Abstract
The lack of scientific evidence on the constituents, properties, and health effects of second-hand waterpipe smoke has fueled controversy over whether public smoking bans should include the waterpipe. The purpose of this study was to investigate and compare emissions of ultrafine particles (UFP, <100 nm), carcinogenic polyaromatic hydrocarbons (PAH), volatile aldehydes, and carbon monoxide (CO) for cigarettes and narghile (shisha, hookah) waterpipes. These smoke constituents are associated with a variety of cancers, and heart and pulmonary diseases, and span the volatility range found in tobacco smoke.
Sidestream cigarette and waterpipe smoke was captured and aged in a 1 m3 Teflon-coated chamber operating at 1.5 air changes per hour (ACH). The chamber was characterized for particle mass and number surface deposition rates. UFP and CO concentrations were measured online using a fast particle spectrometer (TSI 3090 Engine Exhaust Particle Sizer), and an indoor air quality monitor. Particulate PAH and gaseous volatile aldehydes were captured on glass fiber filters and DNPH-coated SPE cartridges, respectively, and analyzed off-line using GC–MS and HPLC–MS. PAH compounds quantified were the 5- and 6-ring compounds of the EPA priority list. Measured aldehydes consisted of formaldehyde, acetaldehyde, acrolein, methacrolein, and propionaldehyde.
We found that a single waterpipe use session emits in the sidestream smoke approximately four times the carcinogenic PAH, four times the volatile aldehydes, and 30 times the CO of a single cigarette. Accounting for exhaled mainstream smoke, and given a habitual smoker smoking rate of 2 cigarettes per hour, during a typical one-hour waterpipe use session a waterpipe smoker likely generates ambient carcinogens and toxicants equivalent to 2–10 cigarette smokers, depending on the compound in question. There is therefore good reason to include waterpipe tobacco smoking in public smoking bans.
Keywords: Environmental tobacco smoke, Aerosol dynamics, Environmental chamber, Shisha, Hookah
1. Introduction
Exposure to second-hand smoke from cigarettes has been found to pose significant health risks due to its toxic and carcinogenic effects (USDHHS, 2006; UKDHSS,1988; ANHMRC,1987; NRC,1986). On this basis, an increasing number of regulatory bodies around the world have banned most forms of tobacco smoking in public indoor spaces such as restaurants, bars, government buildings, and schools, and some have banned it in outdoor places such as public parks and beaches. Smoking bans have stirred controversy with café owners and patrons, regulatory agencies, and tobacco control advocates over whether the bans should apply to waterpipe (narghile, hookah, shisha) smoking. The lack of scientific evidence on the constituents, properties, and health effects of second-hand waterpipe smoke has left the subject open to conjecture, particularly in light of persistent beliefs in the “reduced harm” nature of waterpipe smoking (e.g. Jawaid et al., 2008; Smith-Simone et al., 2008).
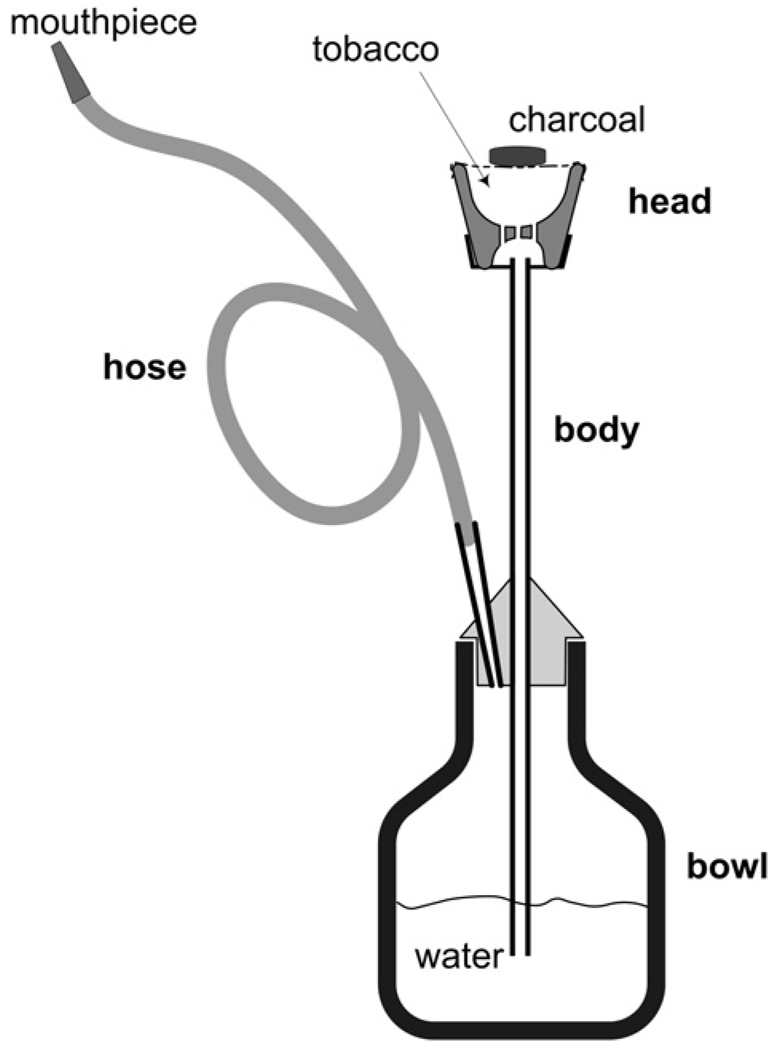
While over the past decades a formidable evidence base has been built about the nature and health effects of second-hand cigarette smoke, little is known about the fumes emitted from tobacco smoking with the narghile waterpipe (aka “shisha”, “hookah”; see Fig. 1), a practice that uses burning charcoal in conjunction with an often heavily-flavored tobacco product to produce the desired smoke. This knowledge gap has become particularly salient in the past decade with the global rise in narghile waterpipe use (Cobb et al., 2010; Pärna et al., 2008; Baska et al., 2008; El-Roueiheb et al., 2008; Jawaid et al., 2008; Eissenberg et al., 2008; Primack et al., 2008; WHO, 2005), which commonly occurs outdoors as well as in homes, restaurants, bars, and cafés. Through “involuntary” or “passive” smoking, occupants of these spaces may be exposed to significant levels of hazardous substances issuing from the waterpipe.
