroin film can already be used effectively to promote angiogenesis when used as a dressing for accelerating wound healing, and probably, after screening and denaturization, can also be developed into a medical biomaterial for gene engineering with wide potential application prospects and the capability to promote skin wound healing and induce angiogenesis using gene transfection techniques.
5. Conclusion
Regenerated silk fibroin film does not have an adverse influence on the growth and biofunction of fibroblasts and vascular endothelial cells. It also does not interfere with the secretion of angiogenesis growth factors such as VEGF, Ang-1, FGF2 and PDGF. Thus, regenerated silk fibroin film is an excellent biomaterial with good biocompatibility.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2005CB623906) and the Medical Development Foundation of Soochow University (No. EE134702), China
References
- 1.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 2.Augustin HG, Breier G. Angiogenesis: molecular mechanisms and functional interactions—2nd Kloster Seeon Meeting of the German Priority Research Grant “Angiogenesis”. Thromb Haemos. 2003;89(1):190–197. [PubMed] [Google Scholar]
- 3.Buffoni F, Banchelli G, Cambi S. Skin wound healing: some biological parameters in guinea pig. J Pharm Pharmacol. 1993;45:784–790. doi: 10.1111/j.2042-7158.1993.tb05685.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Li WJ, Zhong W, Lu YH, Yu TY. pH sensitivity and ion sensitivity of hydrogels based on complex-forming chitosan/silk fibroin interpenetrating polymer network. J Appl Polym Sci. 1997;65(11):2257–2262. doi: 10.1002/(SICI)1097-4628(19970912)65:11<2257::AID-APP23>3.3.CO;2-L. [DOI] [Google Scholar]
- 5.Freddi G, Romano M, Rosaria M, Tsukada M. Silk fibroin/cellulose blend films: preparation, structure and physical properties. J Appl Polym Sci. 1995;56(12):1537–1545. doi: 10.1002/app.1995.070561203. [DOI] [Google Scholar]
- 6.Gong AH, Li MZ, Sheng WH, Xie YF, Miao JC, Jiang HY, Wu WY, Yang JC. Effect of regenerated silk films on cytogenetic properties of rat embryo dermal fibroblasts. Chin J Biomed Eng. 2005;24(1):38–42. (in Chinese) [Google Scholar]
- 7.Halsted W. The employment of fine silk in preference to catgut and the advantage of transfixing tissues and vessels in controlling hemorrhage. Ann Surg. 1892;16:505–526. [Google Scholar]
- 8.Horan RL, Adam LKA, Wang Y, Huang J, Moreau JE, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials. 2005;26(17):3385–3393. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Jiang WG, Harding KG. Enhancement of wound tissue expansion and angiogenisis by matrix-embedded fibroblast (Dermagraft), a role of hepatocyte growth factor/scatter factor. Int J Mol Med. 1998;2(2):203–210. [PubMed] [Google Scholar]
- 10.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 11.Li MZ, Wu ZY, Lu SZ, Jia SX, Yao M. Development of porous fibroin membrane and study of its properties. Silk. 2001;13:10–13. (in Chinese) [Google Scholar]
- 12.Lu SZ, Li MZ, Kang N, Wu ZY. Epoxide cross linker applied to preparation of fibroin membrane. Silk. 2000;3:7–9. (in Chinese) [Google Scholar]
- 13.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28(35):5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26(2):147–155. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Miao JC, Sheng WH, Li MZ, Xie YF, Gong AH, Yang JC. Genotoxic potential of regenerated silk fibroin films by different cross-linking mode. Key Eng Mat. 2007;342-343:257–260. doi: 10.4028/www.scientific.net/KEM.342-343.257. (in Chinese) [DOI] [Google Scholar]
- 16.Mori H, Tsukada M. New silk protein: modification of silk protein by gene engineering for production of biomaterials. Rev Mol Biotechnol. 2000;74(2):95–103. doi: 10.1016/S1389-0352(00)00004-0. [DOI] [PubMed] [Google Scholar]
- 17.Nangia A, Hung CT. Laboratory evaluation of a new hydrogel-type skin substitute. Burns. 1990;16(5):368–372. doi: 10.1016/0305-4179(90)90010-T. [DOI] [PubMed] [Google Scholar]
- 18.Neufelda G, Cohena T, Gengrinovitcha S, Poltoraka Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 19.Ning L, Xue M, Huang HN. Study on biocompatibility of skin reproductive membrane. Chin J Rep Reconstr Surg. 2000;14(1):44–48. (in Chinese) [PubMed] [Google Scholar]
- 20.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, Di Pietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152(6):1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 21.Olofsson B, Jeltsch B, Eriksson U, Alitalo K. Current biology of VEGF-B and VEGF-C. Pharm Biotechnol. 1999;10:528–535. doi: 10.1016/s0958-1669(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 22.Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, et al. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest. 2000;80(1):99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- 23.Sheng WH, Gong AH, Li MZ, Xie YF, Miao JC, Yang JC, Jiang HY, Lu SZ. The study on cytotoxity of regenerated silk fibroin materials. Chin J Biomed Eng. 2005;24(3):277–281. (in Chinese) [Google Scholar]
- 24.Unger RE, Wolf M, Peters K, Motta A, Migliaresi C, Kirkpatrick CJ. Growth of human cells on a non-woven silk fibroin net: a potential for use in tissue engineering. Biomaterials. 2004;25(6):1069–1075. doi: 10.1016/S0142-9612(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 25.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32(8-9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Blasioli JD, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27(25):4434–4442. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27(36):6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]