Abstract
Inorganic phosphate (Pi) plays a critical role in diverse cellular functions, and regulating the Pi balance is accomplished by sodium-dependent Pi co-transporter (NPT). Pulmonary NPT has recently been identified in mammalian lungs. However, to date, many of the studies that have involved Pi have mainly focused on its effect on bone and kidney. Therefore, current study was performed to discover the potential effects of low Pi on the lung of developing transgenic mice expressing the renilla/firefly luciferase dual reporter gene. Two-weeks old male mice divided into 2 groups and these groups were fed either a low PI diet or a normal control diet (normal: 0.5% Pi, low: 0.1% Pi) for 4 weeks. After 4 weeks of the diet, all the mice were sacrificed. Their lungs were harvested and analyzed by performing luciferase assay, Western blotting, kinase assay and immunohistochemistry. Our results demonstrate that low Pi affects the lungs of developing mice by disturbing protein translation, the cell cycle and the expression of fibroblast growth factor-2. These results suggest that optimally regulating Pi consumption may be important to maintain health.
Keywords: Akt, fibroblast growth factor, inorganic phosphate, lung
Introduction
Inorganic phosphate (Pi) is present in bacterial, fungal, plant and animal cells. Pi plays a critical role in diverse cellular functions that are involved in intermediary metabolism and energy-transfer mechanisms. It is a vital component of the phospholipids in membranes and of nucleotides, and both of which provide energy and serve as components of DNA, RNA and the phosphorylated intermediates of cellular signaling [28]. Regulation of the Pi balance is accomplished by the family members of sodium-dependent inorganic phosphate co-transporter (NPT), and these proteins regulate entrance into the cellular membrane [5].
The existence of pulmonary NPT type II (NPT-2b) has recently been identified in the developmental stages of rat lungs, and it plays an important role in producing surfactant through regulating the phosphate uptake [15]. Moreover, a functional characterization study that was based on searching the expressed sequence tag data-base of NPT has revealed that the human lung also contains NPT, which is the ortholog of mouse NPT-2b [12,16]. Lungs are under stress due to countless burdens of external stimuli as well as internal stimuli. Therefore, understanding the cellular/molecular changes involved in how lungs deal with these stimuli may provide critical clues to treat diverse pulmonary diseases.
Since phosphate cannot be synthesized by animal itself, the need for this nutrient should be met by ingesting phosphate in the diet [31]. As a signal molecule, Pi plays an important role in the developing organs [26,28] through regulating cellular differentiation and the expression of multiple genes [2]. Thus, dietary Pi restriction may affect the signal transduction important for normal growth. However, to date, many of the previous studies involving Pi have mainly focused on its effects on bones and kidneys. Our research group has recently reported that Pi controlled lung cell growth and cap-dependent protein translation through the Akt-mediated MEK pathway [6]. However, there has been no study of investigating the response of the lung to low dietary Pi in vivo. Therefore, this current study was performed to discover the potential effects of low Pi on the lungs of young newly weaned transgenic mice that expressed the CMV-LucR-cMyc-IRES-LucF reporter gene. Transgenic mice expressing the CMV-LucR-cMyc-IRES-LucF reporter gene are convenient, powerful tools for confirming the cap-dependent and cap-independent protein translation since LucR (renilla luciferase) and LucF (firefly luciferase) provide a way to measure the level of cap-dependent and cap-independent (internal ribosome entry site-dependent; IRES-dependent) protein translation, respectively [9]. Moreover, an improved understanding of the responses of the developing lung of young animals to stimulation may provide critical functions for coping with diverse changes including alteration of pulmonary function.
Materials and Methods
Animals and diet
Eight 2-week-old transgenic male newly weaned mice that expressed the CMV-LucR-cMyc-IRES-LucF reporter gene were divided into two dietary groups based on their body weight. One group was put on a normal diet containing 0.5% Pi (normal Pi) and the other group was put on a low phosphate diet containing 0.1% Pi (low Pi). All the diets were prepared according to the guideline of the American Institute of Nutrition [23]. The mice were put on the specified diet for 4 weeks until complete physical maturation (6 weeks after birth). At the end of 4 weeks of the diet, all the mice were sacrificed and their lung tissues were harvested and then a lobe of the left lung was fixed in 10% neutral buffered formalin for immunohistochemistry. Remaining lobes of the lung were stored in liquid nitrogen for further use. All animal experiments were performed according to the guideline for the care and use of laboratory animals of Seoul National University.