Figure 1.
Schematic of a narghile waterpipe. The head, body, water bowl, and hose are the primary elements from which the waterpipe is assembled. Tobacco is loaded into the head, and burning charcoal is placed on top of the tobacco. When a user inhales from the mouthpiece, air and hot charcoal fumes are convected through the tobacco, raising its temperature, and generating the desired smoke. The smoke exits from the bottom of the head, into the body, and through the bubbler and hose to the user. The waterpipe illustrated here, and used in this study, is configured for use with sweetened and flavored tobacco, known as ma’ssel. When ma’ssel is used, a relatively deep (approximately 3 cm) head is filled with 10–20 g of the flavored tobacco mixture and covered with an aluminum foil sheet that is perforated for air passage. Burning charcoal is placed on top of the aluminum foil to provide the heat needed to generate the smoke. Similar quantities of charcoal and tobacco mixture are consumed in a typical 1 h café use session. (Figure adapted from Monzer et al., 2008.)
It has been previously found that mainstream smoke (MS) from the narghile waterpipe delivers large quantities of nicotine, particulate matter, CO, PAH, volatile aldehydes and ultrafine particles to the user (Al Rashidi et al., 2008; Sepetdjian et al., 2008; Monn et al., 2007; Shihadeh and Saleh, 2005). It can be reasonably expected that after inhalation, some fraction of these toxicants will be exhaled into the immediate environment of the user, and, combined with sidestream smoke (SS) emitted directly from the waterpipe head (see Fig. 1), will result in an increase in ambient pollutant levels. Indeed, recent studies have found elevated pollutant levels in indoor environments where waterpipes were smoked (Fromme et al., 2009; Maziak et al., 2008; El-Nachef and Hammond, 2008).
The purpose of the current study is to quantify and compare hourly emissions of ultrafine particles (UFP, <100 nm), particulate PAH, CO, and gaseous volatile aldehydes in waterpipe and cigarette SS, and to estimate the total (SS + exhaled MS) hourly emissions of these toxicants for a typical waterpipe use session. Exposure to trace quantities of aldehydes, PAH, and CO has been linked to lung cancer, and respiratory and cardiovascular diseases. In addition, insoluble UFP are capable of translocation from the lung to other sites such as the lymph nodes, spleen, heart, and bone marrow, and their high surface area to mass ratio increases their biological activity in relation to larger particles of the same chemical composition (Oberdörster et al., 2005).
The approach taken in this study was to measure SS emissions using an environmental chamber for which a single-compartment mass or number balance model was rigorously applicable. To do so, time-resolved or total integrated smoke component concentrations were measured in an inert, well-stirred environmental chamber of known particle mass and number deposition rate while the waterpipe or cigarette was machine-smoked. Total emissions – SS plus exhaled MS (eMS) – were then estimated for each smoke component by assuming the smoker absorbs a fraction of any given inhaled MS toxicant, which can at most vary from zero to one.
Use of a smoking-machine/environmental chamber approach affords repeated measurements under highly controlled conditions, with minimal confounding variables (e.g. varying smoking behavior, other sources of airborne pollutants, unknown air change rates, uncharacterized surface deposition and reaction mechanisms). This approach has been previously employed to study second-hand smoke (e.g. Charles et al., 2007; Baek and Jenkins, 2004; Morawaska et al., 1997), and is particularly useful for comparing different smoked products.
2. Methods
SS from awaterpipe or cigarette was generated and routed to an experimental chamber which allows dilution and ageing processes characteristic of an indoor environment. The overall experimental setup is shown in Fig. 2. The waterpipe hose or cigarette is connected to an external smoking machine (see Shihadeh and Azar, 2006), while the rest of the waterpipe or cigarette is placed in a vertically-oriented cylindrical dilution tunnel (24 cm diameter, 67 cm height) fitted with a tapered cone roof. The tunnel captures the smoke coming off the head, dilutes it, and routes it into the 1 × 1 ×1 m ageing chamber. Repeated experiments showed that placement of the waterpipe inside the tunnel had no effect on the amount of tobacco burned in the head, indicating representative combustion conditions inside the tunnel.
Figure 2.
Schematic of the experimental setup used in this study. Air change (1.5 ACH) is driven by computer-controlled sampling trains located on the right hand side of the chamber. SS enters the chamber from the roof of the dilution tunnel, and HEPA-filtered lab air enters at the bottom of the tunnel. All surfaces are Teflon® coated.
The ageing chamber is fitted with a series of ports which are connected to external sampling pumps whose flow rates are monitored and regulated by a series of computer-controlled valves and mass flow meters. The chamber air change rate is set to 1.5 h−1 (1.5 ACH) using the data acquisition and control software, and is verified for every run by CO decay. The temperature, humidity, CO, and CO2 are continuously recorded using a Kanomax IAQ monitor whose sampling wand is suspended from the chamber ceiling. All internal surfaces, including the fan and IAQ wand, are coated with Teflon to minimize surface reactions.
To assure well-mixed conditions and therefore sampling location-independent emissions measurements, detailed consideration of the mixing patterns inside the chamber was made by 3-D numerical simulations of the velocity and concentration fields resulting from the inlet and outlet flows as well as various fan flow rates and placements. For this purpose, the Lagrangian discrete phase model in Fluent 6.3 was used, assuming particles of 250 nm diameter and unit density. Turbulence closure was achieved through application of the two-equation k–ε model. Results were checked at multiple grid resolutions, and second order discretization schemes were used. To check for mixing homogeneity, the simulated chamber volume was divided into 9 equidistant 1 m2 surfaces in the x–y, y–z, and x–z planes and the average particle concentration was computed over each surface. The maximum deviation of any plane from the chamber mean concentration was less than 3%. Further validation was obtained experimentally by measuring total particle matter concentration simultaneously at the four sampling ports of the chamber (Fig. 2), and it was found that the four ports provided the same particle concentration within the sampling uncertainty.
