Abstract
Accumulating evidence indicates that dysfunction in amino acid neurotransmission contributes to the pathophysiology of depression. Consequently, the modulation of amino acid neurotransmission represents a new strategy for antidepressant development. While glutamate receptor ligands are known to have antidepressant effects, mechanisms regulating glutamate cycling and metabolism may be viable drug targets as well. In particular, excitatory amino acid transporters (EAATs) that are embedded in glial processes constitute the primary means of clearing extrasynaptic glutamate. Therefore, the decreased glial number observed in preclinical stress models, and in postmortem tissue from depressed patients provides intriguing, yet indirect evidence for a role of disrupted glutamate homeostasis in the pathophysiology of depression. More direct evidence for this hypothesis comes from studies using magnetic resonance spectroscopy (MRS), a technique that non-invasively measures in vivo concentrations of glutamate and other amino acids under different experimental conditions. Furthermore, when combined with the infusion of 13C-labeled metabolic precursors, MRS can measure flux through discrete metabolic pathways. This approach has recently shown that glial amino acid metabolism is reduced by chronic stress, an effect that provides a link between environmental stress and the decreased EAAT activity observed under conditions of increased oxidative stress in the brain. Furthermore, administration of riluzole, a drug that enhances glutamate uptake through EAATs, reversed this stress-induced change in glial metabolism. Because riluzole has antidepressant effects in both animal models and human subjects, it may represent the prototype for a novel class of antidepressants with the modulation of glial physiology as a primary mechanism of action.
Keywords: Depression, Excitatory amino acid transporter (EAAT), Glia, Magnetic resonance spectroscopy, Riluzole, Stress
1. Overview
The initial scientific observation that amino acids could behave as bona fide neurotransmitters in the vertebrate CNS was met with incredulity when reported in the late 1950s. Because amino acids are present in all cells, as both metabolic intermediates and precursors for protein synthesis, amino acids lacked the distinct CNS distribution patterns exhibited by the monoamine neurotransmitters. In addition, amino acids did not act stereo-selectively on neuronal physiology, a defining feature of ligand-receptor specificity. Today, amino acid neurotransmitters (AANt) are recognized as the most abundant in the CNS, with glutamate (Glu) and gamma-aminobutyric acid (GABA) serving as the principle neurotransmitters for fast excitatory and inhibitory transmission respectively. Historically, translational research on glutamatergic neurotransmission has been of primary interest to the field of neurology where glutamatergic abnormalities are thought to contribute to the pathophysiology of a variety of neurological disorders including epilepsy, stroke, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD). However, accumulating evidence now suggests that disrupted Glu homeostasis may be a more generalized mechanism of brain dysfunction that also underlies various psychiatric disorders, including major depressive disorder (MDD). Although a seminal paper by Olney et al. published in 1971 linked the neuroexcitatory and neurotoxic properties of ‘cytotoxic amino acids’ and suggested that ‘…the potential role of acidic amino acids in the pathogenesis of etiologically obscure syndromes of mental retardation or other forms of aberrant mental functioning warrants consideration’ [1], a sustained interest in studying the glutamatergic contributions to psychiatric disorders only began in the 1990s with a glutamatergic hypothesis of schizophrenia. Recently, there has been a surge of interest in the contribution of AANt to the pathophysiology of mood disorders as the unmet need for true innovation in their treatment becomes increasingly apparent.
The accumulating evidence for a role of AANt in the pathophysiology of mood disorders suggests that their clinical therapeutics might be improved by targeting glutamatergic neurotransmission. Indeed, several recent clinical trials have demonstrated antidepressant effects of NMDA antagonists, and modulators of other classes of Glu receptors have antidepressant-like effects in preclinical models (See recent reviews [2–5]). While these findings provide a strong impetus for continued research on the antidepressant potential of receptor ligands, the mechanisms regulating the synaptic clearance and metabolism of Glu may also provide viable drug targets. This commentary will present emerging evidence implicating dysregulated glutamatergic cycling in the pathogenesis and pathophysiology of MDD and the novel pharmacological targets for antidepressant development brought to light by this work.