Luciferase assay
The luciferase activities in the tissue extracts were measured by using a luminometer (EG&G Berthold, Australia). Briefly, the lungs were homogenized in passive lysis buffer (Promega, USA). The homogenates were centrifuged for 20 min at 4,500 rpm at 4℃, and the supernatant was centrifuged for an additional 15 min at 13,000 rpm at 4℃. The LucF and LucR activities were measured using a dual luciferase assay kit (Promega, USA).
Western blot analysis
After measuring the protein concentration of the homogenized lysates with using a Bradford kit (Bio-Rad, USA), equal amounts (50 µg) of protein were separated on sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) and the proteins were then transferred to nitrocellulose membranes. The membranes were blocked in TBST (Tris-buffered saline + Tween 20) containing 5% skim milk for 1 h; immunoblotting was performed by incubating the membranes overnight with their corresponding primary antibodies at 4℃ in 5% skim milk. Anti-NPT2b was obtained from Alpha Diagnostic International (USA). Anti-Akt1 and anti-phospho-Akt (Ser473) monoclonal antibodies were created by the methods described elsewhere [13]. Anti-phospho-Akt (Thr308), anti-eukaryotic initiation factor 4E binding protein 1 (4E-BP1), anti-phospho-4E-BP1, anti-cyclin D3, anti-cyclin-dependent kinase 4 (CDK4), anti-proliferating cell nuclear antigen (PCNA), anti-p53, anti-p27, anti-p21 and anti-FGF-2, anti-α-tubulin antibodies were purchased from Santa Cruz Biotechnology (USA). The antibody against mammalian target of rapamycin (mTOR) was obtained from Cell Signaling (USA). After washing in TBST, the membranes were incubated with a horseradish peroxidase (HRP)-labeled secondary antibody for 1 h at room temperature. The bands-of-interests were detected using a luminescent image analyzer LAS-3000 (Fujifilm, Japan). The results were quantified using the measurement program of the LAS-3000.
Immunoprecipitation and kinase assays
Immunoprecipitation of mTOR and eukaryotic translation initiation factor (eIF4E) was carried out using a Seize primary mammalian immunoprecipitation kit (Pierce, USA) according to the manufacturer's guide. The mTOR kinase assay was performed with 300 µmol/ATP and 1 µl PHAS I (Calbiochem, USA) for 30 min at 30℃. The reactions were terminated by adding ×5 sample buffer and then boiling the mixture. The samples were analyzed by performing 15% SDS-PAGE. The kinase activity of Akt was examined with using the Akt kinase assay kit (Cell Signaling Technology, USA) according to the manufacturer's instructions.
Immunohistochemistry
The formalin-fixed, paraffin-embedded tissue sections (4 µm) were transferred to plus slides (Fisher Scientific, USA). The tissue sections were deparaffinized in xylene and rehydrated through a graded series of alcohol solutions and they were incubated in 200 µl of proteinase K, and then they were washed and incubated in 3% hydrogen peroxide (AppliChem, Germany) for 30 min to quench the endogenous peroxidase activity. After washing in 1 × PBS, the tissue sections were incubated with 5% BSA in 1 × PBS for 1 h at room temperature to block the non-specific binding sites. The primary antibodies were applied on the tissue sections overnight at 4℃. The following day, the tissue sections were washed and incubated with the secondary HRP-conjugated antibodies (1:50) for 1 h at room temperature. After careful washing, the tissue sections were counterstained with Mayer's Hematoxylin (Dako, USA) and then they were washed with xylene. Cover slips were mounted using Permount (Fisher, USA), and the slides were reviewed using a light microscope (Carl Zeiss, USA). The FGF-2 and PCNA positive staining was determined by counting 5 randomly chosen fields per section and determining the percentage of DAB-positive cells per 100 cells at ×400 by the method described by Zhang et al. [32].