2.1. Determination of emission factors
Emission factors were defined as total mass of pollutant emitted per waterpipe use session. For carbon monoxide and ultrafine particles, emitted mass was determined by mass balance using the time-resolved concentration measurements. For a well-stirred chamber of constant volume Vc, it can be shown that the emitted mass (or number), me, of any constituent during the sampling time interval ts can be determined as
| (1) |
where C is the mass (number) concentration and Q is the volume flow rate through the chamber, and md is the mass (number) lost by deposition or surface reactions on the chamber walls. For volume-integrated determination of volatile aldehyde and PAH compounds, the sampling time after smoking was sufficiently large (e.g. 3 air changes) that the first term on the right hand side of equation (1) could be neglected. In this case, the mass emitted by the waterpipe was computed as
| (2) |
where C̄ is the mass concentration determined by off-line chemical analysis of the sampling filter (PAH) or cartridge (aldehydes) and ΔV is the total volume of air displaced through the chamber from the start of the smoking session to the end of the sampling period (ΔV = t·ACH·Vc).
While md was assumed to be zero for gaseous species due to the inert Teflon coating, diffusiophoresis and impaction may result in significant surface losses for the particle-phase components (e.g. UFP, PAH). Particle number and mass deposition in the chamber was assessed by measuring number and volume concentration decay rates (see Jamriska and Morawska, 2003) with tobacco smoke and with condensation-generated diethylhexylsebecate (DEHS) test aerosol of size characteristics similar to tobacco smoke. For UFP number emissions, number deposition was inferred from waterpipe smoke decay measurements following removal of the waterpipe from the dilution tunnel or following the end of DEHS injection. More specifically, the total number concentration decay rate was compared to that predicted by a sectional numerical solution of the general dynamic equation (Friedlander, 2000) for an inert, coagulating, and convecting aerosol in a well-stirred chamber with no wall deposition:
| (3) |
where Nk is the number concentration in size bin k, v is particle volume, β is the Brownian collision frequency function, and γ is the fraction of particles removed per unit time due to air change. Equation (3) was summed over all size bins at each time step to obtain the total number concentration in the chamber, from which the resulting theoretical decay rate could be obtained. The initial size distribution was given by the spectrometer measurement at the start of the decay period. The differences between measured and theoretical particle number decay rate were attributed to wall deposition, and in six repeated trials (3 for smoke, 3 for DEHS) was found to be less than 4% of the ventilation rate, and therefore had a negligible impact on computed number emission factors. Similar results were obtained for particle mass decay with DEHS. We used DEHS to determine excess mass decay by surface deposition because, unlike waterpipe smoke, it is non-volatile. In summary, both number and mass surface deposition losses were negligible relative to ventilation.
2.2. Smoke generation
For all waterpipe smoking sessions, the hose of the waterpipe was connected to a smoking machine programmed to produce 171 puffs of 2.6 s duration, 0.53 L volume, and 17 s interpuff interval. These puff parameters were based on analysis of smoking topography data for 52 waterpipe users in Beirut-area cafés (see Shihadeh et al., 2004). The waterpipe geometry and preparation methodology were as described in Shihadeh (2003) and Shihadeh and Saleh (2005). Briefly, 10 g of Nakhla brand (Cairo, Egypt), Two Apples flavor ma’ssel tobacco mixture was loaded into the waterpipe head. A single quick light charcoal briquette (Three Kings, Holland) commonly used for waterpipe smoking was placed on the waterpipe at the start of the smoking session, and a half-briquette added at the 105th puff. The charcoal briquettes were lit under an exhaust snorkel and allowed to burn for an additional minute prior to placement on the waterpipe head. The leather waterpipe hose used in the study exhibited an infiltration rate of 2 lpm while drawing a puff (see Saleh and Shihadeh, 2008 for infiltration measurement methods).
For cigarette tests, the Massachusetts “intense” protocol was followed except that ventilation holes were not blocked. All tests were conducted with Marlboro Red cigarettes purchased at retail outlets adjacent to the American University of Beirut.
2.3. Sampling and chemical analysis
Particle size distributions in the range 5.6–560 nm were measured at a frequency of 1 Hz using a fast particle mobility spectrometer (TSI 3090 Engine Exhaust Particle Sizer) attached to one of the chamber’s sampling ports. The spectrometer provides number concentration in 32 geometrically spaced size bins spanning the range 5.6–560 nm. Time-resolved concentration of ultra-fine particles (<99.5 nm) was determined by summing particle counts in the relevant size bins at each point in time.
For PAH, duplicate samples were generated for each experiment using 47 mm glass fiber filters (Pall type A/E) installed on two separate sampling ports of the chamber, each operating at a flow rate of 8 lpm. At the end of each run, the filters were removed and each was sonicated in 10 ml of toluene for 2 h, and the resulting solution was concentrated to 1 ml under a flow of nitrogen. The concentrated solution was eluted with 10 ml of hexane through a conditioned SPE silica cartridge, and evaporated to a final volume of 100–200 µl before injection in the GC–MS. Chromatographic separation was achieved with an Alltech AT-5ms column (30m × 0.25mm, 0.25 µm film thickness), using helium gas as the carrier phase. Quantification was done in the selected ion current mode, using calibration curves derived from area determinations of filters spiked with PAH standards at concentrations of 0.2–0.4–1–1.6–2 ppm. Correlation coefficients (R2) of extracted calibration curves ranged between 0.991 and 0.999. Additional details of the quantification procedure are given in Sepetdjian et al. (2008).