2. Glutamate metabolism and neuron–glia interactions
In order to consider a potential role for glutamatergic dysregulation in the pathophysiology of depression, it is important to review several basic aspects of glutamate metabolism. Upon depolarization of glutamatergic neurons, vesicular Glu is released in a calcium-dependent manner into the synapse and removed from the extracellular space by transport through transmembrane proteins, the same mechanism used at monoaminergic synapses. However, unlike monoamine transmitters that are transported into presynaptic nerve terminals, Glu is cleared predominately by glial cells through the activity of highly efficient excitatory amino acid transporters (EAAT1–2). In addition, while monoamine metabolism results in excreted waste products, Glu is efficiently recycled through a Glutamate/Glutamine (Glu/Gln) metabolic cycle. After uptake by EAATs, Glu is rapidly converted into the ‘inert’ intermediate Gln within glia by the enzyme glutamine synthetase. Gln is then transferred to neurons where it is converted back into Glu and packaged into synaptic vesicles through vesicular glutamate transporters (VGLUT1–3). Consequently, there are two primary modes of Glu production within neurons, (1) de novo synthesis from glucose through transamination of the tricarboxylic acid (TCA) cycle intermediate alpha-oxoglutatrate, and (2), the conversion of glutamine (Gln) into Glu by glutaminase located in neuronal mitochondria. Therefore, glial cells are not only responsible for protecting neurons from the deleterious effects of elevated Glu levels, they are also the principle source of this synaptically released Glu as a key participant in the Glu/Gln cycle.
3. Alterations in glutamatergic neurotransmission in depressed subjects
Dysfunction in glutamatergic neurotransmission has been implicated in the pathophysiology of various psychiatric disorders including schizophrenia, obsessive-compulsive disorder, and alcohol dependence [6–8]. In addition, multiple lines of clinical evidence suggest that the glutamatergic system plays an important role in the pathophysiology of major depressive disorder (see Ref. [2] for more complete review). First, glutamatergic abnormalities have been identified in the peripheral tissues of MDD subjects with several, but not all, studies reporting increased plasma levels of Glu in MDD (see Ref. [9] for review). Furthermore, postmortem assessments of Glu concentrations in brain tissue from depressed subjects have also been performed, in addition to a study on brain biopsy specimens. While no clear differences were observed in neurosurgical samples of frontal cortex obtained from chronically depressed subjects [10], a more recent postmortem study demonstrated significantly elevated Glu content in the frontal cortex from individuals with MDD [11].
Additional evidence for glutamatergic abnormalities in MDD is provided by gene expression studies on brain tissue from depressed subjects where a downregulation of GLUL, the gene that codes for glutamine synthetase, and SLC1A2 and SLC1A3, the genes that code for the EAAT2 and EAAT1 transporters respectively, has been identified [12]. In fact, abnormal expression of genes associated with the regulation of glutamate clearance and metabolism has consistently been found to be associated with several neuropsychiatric disorders (please see Ref. [13] for review).
Unfortunately, the numerous complications surrounding postmortem studies places major limitations on the interpretation of these data. Many confounds in postmortem studies can be overcome by using proton magnetic resonance spectroscopy (1H-MRS), a technique that measures the concentration of specific molecules in the living brain. The basic technology for obtaining this chemical information is similar to that used for constructing visual representations of brain structure through magnetic resonance imaging (MRI). In 1H-MRS, differences in the chemical environment of protons, both between and within compounds, are detected as differences in the frequency of response to experimentally applied electromagnetic energy. This ‘chemical shift’ is a result of differences in the amount of shielding experienced by protons from the surrounding electron clouds of other atoms. At high magnetic field strengths, amino acids that are unambiguously resolved by 1H-MRS include Glu, aspartate, GABA, and glutamine. Using standard clinical field strength magnets (1.0–3.0 T), the unique identification of these metabolites is limited by multiple factors making it difficult to assign unequivocal resonance peaks to particular amino acids. This has led to the use of a combined measure termed Glx containing overlapping resonance signals from Glu, Gln, and GABA, the greatest proportion of which reflects Glu concentration.