Statistical analysis
Quantification of the Western blot analysis was performed using the Multi Gauge version 2.02 program (Fujifilm, Japan). All the results are given as means ± SE. The results were analyzed by unpaired Student's t-tests (GraphPad Software, USA). p values < 0.05 were considered significant and p values < 0.01 were considered highly significant as compared to the corresponding control.
Results
Low dietary Pi decreased the pulmonary NPT-2b
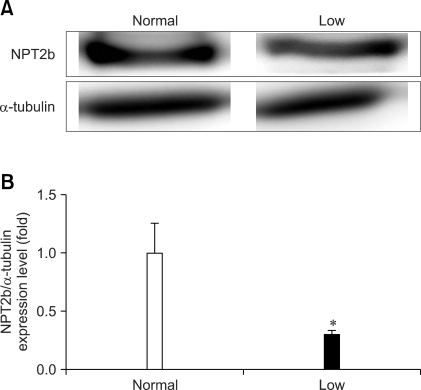
Potential effects of low dietary Pi on the lung-specific NPT2b protein expression were evaluated by Western blotting. The animals fed the low dietary Pi expressed significantly less NPT-2b protein in their lungs than did the controls (Fig. 1A). Densitometric analysis clearly reconfirmed the reduction of the NPT-2b protein expression in the low Pi diet group (Fig. 1B).
Fig. 1.
Western blot analysis of NPT2b protein in the lungs of mice that were fed a low inorganic phosphate (Pi) diet (0.1% Pi) or a normal (0.5% Pi) diet for 4 weeks. (A) The expression of NPT2b protein. (B) The bands-of-interests were further analyzed by using a densitometer. *p values < 0.05 showed a significant difference (mean ± SE, n = 4).
Low dietary Pi increased the pulmonary Akt activity
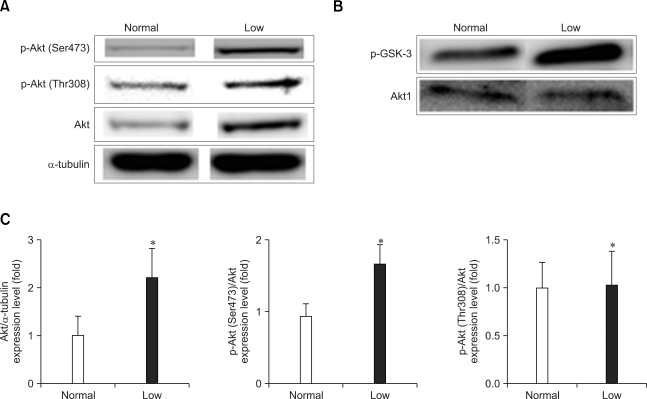
Low dietary Pi significantly increased the total protein expression of Akt1, and it increased Akt phosphorylation at Ser473. In contrast, the Akt phosphorylation at Thr308 remained unchanged (Figs. 2A and C). For clearly detecting the effect of low dietary Pi on Akt activity, an Akt kinase assay was performed. Our results clearly demonstrated that Akt kinase activity was increased about 7 fold in low dietary Pi group than normal diet group (p < 0.01) (Fig. 2B).
Fig. 2.
Western blot analysis of the Akt and phospho-Akt protein in the lungs of mice fed a low Pi diet (0.1% Pi) or a normal (0.5% Pi) diet for 4 weeks. (A) The expressions of Akt and phospho-Akt protein in the lungs. (C) The bands-of-interests were further analyzed by using a densitometer. (B) The Akt kinase activity was measured in the lung homogenates. *p values < 0.05 showed a significant difference compared with normal (mean ± SE, n = 4).