Volatile aldehyde compounds were sampled from a single port of the chamber operating at 0.5 lpm. The aldehydes were collected and derivatized to stable hydrazone species using 2,4-dinitrophenylhydrazine (DNPH) treated SPE cartridges (H10 Lp-DNPH, Supelco©). To prevent particulate matter from blocking the SPE cartridge and its ozone trap, smoke drawn from the chamber was passed through a single 47 mm glass fiber filter (Pall type A/E) located immediately upstream of the SPE cartridge. Prior to adopting this procedure, we verified that inclusion of the upstream particulate filter did not bias the measurement by separately assessing gas and particle-bound aldehydes using the methods presented in Al Rashidi et al. (2008). After sampling, both filters and cartridges were covered with aluminum foil and stored at 4 °C, normally for less than 24 h.
H10 Lp-DNPH cartridges were eluted with 5 ml of HPLC grade acetonitrile, filtered, and analyzed using HPLC–MS system (Agilent 1100) equipped with a photodiode array detector set at λ = 360 nm. Gradient elution on a reverse phase C-18 column (25 cm × 4.6 mm, 5 µm) was performed. The solvents used were (A) water/acetonitrile/THF (6:3:1 v/v/v), (B) water/acetonitrile (2:3 v/v), and (C) acetonitrile. The MS analysis was conducted using atmospheric pressure photo-ionization (APPI). Carbonyl compounds were identified based on their chromatographic retention times as well as the mass spectrometric fragmentation patterns as compared to the calibration standards. Their concentrations were determined using recovered standard calibration curves which accounted for any losses during elution or extraction. Further details are given in Al Rashidi et al. (2008).
2.4. Data analysis
Mean and 95% confidence intervals were computed for all measures. Confidence intervals were computed using two-tailed Student’s t-distribution. Uncertainties in total PAH and aldehydes were calculated using a first-order error propagation method (Figliola and Beasley, 1995). In addition, differences in means between cigarette and waterpipe were analyzed for significance also using two-tailed Student’s t-distribution. Probability values below 0.05 were taken to indicate a statistically significant difference.
3. Results
3.1. Ultrafine particles and size distribution
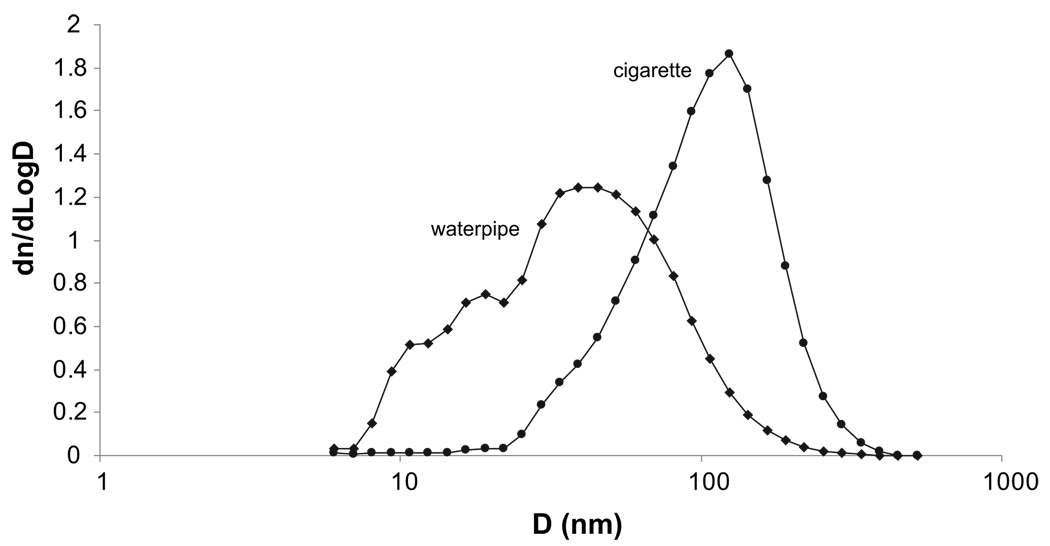
Average ultrafine sidestream particle emissions for 4 repeated waterpipe smoking sessions was 3.99 ± 0.60 × 1012 particles/waterpipe, while for 4 repeated cigarette trials it was 0.638 ± 0.188 × 1012 particles/cigarette. Fig. 3 shows typical particle size frequency functions averaged over the smoking period for a waterpipe and cigarette, where it can be seen that waterpipe emissions contain a significantly larger proportion of particles below 100 nm. This is reflected in the smaller count median diameter for the waterpipe (37.9 ± 4.1 nm for the waterpipe versus 132 ± 11 nm for the cigarette). Among other potential factors, the smaller size range of waterpipe smoke likely reflects the differing sources (e.g. charcoal in addition to tobacco), tobacco aerosol generation conditions (e.g. lower temperature; see Shihadeh, 2003), tobacco additive ingredients, and probably higher volatility of the waterpipe smoke condensates relative to cigarette smoke.
Figure 3.
Typical frequency functions for cigarette and waterpipe SS. A larger fraction of waterpipe SS is found below 100 nm.