To date, there have been several reports of increased Glu or Glx concentrations in the occipital cortex of MDD patients [14,15], and elevated levels of Glx have also been found in the frontal lobe and basal ganglia of depressed bipolar children [16]. In addition, several studies have observed decreased Glx levels in the anterior cingulate and PFC of depressed subjects that appeared to increase following treatment with ECT [17,18]. In one of the more unique 1H-MRS studies, decreases in Glx levels were observed to coincide with the transient experience of suicidal depression in patient’s receiving Taxol and Neupogen chemotherapy [19].
While 1H-MRS has many advantages over postmortem and plasma analyses of Glu levels, most in vivo MRS studies have been confounded by indirect measurements of Glu through Glx, incomplete accounting of all macromolecular contributions to resonance peaks, and inadequate characterization of the relative contributions from gray and white matter. In addition, even at higher magnetic field strengths, 1H-MRS studies cannot differentiate among intracellular and extracellular Glu pools and provide only static measures of total tissue Glu.
In sum, although there is now strong reason to believe that abnormalities in the glutamatergic neurotransmitter system are associated with MDD, there remains no clear understanding of the mechanism responsible for the abnormal Glu levels in the brain tissue of depressed patients or even the physiological meaningfulness of the findings. The remaining sections will outline the evidence supporting the hypothesis that disrupted glutamate cycling contributes to the pathophysiology of MDD and other stress-related neuropsychiatric disorders, and how the mechanisms regulating glutamate cycling are viable targets for anti-depressant drug development.
4. Glutamate as a mediator of stress-induced neural change
A primary assumption of preclinical depression research is that of a ‘stress–depressive diathesis’ in which the neurobiological effects of stress exposure simulates the conditions promoting depressive illness in humans. Stress has long been implicated in the pathogenesis of mood disorders [20,21] with numerous identified mediators including alterations in various hormones, neurogenesis, angiogenic factors, neurotrophic factors, and monoaminergic transmission. However, multiple lines of preclinical evidence suggest that Glu is an additional mediator of a stress–depressive diathesis. While initial studies examining brain levels of Glu following exposure to stressors yielded inconclusive results, more recent studies have demonstrated a rapid efflux of Glu into the extrasynapatic space of the frontal cortex, hippocampus and amygdala of rodents following exposure to a variety of acute stressors including saline injections, restraint, tail pinch, swim, and beta-carboline administration [22]. The fact that locally perfused tetrodotoxin was able to block these stress-induced increases suggests that the elevation in Glu levels was secondary to increased neuronal release. Surprisingly, little attention has been given to the effects of repeated stress on glutamatergic neurotransmission. At least one preclinical microdialysis study has examined the effect of repeated tail pinches on Glu efflux in the prefrontal cortex (PFC) and hippocampus. A rapid increase in extracellular Glu was observed (within 1 min of the initial tail pinch) but the effect was attenuated, especially in the frontal cortex, upon subsequent pinches over a 3-h period [23]. This may represent a compensatory response to stress-induced increases in extracellular Glu that reduces the probability of excitotoxic effects from excessive Glu receptor activation. Under normal conditions, glia cells are a biologically plausible cellular mediator of this compensation to repeated stress as they are the primary means of removing Glu from excitatory synapses. However, under conditions of prolonged stress, the capacity of glial cells to clear Glu may be diminished secondary to decreases in glial number and/or decreases in the activity or expression of EAATs.