Low dietary Pi facilitated cap-dependent protein translation
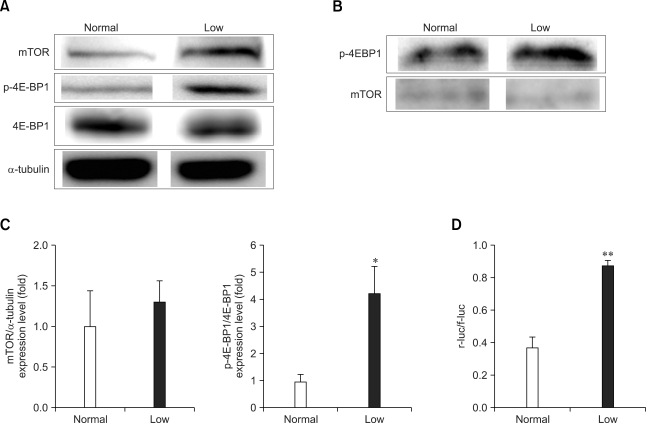
Our results demonstrated that low dietary Pi increased the phosphorylation of 4E-BP1, while the protein expression of mTOR was slightly increased without statistical significance (Figs. 3A and C). However, the net results were an increase of mTOR kinase activity (Fig. 3B) and facilitated cap-dependent protein translation, as was shown on the dual luciferase assay (normal group: 0.38 ± 0.12; low dietary group: 0.87 ± 0.07) (Fig. 3D).
Fig. 3.
Western blot analysis of the mammalian target of rapamycin (mTOR), 4E-PB1 and p-4E-BP1 protein in the lungs of mice fed a low Pi diet (0.1% Pi) or a normal (0.5% Pi) diet for 4 weeks. (A) The expressions of mTOR, 4E-PB1 and p-4E-BP1 protein in the lungs. (B) The mTOR kinase activity and phosphorylation ratio for 4E-BP1 were measured in the lung homogenates. (C) The bands-of-interests were further analyzed by using a densitometer. (D) The luciferase activities were measured in the tissue homogenate from lung, and the ratios of the cap-dependent (r-luc) to the IRES dependent (f-luc) protein translation are shown. p values (*p < 0.05, **p < 0.01) indicate a significant difference compared with normal (mean ± SE, n = 4).
Low dietary Pi affected the signals important for cell cycle control
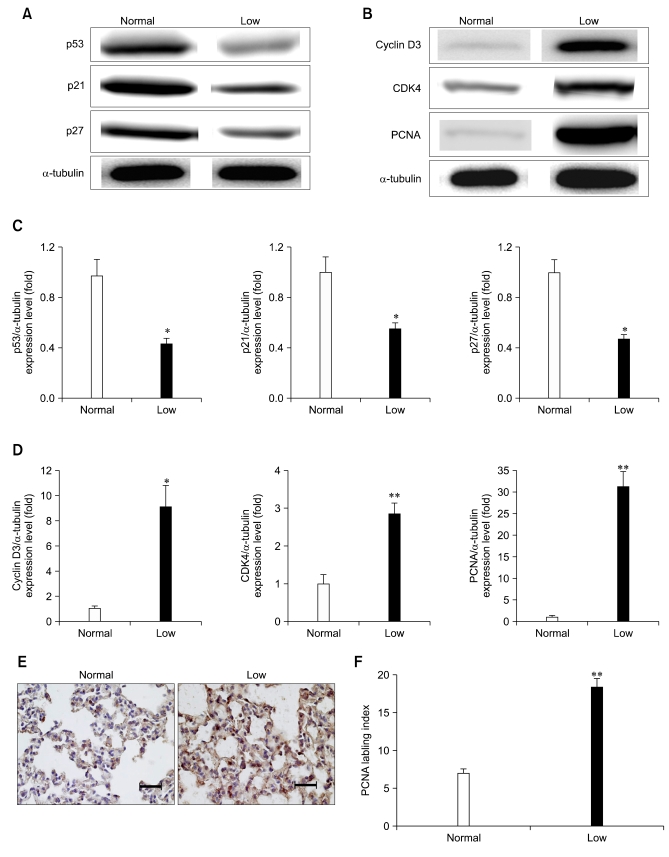
Low Pi decreased the protein expressions of p53, p21 and p27 (Figs. 4A and C). In contrast, low Pi significantly increased the protein expressions of cyclin D3, CDK4 and PCNA (Figs. 4B and D). IHC analysis of PCNA clearly showed that low dietary Pi stimulated lung cell proliferation in the lungs of the dual luciferase reporter mice (Figs. 4E and F).