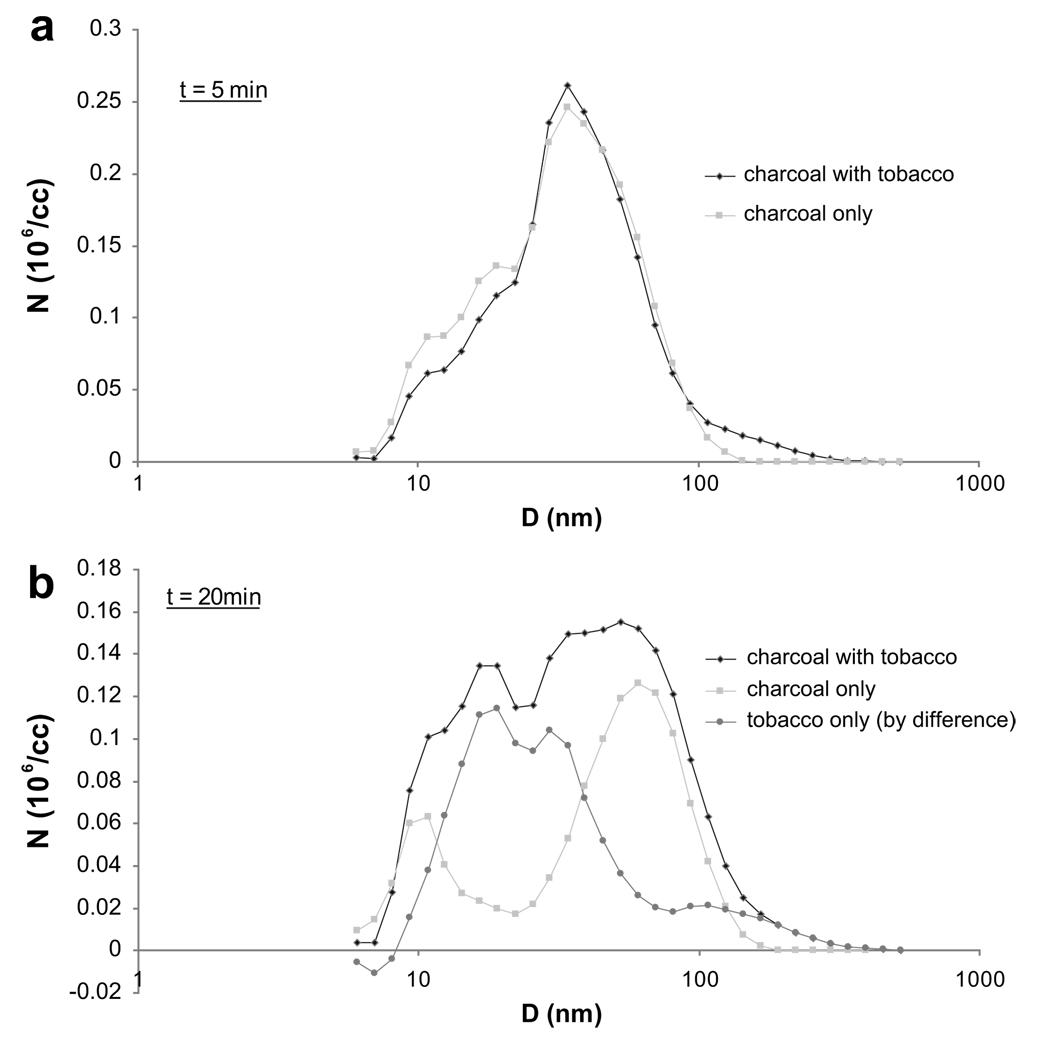
An indication of the relative role of the charcoal in the production of UFP can be gleaned from Fig. 4a and b, where particle size distribution for SS of a charcoal-only condition (i.e. where the waterpipe is smoked without any tobacco in the head) is compared to that of a normally prepared waterpipe. It can be seen that at 5 min into the smoking session, virtually all of the particulate matter is accounted for by the charcoal, whereas after 20 min a significant fraction derives from the tobacco. These comparisons are only indicative because they assume frozen particle size distribution dynamics.
Figure 4.
(a and b) Particle number distribution in chamber for standard waterpipe smoking condition (charcoal with tobacco), charcoal-only condition (waterpipe smoked with no tobacco in the head), and tobacco alone (by difference) at t = 5 and 20 min after start of smoking session. Charcoal is shown to be a major contributor of UFP in waterpipe SS.
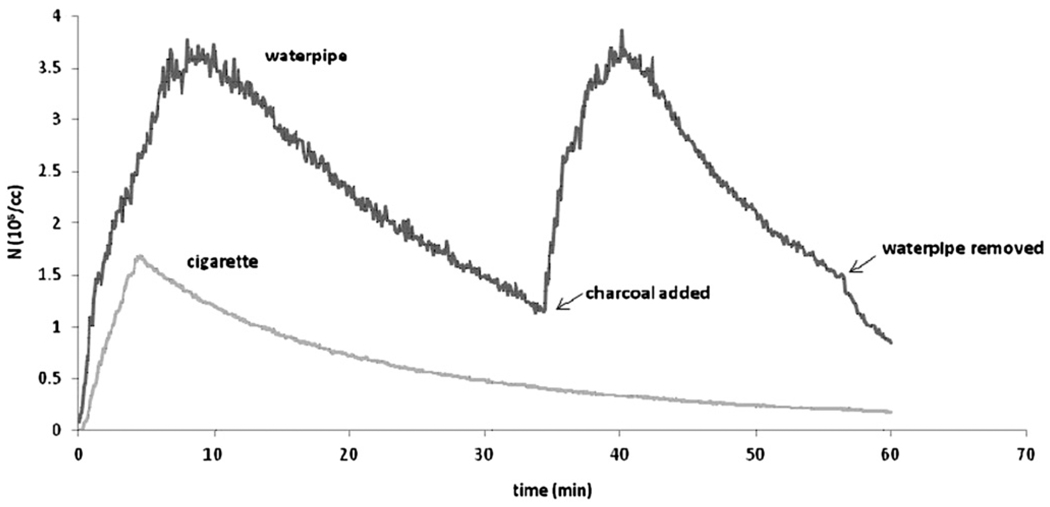
Sample time traces of total particle concentration (5.6–560 nm) in the chamber are given in Fig. 5 for a one-hour period for a single cigarette (smoked during the 0–5 min period) and a single waterpipe. It can be seen that even during the first 5 min, the total particle concentration in the chamber is greater for the waterpipe than for the cigarette case (i.e. even if the waterpipe were to be smoked for only 5 min, it would emit a larger number of particles into the environment than a cigarette). It can also be seen that the waterpipe emissions rise then decay until a second piece of charcoal is added. The particle concentration decay rate is significantly lower than the air displacement rate, indicating that even during decay periods, particles continue to be emitted into the chamber, albeit at a lower rate.
Figure 5.
Typical total (5.6–560 nm) particle concentration versus time for waterpipe and cigarette cases. An additional half charcoal briquette is added at 30 min, resulting in a second concentration peak. Waterpipe is removed from the tunnel at 56 min.
3.2. Carbon monoxide
Determinations of carbon monoxide for 13 replicate waterpipe smoking sessions yielded an average of 2269 ± 108 mg/waterpipe. Carbon monoxide for 9 replicate cigarette sessions was 65.5 ± 5.5 mg/cigarette.