5. Stress and glia
Decreases in glial cell number and density have been repeatedly observed in postmortem analyses of brain tissue from ‘fronto-limbic’ regions of depressed patients (see Ref. [24] for complete review). Given the reproducibility of this finding, these glial alterations likely reflect an important neurobiological aspect of MDD. However, postmortem studies cannot determine whether the glial changes are a cause or consequence of the disease and, the glial subtypes involved remain poorly characterized. Therefore, preclinical studies on the molecular, cellular and behavioral consequences of glial dysfunction might improve the modeling of the etiological and pathophysiological processes underlying MDD. The possibility that glial dysfunction is an etiological factor is supported by observations of glial loss after exposure to a variety of environmental stressors. For example, chronic psychosocial stress (5 weeks) in the tree shrew is associated with a 25% reduction in the number and volume of hippocampal astrocytes, changes that correlated with the hippocampal volume loss identified in this study [25]. In addition, chronic social stress in rats is associated with decreased gliogenesis in the mPFC [26] and chronic unpredictable stress is associated with both a decrease in glial proliferation in the mPFC and hippocampus and a decrease in glial fibrillary acidic protein (GFAP) mRNA expression in the mPFC of adult rats [27]. Furthermore, a recent study demonstrated that the infusion of the astrocyte-specific gliotoxin, L-alpha-amino-adipic acid (L-AAA), directly into the prefrontal cortex of adult rats induced depressive-like behaviors in several models of depression [28].
Given that up to 90% of extracellular Glu clearance in rodents is performed by astrocytes through the activity of excitatory amino acid transporter GLT1, the decreases in both glial number and in astrocytic markers observed in animal studies, combined with the decreases in the astrocytic marker GFAP [29,30] found in the brains of depressed subjects implicate decreased Glu clearance as a stress-induced neural change underlying MDD [29,30]. That astrocytic dysfunction is involved in the neurobiology of MDD is further supported by the recent finding of reduced EAAT1, EAAT2, and glutamine synthetase gene expression in frontal brain regions of postmortem tissue from depressed individuals [12]. In addition, chronic blockade of Glu uptake through infusion of the EAAT antagonist L-trans-pyrrolidine-2,4-dicarboxylic acid (L-trans 2,4 PDC) into the rat amygdala results in a dose-dependent reduction in social exploratory behavior and disrupts circadian activity, behavioral states consistent with those observed in patients with MDD. Finally, injection of the selective GLT-1 blocker dihydrokainic acid (DHK) into the amygdala also reduces social interaction [52]. Collectively, these studies suggest that stress-induced changes in glial physiology contribute to the pathophysiology of depression.
6. Role of glial EAATs in excitatory synaptic physiology and plasticity
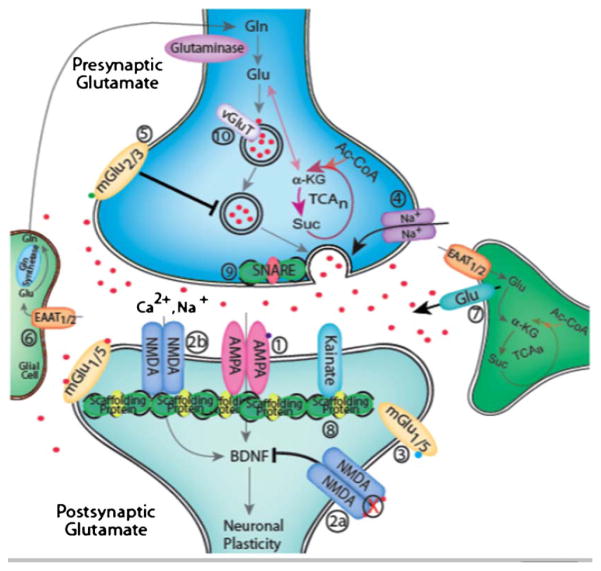
In the vertebrate CNS, the extracellular levels of Glu are primarily controlled by five different EAAT isoforms. The first three subtypes identified were the glial transporters GLAST and GLT1 (in rodents) and the neuronal transporter EAAC1 (in rabbit). The human homologues of these transporters are EAAT1, EAAT2 and EAAT3 respectively. EAAT4 is primarily restricted to cerebellar Purkinje cells while EAAT5 is restricted to retinal neurons and Muller cells. In contrast, EAATs 1–3 are enriched in the forebrain regions of mammals, and consequently have the highest probability of modulating neural systems implicated in the pathophysiology of MDD. EAATs use sodium and potassium gradients to