Fig. 4.
Western blot analysis of the cell cycle signaling proteins. The lungs of mice fed a low Pi diet (0.1% Pi) or a normal (0.5% Pi) diet for 4 weeks. (A) The expressions of p53, p21 and p27 protein in lung. (B) The expressions of cyclin D3, cyclin-dependent kinase 4 (CDK4) and proliferating cell nuclear antigen (PCNA) protein in lung. (C, D) The bands-of-interests were further analyzed by using a densitometer. (E) Immunohistochemical measurement of PCNA in the lung. The dark brown color indicates the PCNA expression (scale bar = 100 µm). (F) Comparison of the PCNA labeling index in the lungs. p values (*p < 0.05, **p < 0.01) indicate a significant difference compared with normal (mean ± SE, n = 4).
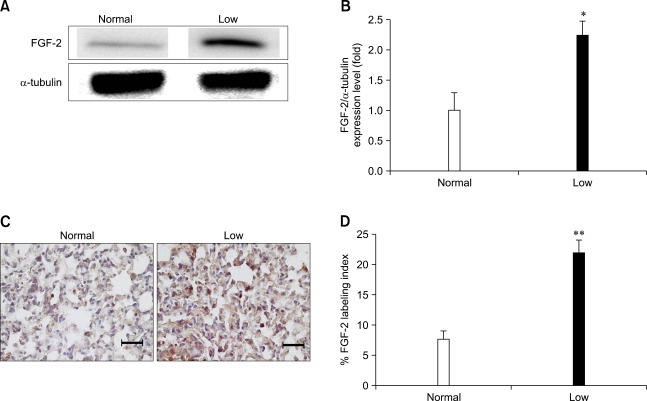
Low dietary Pi increased the FGF-2 protein expression
Low dietary Pi significantly increased the FGF-2 protein expression as shown on Western blotting and densitometric analysis (Figs. 5A and B). Such an overexpression of FGF-2 was clearly demonstrated by the IHC study. As shown Figs. 5C and D, FGF-2 expression was increased about 7.5 fold in the low Pi diet group than control group.
Fig. 5.
Analysis of fibroblast growth factor 2 (FGF-2) protein in the lungs of mice fed a low Pi diet (0.1% Pi) or a normal (0.5% Pi) diet for 4 weeks. (A) The expression of FGF-2 protein in the lung. (B) The bands-of-interests were further analyzed by using a densitometer. (C) Immunohistochemical measurement of FGF-2 in the lung of transgenic mice. The dark brown color indicates the expression of FGF-2 (scale bar = 100 µm). (D) Comparison of the FGF-2 labeling index in the lungs. p values (*p < 0.05, **p < 0.01) indicate a significant difference compared with normal (mean ± SE, n = 4).
Discussion
Non-oncogenic lung tissues as well as oncogenic lung tissues often display alterations of the gene expressions in the signal transduction pathways that are responsible for homeostasis, yet the exact mechanisms by which the genes modulate abnormal cell growth/differentiation have yet to be determined. Such alterations are likely associated with cellular changes that involve an imbalance between cell proliferation, DNA repair and cell death. Additionally, these alterations may result from cellular aging and/or insults from endogenous or exogenous chemical exposure [6].
Pi is normally taken from the diet, and the intestinal absorption of Pi is efficient and well regulated. The kidney is a major regulator of Pi homeostasis and it can increase or decrease its Pi reabsorptive capacity to accommodate the need for Pi. The bulk of filtered Pi is reabsorbed in the proximal tubule where the sodium-dependent Pi transport system in the brush-border membrane mediates the rate-limiting step in the overall Pi reabsorptive process [27]. As mentioned previously, Pi plays a key role in diverse physiological functions. Several lines of research have indicated that Pi works as a stimulus that is capable of increasing or decreasing the expression of several pivotal genes such as transcriptional regulators, signal transducers and cell cycle regulators through controlling the sodium/phosphate co-transporter 2 (NPT-2) expression in the lung [6,17]. Together, the potential importance of Pi, as a novel signaling molecule, and the pulmonary expression of NPTs together with the poor prognosis of many diverse lung diseases have prompted us to begin defining the pathways by which low dietary Pi regulates lung cell growth.