3.3. Particle polyaromatic hydrocarbons
Good sensitivity and chromatographic resolution was exhibited for all PAH analytes, except for the overlapping peaks of benzo(k)fluoranthene and benzo(b)fluoranthene. These overlapping components were subsequently quantified by combining their chromatogram areas and reporting the total concentrations. The waterpipe smoke chromatograms showed considerably lower background signals than we previously reported (Sepetdjian et al., 2008) when measuring mainstream smoke, indicating that the sidestream smoke matrix is less complex. Determinations of PAHs in 11 replicate sidestream waterpipe smoke sessions yielded, per waterpipe smoked, 155 ± 72 ng benzo(a)pyrene, 398 ± 171 ng benzo(b + k)fluoranthenes, 52 ± 11 ng dibenz(a,h)anthracene, 266 ± 95 ng benz(g,h,i)perylene, and 322 ± 87 ng indeno(1,2,3-cd)pyrene. Measurements of cigarette SS for three replicate smoking sessions yielded, per cigarette, 96 ± 31 ng benzo(a)pyrene, 81 ± 28 ng benzo(b + k)fluoranthenes, 30 ± 7 ng dibenz(a,h)-anthracene, 57 ± 17 ng benz(g,h,i)perylene, and 41 ± 19 ng indeno(1,2,3-cd)pyrene.
3.4. Volatile aldehydes
Well resolved chromatograms allowed the identification and quantification of C1–C4 aldehydes. While the identification of the aldehydes was confirmed by mass spectrometry, their quantification was based on comparing each component to an extracted curve prepared with each run. Analysis of SS from six replicate waterpipe sessions yielded 5234 ± 1011 µg formaldehyde, 5084 ± 1211 µg acetaldehyde, 1135 ± 297 µg acrolein, 441 ± 129 µg propionaldehyde, and 110 ± 30 µg methacrolein per waterpipe. Analysis of SS from 5 replicate cigarette sessions yielded 357 ± 143 µg formaldehyde, 2136 ± 384 µg acetaldehyde, 144 ± 21 µg acrolein, 213 ± 65 µg propionaldehyde, and 104 ± 11 µg methacrolein per cigarette.
4. Analysis and discussion
This study was undertaken to address the dearth of information regarding the potential hazards of second-hand narghile waterpipe smoke. Emissions from the waterpipe head were measured and found to include large quantities of ultrafine particles, carcinogenic polyaromatic hydrocarbons, volatile aldehydes, and carbon monoxide, key classes of toxic or carcinogenic substances that span the particle, vapor, and gas phases. Limitations of the study include the use of only one tobacco and charcoal type with the waterpipe, use of only one model smoking topography each for the waterpipe and cigarette, and neglect of potential changes in chemistry due to mixing of SS and eMS in real environments. The data are summarized in Table 1. It can be seen that a single waterpipe smoking session emits in the SS approximately 4 times the PAH and aldehydes, 5 times the ultrafine particles, and about 35 times the carbon monoxide emitted in the SS of a single cigarette. It can also be seen in Table 1 that the cigarette emissions measured in this study are broadly consistent with those reported previously.
Table 1.
Measured waterpipe and cigarette sidestream smoke emissions, expressed as mass or number per waterpipe or cigarette smoked. Data from previous sidestream cigarette smoke studies shown for comparison. All differences in reported means between cigarette and waterpipe measures are statistically significant except benzo(a)pyrene and methacrolein. Data reported as mean ± 95% confidence interval.
| Current study | Previous studies |
||
|---|---|---|---|
| Waterpipe SS | Cigarette SS | Cigarette SS | |
| N = 12 | N = 9 | ||
| Carbon monoxide, mg | 2269 ± 108 | 65.5 ± 5.5 | 61.6–61.7b |
| PAH, ng | N = 11 | N = 3 | |
| Benzo[a]pyrene | 155 ± 72 | 95.7 ± 30.7 | 62.7–91.7b |
| Benzo[b + k]fluoranthenes | 398 ± 171 | 80.8 ± 28.0 | 99.1–124.2b |
| Dibenz[a,h]anthracene | 52.3 ± 10.5 | 29.9 ± 7.1 | 13.8–13.9b |
| Benzo[g,h,i]perylene | 266 ± 95 | 57.3 ± 17.0 | 32.8–41.7b |
| Indeno[1,2,3-cd]pyrene | 322 ± 87 | 40.9 ± 18.8 | 32.8–44.7b |
| Total PAH | 1193 ± 226 | 305 ± 49 | |
| Particle number emissions | N = 4 | N = 4 | |
| Ultrafine particles 5.6–99.5 nm,/1012 |
3.99 ± 0.60 | 0.639 ± 0.188 | |
| Total particles 5.6–560 nm,/1012 |
4.38 ± 0.66 | 1.68 ± 0.27 | |
| Count median diameter, nm | 37.9 ± 4.1 | 130 ± 8 | 140.7,a 135d |
| Count geometric standard deviation |
2.07 ± 0.10 | 1.47 ± 0.04 | 1.53,a 1.58d |
| Volume median diameter, nm | 134 ± 22 | 204 ± 13 | 292d |
| Volume geometric standard deviation |
1.77 ± 0.10 | 1.47 ± 0.06 | 1.55d |
| Volatile aldehydes,µg | N = 6 | N = 5 | |
| Formaldehyde | 5234 ± 1011 | 357 ± 143 | 662–886,b 234c |
| Acetaldehyde | 5084 ± 1211 | 2136 ± 384 | 1170–1587, 463c |
| Acrolein | 1135 ± 297 | 144 ± 21 | 316–437, 189c |
| Propionaldehyde | 441 ± 129 | 213 ± 65 | 116–121, 34.7c |
| Methacrolein | 110 ± 30 | 104 ± 11 | |
| Total aldehydes | 12,000 ± 1610 | 2954 ± 416 | |
We have previously reported mainstream waterpipe smoke toxicant and carcinogen yields using the same products, smoking protocol, and analytical instruments used in this study (Sepetdjian et al., 2008; Al Rashidi et al., 2008; Shihadeh and Saleh, 2005). The SS:MS ratios for CO, aldehydes, and PAH using current SS and previous MS results are given in Table 2. It can be seen that for these 3 classes of toxicants, waterpipe SS emissions generally exceed MS yields, as is the case with cigarettes (Jenkins et al., 2000). On the other hand, we expect, but have yet to verify, that ultrafine particles in waterpipe MS will greatly exceed those in waterpipe SS.