concentrate Glu intracellularly to levels 10,000 times higher than found extracellularly under resting conditions (less than 1 μM). This high capacity is necessary to prevent triggering excitotoxic signaling cascades that can occur when extracellular Glu levels exceed 1–3 μM [31]. In addition to excitotoxic effects of excessive extracellular Glu, even relatively small changes in Glu concentration outside the synaptic cleft can effect a variety of additional physiological functions including the induction of hippocampal long-term depression (LTD) [32] and activation of several signal transduction pathways involved in the regulation of neurotrophic factor expression, neuroplasticity, and cellular resilience [33–35]. Under normal conditions, intrasynaptic Glu is rapidly cleared by EAATs. However, under pathological conditions of excessive Glu release and/or impaired EAAT activity, Glu can ‘spillover’ from the synaptic cleft, a situation that both reduces the input specificity of neural signaling and activates extrasynaptic receptors. If elevated extrasynaptic Glu levels chronically activate presynaptic group II mGlu receptors, Glu release into synapses might be reduced thereby amplifying the reduction in neuroplasticity and cellular resilience associated with decreased synaptic activation of NMDA [33–35] and AMPA [5] receptors (see Fig. 1). However, this scenario creates an inconsistency between MDD models of glutamatergic dysfunction that are based on pharmacological studies. Both preclinical and clinical studies have shown that NMDA antagonists (such as MK-801 and ketamine) have antidepressant properties [9,36,37] suggesting that MDD is associated with a hyper-glutamatergic state. In contrast, other studies have shown that both AMPA potentiators [38] and mGlu2/3 antagonists, compounds that increase presynaptic Glu release, also possess antidepressant-like properties [39] suggesting MDD is associated with a hypo-glutamatergic state. A model of MDD centered on the deleterious consequences of excessive extrasynaptic glutamate secondary to insufficient intrasynaptic clearance by EAATs resolves this apparent contradiction. In this model, Glu spillover will increasingly activate extrasynaptic mGlu2/3 receptors, and thereby pathologically dampen stimulated presynaptic release of Glu. Consequently, the antidepressant effects of mGlu2/3 antagonists and AMPA potentiators may be secondary to promotion of excitatory activity at synapses. In addition, this model accounts for the antidepressant properties of ketamine, a drug with mixed effects on glutamatergic signaling. The potential for a preferential blockade of extrasynaptic NMDA receptors by ketamine in the prefrontal cortex would be neuroprotective with attendant antidepressant effects secondary to blocking the over-activation of NMDA receptors that promotes excitotoxicity and decreases cellular resilience. In addition, multiple studies have demonstrated that many of the effects of NMDA receptor antagonists are actually related to an increased presynaptic Glu release [40], similar to the effects of mGlu2/3 antagonists. This enhanced presynaptic Glu release might acutely increase the ratio of AMPA/NMDA activity, a mechanism implicated in the anti-depressant effects of several different compounds [41]. Finally, a recent study has demonstrated that AMPA receptor activation is associated with antidepressant actions of ketamine [42]. As shown in Fig. 1, the mechanisms regulating Glu signaling at excitatory synapses represent a variety of targets for developing novel pharmacological agents for MDD and other psychiatric disorders. While the rationale behind some mechanisms are currently speculative, other targets, such as the EAATs, are showing great promise in both preclinical and clinical studies.
Fig. 1.