Among the 3 classes of NPTs (Types 1, 2 and 3), two types (Types 2 and 3) have been identified in mammalian lung and there has been considerable progress in understanding their function and regulation. Pi transport into the lung cells is mainly regulated by the dietary Pi value through controlling the NPT expression [28]. In our study, low dietary Pi suppressed the protein expression of NPT-2b in the lungs of developing mice, and this suggests that low dietary uptake of Pi for a critical period may disturb the function of lung. In fact, our finding is supported by the recent report that NPT-2b may function in alveolar type II cells as a surfactant producer because phosphate in the alveolar type II cells is an essential constituent of phospholipids, which are a major component of surfactant [15]. Moreover, another line of evidence has demonstrated that the availability of phosphate for surfactant synthesis might be accompanied by sodium-dependent phosphate uptake [7]. Together, pulmonary NPTs may play a critical role in collecting inorganic phosphate for important functions. Further studies that will focus on the effects of low Pi on the pulmonary function would clarify the eventual molecular/cellular events in lung development.
Akt is a serine/threonine kinase that is a crucial mediator in signaling pathways [3], and Akt signaling plays an important role for mouse lung development through regulating cell survival and cell proliferation [30]. Low Pi induced Akt phosphorylation at Ser473 with an increase of the total Akt protein expression and increased Akt activity. Jin et al. [18] also showed that low dietary Pi significantly increased the Akt phosphorylation at Ser 473 in murine brain cells. Together, these data suggest that low Pi may play a key role in cell proliferation as well as cell differentiation through controlling the Akt activity. A recent report also indicated that activation of NPTs mediates the activation of multiple signaling pathways, including PI3/Akt signaling [22]. Moreover, our group reported that nano-aerosol delivery of the wild type Akt controls protein translation in a way to preferentially increase the cap-dependent protein translation through the increase of Akt phosphorylation at Ser473 and 4E-BP1 in the lungs of mice [29]. Our current results very well match with the previous findings such that low Pi caused the selective increase of cap-dependent protein translation.
As previously mentioned, Akt/mTOR is involved in complex regulation of the cell cycle [20]. As shown in Fig. 4, many signals were up-regulated (cyclin D3, CDK4 and PCNA) or down-regulated (p53, p27 and p21) by low dietary Pi. The p53 protein is a major tumor suppressor, and it exerts its effects on the cell cycle and apoptosis primarily via its activity as a transcription factor that controls over a hundred genes [11,29]. Notably, one of the genes regulated by p53 is p21, which is a cell cycle inhibitor that acts by inhibiting cyclin-dependent kinases [1]. Remarkably, p21 controls PCNA through binding at the site of polδ [10]. Our results strongly demonstrated that low dietary Pi may disturb the control of the cell cycle by loss of the key function of cell cycle arrest. The p53 protein has been termed the 'Guardian of the Genome' [14], and so its down-regulated role in the controlling the cell cycle due to low dietary Pi may be a molecular manifestation of this function.
Members of the FGF family have functions for cell division and migration, thus, they affect the developmental process, angiogenesis, wound healing and tumorigenesis [21]. FGFs are also known to play a prominent role in lung development [8,19] as well as in alveolar type II cell-specific activities [4,24]. FGF-2 is also expressed by alveolar type II cells [25] and FGF-2 regulates cell proliferation and survival through the activation of multiple signaling pathways, including Akt [21]. Our results strongly suggest that low dietary Pi during physical maturation after weaning may affect the normal lung development by disturbing the Akt-FGF-2 signals. Our findings were supported by the recent report that FGF stimulated signal transduction via the Akt pathways in primary rat alveolar type II cells [21]. Further works to uncover the crosstalk between FGF-2 and Akt would provide detailed information on how such signals function in the development of lung.