Table 2.
Sidestream emissions to mainstream smoke toxicant yield ratios for narghile waterpipe and cigarette.
| Waterpipe SS/MS | Cigarette SS/MS | |
|---|---|---|
| Volatile aldehydes | ||
| Formaldehyde | 8.3 | 4.4 |
| Acetaldehyde | 2 | 1.8 |
| Acrolein | 0.7 | 3.5 |
| Propionaldehyde | 1.1 | 1.7 |
| Methacrolein | 1 | NR |
| PAH | ||
| Benzo[a]pyrene | 0.5 | 6.4 |
| Benzofluoranthenes [k + b] | 1.1 | 8.7 |
| Dibenz[a,h]anthracene | 0.3 | 12.1 |
| Benzo[g,h,i]perylene | 1.9 | 11.1 |
| Indeno[1,2,3-cd]pyrene | 1.8 | 9.7 |
| CO | 14.6 | 2.9 |
From a public policy perspective, perhaps the most pertinent question is how emissions from the waterpipe–smoker system (i.e. SS + eMS) compare to those of the cigarette–smoker system. That is, with a cigarette smoker and waterpipe user sitting side-by-side in a café for an hour, will the waterpipe user contribute significantly to the levels of airborne carcinogens, CO, and UFP given the presence of the cigarette smoker? To address this question, we need to know a) the total (SS + eMS) waterpipe emissions over a typical one-hour use period, and b) the hourly total emission rates for cigarette smoking. The later can be obtained from recent data by Bi et al. (2005), assuming an hourly “habitual smoker” cigarette consumption rate of 2 per hour (Repace and Lowrey, 1980). To estimate the former, we calculated a lower and upper bound total emission rate corresponding to the waterpipe user exhaling zero or 100% of the inhaled MS toxicants. We also computed a “best estimate” of exhaled waterpipe MS emissions using toxicant-specific absorption fractions (70% particle PAH, 60% CO, 95% aldehydes) reported in a recent review by Baker and Dixon (2006). The results are given in Table 3, where it can be seen that just the SS emissions (i.e. the lower bound) of a single waterpipe use session exceed the combined SS and exhaled MS emissions of a person smoking two cigarettes over the same period. In fact, the waterpipe smoker likely emits as much aldehydes and PAH into the immediate environment as do two cigarette smokers, and as much CO as 10 cigarette smokers.
Table 3.
Calculated SHS emissions from one person-hour of waterpipe and cigarette smoking. Cigarette smoker is assumed to consume two full-flavored cigarettes per hour and is based on human subject measurements of SHS by Bi et al. (2005). Theoretical waterpipe range corresponds to the smoker exhaling none or all of the inhaled toxicant. “Best estimate” assumes toxicant fractions exhaled as previously reported (Baker and Dixon, 2006) for cigarette smokers. Reported totals for PAH and aldehydes correspond to compounds shown in Table 1, excluding dibenz[a,h]-anthracene and methacrolein, respectively, for commensurability with Bi et al., 2005.
| Emissions from 1 person-hour |
Waterpipe smoking | Cigarette smoking |
Waterpipe/cigarette | |
|---|---|---|---|---|
| Theoretical range |
Best estimate |
Bi et al.,2005 | Best estimate | |
| Total PAH, µg h−1 | 1.14–2.14 | 1.84 | 1.09 | 1.7 |
| Total volatile aldehydes, mg h−1 |
11.9–16.3 | 12.1 | 5.7 | 2.1 |
| CO, g h−1 | 2.28–2.42 | 2.30 | 0.212 | 10.8 |
5. Conclusions
The available evidence therefore indicates that waterpipe smoking results in environmental emissions of ultrafine particles, aldehydes, PAHs, and carbon monoxide well in excess of those resulting from cigarette smoking, regardless whether the comparison is made per unit smoked or smoker per unit time. The data thus provide strong justification for including narghile waterpipe smoking in public smoking bans.
Acknowledgements
The authors thank the staff of the Faculty of Engineering and Architecture machine shop at the American University of Beirut for assistance in fabricating the experimental setup. This work was supported by Research for International Tobacco Control, a secretariat of the Canadian IDRC, and by United States Public Health Service Grant R01CA120142.