In summary, our results suggest that low dietary Pi may affect the normal lung development of young newly weaned mice through altering the processes of protein translation and cell cycle regulation and the expression of FGF-2. The control of dietary Pi on such pivotal signaling pathways may be involved in numerous biological processes during development, and the deregulation of this control by low levels of Pi may cause various pulmonary diseases. Extensive studies to determine the precise effects and mechanisms, including the shift in cap-dependent versus cap-independent translation, of such activated signals on both the development of the lung and the pathogenesis of lung disease are currently underway. Our results suggest that the optimal regulation of Pi consumption may be one of the most cost-effective approaches to maintain health.
Acknowledgments
This work was partially supported by the grants from the KOSEF (M20704000010-07M0400-01010) of the Ministry of Science and Technology in Korea. S.H.C., M.W., M.S.N. and M.H.C. were supported by the Nano Systems Institute-National Core Research Center (NSI-NCRC) program of the KOSEF. C.X.X., H.J., Y.S.C., J.Y.S., S.K.H., J.T.K., S.J.P., E.S.L. and A.M.T. are also grateful for being awarded the BK21 fellowship. K.H.L was supported by the 21C Frontier Functional Human Genome Project (FG03-0601-003-1-0-0) and the National Nuclear R&D Program from the Ministry of Science and Technology.
References
- 1.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Beck GR, Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 3.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 4.Cardoso WV, Itoh A, Nogawa H, Mason I, Brody JS. FGF-1 and FGF-7 induce distinct patterns of growth and differentiation in embryonic lung epithelium. Dev Dyn. 1997;208:398–405. doi: 10.1002/(SICI)1097-0177(199703)208:3<398::AID-AJA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Caverzasio J, Bonjour JP. Characteristics and regulation of Pi transport in osteogenic cells for bone metabolism. Kidney Int. 1996;49:975–980. doi: 10.1038/ki.1996.138. [DOI] [PubMed] [Google Scholar]
- 6.Chang SH, Yu KN, Lee YS, An GH, Beck GR, Jr, Colburn NH, Lee KH, Cho MH. Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells. Am J Respir Cell Mol Biol. 2006;35:528–539. doi: 10.1165/rcmb.2005-0477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerici C, Soler P, Saumon G. Sodium-dependent phosphate and alanine transports but sodium-independent hexose transport in type II alveolar epithelial cells in primary culture. Biochim Biophys Acta. 1991;1063:27–35. doi: 10.1016/0005-2736(91)90349-d. [DOI] [PubMed] [Google Scholar]
- 8.Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128:2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- 9.Créancier L, Mercier P, Prats AC, Morello D. c-myc Internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Mol Cell Biol. 2001;21:1833–1840. doi: 10.1128/MCB.21.5.1833-1840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Deiry WS. p21/p53, cellular growth control and genomic integrity. Curr Top Microbiol Immunol. 1998;227:121–137. doi: 10.1007/978-3-642-71941-7_6. [DOI] [PubMed] [Google Scholar]
- 11.Fang MZ, Mar WC, Cho MH. Cell cycle was disturbed in the MNNG-induced initiation stage during in vitro two-stage transformation of Balb/3T3 cells. Toxicology. 2001;163:175–184. doi: 10.1016/s0300-483x(01)00400-0. [DOI] [PubMed] [Google Scholar]
- 12.Feild JA, Zhang L, Brun KA, Brooks DP, Edwards RM. Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem Biophys Res Commun. 1999;258:578–582. doi: 10.1006/bbrc.1999.0666. [DOI] [PubMed] [Google Scholar]
- 13.Fuller SA, Takahashi M, Hurrell JGG. Preparation of monoclonal antibodies. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York: John Wiley and Sons; 2007. pp. 11.4.1–11.11.5. [Google Scholar]
- 14.Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Wang DY, Kamo T, Zhu Y, Tsujiuchi T, Konishi Y, Tanaka M, Sugimura H. Isolation and localization of type IIb Na/Pi cotransporter in the developing rat lung. Am J Pathol. 2000;157:21–27. doi: 10.1016/S0002-9440(10)64512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci USA. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Chang SH, Xu CX, Shin JY, Chung YS, Park SJ, Lee YS, An GH, Lee KH, Cho MH. High dietary inorganic phosphate affects lung through altering protein translation, cell cycle, and angiogenesis in developing mice. Toxicol Sci. 2007;100:215–223. doi: 10.1093/toxsci/kfm202. [DOI] [PubMed] [Google Scholar]
- 18.Jin H, Hwang SK, Kwon JT, Lee YS, An GH, Lee KH, Prats AC, Morello D, Beck GR, Jr, Cho MH. Low dietary inorganic phosphate affects the brain by controlling apoptosis, cell cycle and protein translation. J Nutr Biochem. 2008;19:16–25. doi: 10.1016/j.jnutbio.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Lane DP. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 21.Newman DR, Li CM, Simmons R, Khosla J, Sannes PL. Heparin affects signaling pathways stimulated by fibroblast growth factor-1 and -2 in type II cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L191–L200. doi: 10.1152/ajplung.00284.2003. [DOI] [PubMed] [Google Scholar]
- 22.Nishiwaki-Yasuda K, Suzuki A, Kakita A, Sekiguchi S, Asano S, Nishii K, Nagao S, Oiso Y, Itoh M. Vasopressin stimulates Na-dependent phosphate transport and calcification in rat aortic smooth muscle cells. Endocr J. 2007;54:103–112. doi: 10.1507/endocrj.k06-093. [DOI] [PubMed] [Google Scholar]
- 23.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee of the reformulation on the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 24.Sannes PL, Khosla J, Cheng PW. Sulfation of extracellular matrices modifies responses of alveolar type II cells to fibroblast growth factors. Am J Physiol. 1996;271:L688–L697. doi: 10.1152/ajplung.1996.271.5.L688. [DOI] [PubMed] [Google Scholar]
- 25.Sannes PL, Khosla J, Li CM, Pagan I. Sulfation of extracellular matrices modifies growth factor effects on type II cells on laminin substrata. Am J Physiol. 1998;275:L701–L708. doi: 10.1152/ajplung.1998.275.4.L701. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki O, Kamakura S, Katagiri T, Nakamura M, Zhao B, Honda Y, Kamijo R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials. 2006;27:2671–2681. doi: 10.1016/j.biomaterials.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Takeda E, Taketani Y, Morita K, Tatsumi S, Katai K, Nii T, Yamamoto H, Miyamoto K. Molecular mechanisms of mammalian inorganic phosphate homeostasis. Adv Enzyme Regul. 2000;40:285–302. doi: 10.1016/s0065-2571(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 28.Takeda E, Yamamoto H, Nashiki K, Sato T, Arai H, Taketani Y. Inorganic phosphate homeostasis and the role of dietary phosphorus. J Cell Mol Med. 2004;8:191–200. doi: 10.1111/j.1582-4934.2004.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tehrani AM, Hwang SK, Kim TH, Cho CS, Hua J, Nah WS, Kwon JT, Kim JS, Chang SH, Yu KN, Park SJ, Bhandari DR, Lee KH, An GH, Beck GR, Jr, Cho MH. Aerosol delivery of Akt controls protein translation in the lungs of dual luciferase reporter mice. Gene Ther. 2007;14:451–458. doi: 10.1038/sj.gt.3302879. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Ito T, Udaka N, Okudela K, Yazawa T, Kitamura H. PI3K-AKT pathway mediates growth and survival signals during development of fetal mouse lung. Tissue Cell. 2005;37:25–35. doi: 10.1016/j.tice.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Weiner ML, Salminen WF, Larson PR, Barter RA, Kranetz JL, Simon GS. Toxicological review of inorganic phosphates. Food Chem Toxicol. 2001;39:759–786. doi: 10.1016/s0278-6915(01)00028-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Liu Q, Lantry LE, Wang Y, Kelloff GJ, Anderson MW, Wiseman RW, Lubet RA, You M. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 2000;60:901–907. [PubMed] [Google Scholar]