References
- Al Rashidi M, Shihadeh A, Saliba NA. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food and Chemical Toxicology. 2008;46:3546–3549. doi: 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian National Health and Medical Research Council. Effects of Passive Smoking on Health. Report of the NHMRC Working Party on the Effects of Passive Smoking on Health. Canberra: Australia Government Publishing Service; 1987. [Google Scholar]
- Baek SO, Jenkins RA. Characterization of trace organic compounds associated with aged and diluted sidestream tobacco smoke in a controlled atmosphere – volatile organic compounds and polycyclic aromatic hydrocarbons. Atmospheric Environment. 2004;38:6583–6599. [Google Scholar]
- Baska T, Pudule I, Tilgale N, Warren CW, Lee J, Lea V, Jones NR. Smoking tobacco in waterpipes among adolescents in Europe: the case of Latvia and Slovakia. Tobacco Control. 2008;17:432. doi: 10.1136/tc.2008.027128. [DOI] [PubMed] [Google Scholar]
- Baker R, Dixon M. The retention of tobacco smoke constituents in the human respiratory tract. Inhalation Toxicology. 2006;17:255–294. doi: 10.1080/08958370500444163. [DOI] [PubMed] [Google Scholar]
- Bi X, Sheng G, Feng Y, Fu J, Xie J. Gas- and particulate-phase specific tracer and toxic organic compounds in environmental tobacco smoke. Chemosphere. 2005;61:1512–1522. doi: 10.1016/j.chemosphere.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Charles S, Batterman S, Chunrong J. Composition and emissions of VOCs in main- and side-stream smoke of research cigarettes. Atmospheric Environment. 2007;41:5371–5384. [Google Scholar]
- Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. Waterpipe tobacco smoking: an emerging health crisis in the United States. American Journal of Health Behavior. 2010;34:275–285. doi: 10.5993/ajhb.34.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nachef WN, Hammond SK. Exhaled carbon monoxide with waterpipe use in US students. Journal of American Medical Association. 2008;299(1):36–38. doi: 10.1001/jama.2007.6. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Ward KD, Smith-Simone S, Maziak W. Waterpipe tobacco smoking on a U.S. college campus: prevalence and predictors. Journal of Adolescent Health. 2008;42:526–529. doi: 10.1016/j.jadohealth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Roueiheb Z, Tamim H, Kanj M, Jabbour S, Alayan I, Musharrafieh U. Cigarette and waterpipe smoking among Lebanese adolescents, a cross-sectional study, 2003–2004. Nicotine and Tobacco Research. 2008;10:309–314. doi: 10.1080/14622200701825775. [DOI] [PubMed] [Google Scholar]
- Figliola R, Beasley D. Theory and Design of Mechanical Measurements. second ed. New York: Wiley & Sons; 1995. [Google Scholar]
- Friedlander SK. Smoke, Dust, and Haze. Fundamentals of Aerosol Dynamics. second ed. Oxford University Press; 2000. [Google Scholar]
- Fromme H, Dietrich S, Heitmann D, Dressel H, Diemer J, Schulz T, Jörres RA, Berlin K, Völkel W. Indoor air contamination during a waterpipe (narghile) smoking session. Food and Chemical Toxicology. 2009;47(7):1636–1641. doi: 10.1016/j.fct.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Jamriska M, Morawska L. Quantitative assessment of the effect of surface deposition and coagulation on the dynamics of submicrometer particles indoors. Aerosol Science and Technology. 2003;37(5):425–436. [Google Scholar]
- Jawaid A, Zafar AM, Rehman TU, Nazir MR, Ghafoor ZA, Afzal O, Khan JA. Knowledge, attitudes and practice of university students regarding waterpipe smoking in Pakistan. International Journal of Tuberculosis and Lung Disease. 2008;12:1077–1084. [PubMed] [Google Scholar]
- Jenkins R, Guerin M, Tomkins B. The Chemistry of Environmental Tobacco Smoke: Composition and Measurement. Boca Raton: Lewis Publishers; 2000. [Google Scholar]
- Li W, Hopke PK. Initial size distributions and hygroscopicity of indoor combustion aerosol particles. Aerosol Science and Technology. 1993;19(3):305–316. [Google Scholar]
- Maziak W, Rastam S, Ibrahim I, Ward K, Eissenberg T. Waterpipe-associated particulate matter emissions. Nicotine and Tobacco Research. 2008;10(3):519–523. doi: 10.1080/14622200801901989. [DOI] [PubMed] [Google Scholar]
- Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chemical Research in Toxicology. 2008;21(2):494–502. doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- Monn C, Kindler P, Meile A, Brändli O. Ultrafine particle emissions from waterpipes. Tobacco Control. 2007;16:390–393. doi: 10.1136/tc.2007.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer B, Sepetdjian E, Saliba N, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food and Chemical Toxicology. 2008;46:2991–2995. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Morawaska L, Jamriska M, Bofinger N. Size characteristics and ageing of the environmental tobacco smoke. The Science of the Total Environment. 1997;196:43–55. [Google Scholar]
- National Research Council, NRC. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington, DC: National Academy Press; 1986. [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärna K, Usin J, Ringmets I. Cigarette and waterpipe smoking among adolescents in Estonia: HBSC survey results, 1994–2006. BMC Public Health. 2008;8:392. doi: 10.1186/1471-2458-8-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primack B, Sidani J, Agarwal A, Shadel W, Donny E, Eissenberg T. Prevalence of and associations with waterpipe tobacco smoking among U.S. university students. Annals of Behavioral Medicine. 2008;36:81–86. doi: 10.1007/s12160-008-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repace J, Lowrey A. Indoor air pollution, tobacco smoke, and public health. Science. 1980;208(4443):464–472. doi: 10.1126/science.7367873. [DOI] [PubMed] [Google Scholar]
- Saleh R, Shihadeh A. Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food and Chemical Toxicology. 2008;46(5):1461–1466. doi: 10.1016/j.fct.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Sepetdjian E, Shihadeh A, Saliba NA. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food and Chemical Toxicology. 2008;46:1582–1590. doi: 10.1016/j.fct.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Shihadeh A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food and Chemical Toxicology. 2003;41:143–152. doi: 10.1016/s0278-6915(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S, Antonios C, Haddad A. Towards a topographical model of narghile water-pipe cafe smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacology Biochemistry and Behavior. 2004;79:75–82. doi: 10.1016/j.pbb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food and Chemical Toxicology. 2005;43:655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S. A closed-loop control “ “playback” smoking machine for generating mainstream smoke aerosols. Journal of Aerosol Medicine: Deposition, Clearance, and Effects in the Lung. 2006;19(2):137–147. doi: 10.1089/jam.2006.19.137. [DOI] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward K, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs and behavior in two U.S. samples. Nicotine and Tobacco Research. 2008;10:393–398. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Department of Health and Social Security. Fourth Report of the Independent Scientific Committee on Smoking and Health. London: HMSO; 1988. [Google Scholar]
- US Department of Health and Human Services. [Accessed online 01.07.09];Washington, DC: DHHS; The Health Consequences of Involuntary Exposure to Tobacco Smoke: a Report of the Surgeon General. 2006 http://www.surgeongeneral.gov/library. [PubMed]
- World Health Organization, WHO. Switzerland: Geneva; TobReg Advisory Note. Waterpipe Tobacco Smoking: Health Effects, Research Needs and Recommended Actions by Regulators. 2005