Potential targets for drug development based on a hypothesis of impaired glutamate clearance and cycling. (1). AMPA receptor modulation – Enhancing signaling through AMPA receptors using AMPA receptor potentiators, ARPs (Aniracetam, Ampalex, LY392098, LY 451616, and S18986) is associated with antidepressant-like properties in preclinical studies [5,38]. (2). NMDA receptor modulation – Acute administration of NMDA receptor antagonists such as ketamine rapidly produce antidepressant-like responses in both clinical and preclinical studies [36,37,42]. The development of agents targeting NR2 subunits may provide the ability to selectively modulate extrasynaptic (2a) and synaptic (2b) NMDA receptors and thereby improve the side effect profile of this class of medications. A recent study has demonstrated antidepressant effects secondary to NR2B subunit antagonism [78]. (3). Group I metabotropic receptor modulation – mGluR1/5 antagonists such as 2-methyl-6-(phenylethynyl) pyridine (MPEP), 3-[(2-methyl-1,3-thiazol-4-ylethynyl]pyridine (METP), and several others have been shown to have anxiolytic and antidepressant-like activity in preclinical animal models [4,79]. However, to date there are no clinical studies demonstrating the effectiveness of this drug class. (4). Voltage dependent Na+ channel modulation – Inhibition of stimulated Glu release via actions on the voltage dependent sodium channel is believed to be the primary mechanism related to the mood stabilizing action of lamotrigine that is currently indicated for maintenance treatment of bipolar I disorder [80]. This mechanism may also be related to the antidepressant and anti-anxiolytic effects of riluzole in clinical and preclinical studies. (5). Group II metabotropic receptor modulation – mGluR2/3 receptor agonists have been shown to have anxiolytic properties [81,82] while mGluR2/3 receptor antagonists have demonstrated antidepressant activity ([4,39,79] for reviews). Early phase clinical trials have recently been initiated examining the effectiveness and safety of this drug class for the treatment of neuropsychiatric disorders [81]. (6). Facilitation of Glutamate Clearance by EAATs – Evidence suggests that facilitation of glutamate clearance can protect against neurotoxicity [83]. Recent studies suggest that this mechanism may also be associated with antidepressant-like effects, as seen with the β-lactam antibiotic ceftriaxone [49], and possibly riluzole [65,76]. Exploratory clinical trials are now underway to further explore the utility of this approach to treating treatment resistant mood disorders [84–88]. (7). Modulating extrasynaptic glutamate release – Recent studies have shown the importance of extrasynaptic mGluR5 receptors in regulating Ca (2+)-dependent release of excitatory transmitters from glia [89], and in the actions of the cystine–glutamate antiporter in controlling extrasynaptic glutamate content [90]. Agents modifying glial and extrasynaptic release of Glu such as N-acetyl cystine [91,92] and mGluR5 antagonists (see above) have recently gained attention as potential treatments of neuropsychiatric disorders. (8). Post synaptic density (PSD) proteins – There is increasing evidence suggesting that abnormalities exist in the PSD of individuals suffering with mood disorder (see Ref. [2] for review). Other work suggests the interaction between Glu receptors and the PSD proteins is critical in determining Glu receptor trafficking, stabilization, and signaling. While remaining largely a theoretical mechanism to date, it is possible that agents capable of modifying the expression or function of PSD proteins could be used to treat mood disorders. (9). Modulation of presynaptic vesicular glutamate release – There is increasing evidence that chronic antidepressant treatment results in modulation of presynaptic glutamate release [93]. Some evidence suggests this is the result of effects on the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) complex that controls the structural and biochemical aspects of synaptic vesicle exocytosis [94]. Targeting the expression and function of these SNAREs may prove to be a useful tool in the development of novel biomarkers and therapies [95]. (10). Modulation of presynaptic vesicular loading of glutamate – Synaptic vesicle loading is mediated by transport proteins known as vesicular glutamate transporters (vGluTs). Evidence suggests that vesicular Glu content is increased following stress secondary to increases in vGluT levels [96]. Recent studies on vGluT1-deficient mice have informed models postulating roles for vGluTs in synaptic physiology, such as presynaptic regulation of quantal size and activity-dependent short-term plasticity [97]. Mice heterozygous for VGluT1 (VGluT1+/−), exhibit decreased cortical and hippocampal levels (35–45%) of the inhibitory neurotransmitter GABA as well as decreased activity in the forced swimming test [98]. Repeated antidepressant treatment increases VGluT1 protein expression [99]. Controlling the activity of these transporters could potentially modulate the efficacy of glutamatergic neurotransmission and serve as a mechanism of action for antidepressant and mood stabilizing drugs [100].
7. EAAT dysfunction in disease models
Given the crucial role of EAATs in regulating synaptic physiology, deleterious neurobiological effects might be expected from disrupted EAAT function. Indeed, studies have demonstrated an increased sensitivity of brain tissue to Glu-mediated neural injury when EAAT activty is reduced. For example, injection of L-beta-THA or L-Trans 2,4-PDC, molecules that inhibit multiple EAAT subtypes, induce neuronal injury when infused directly into rat striatum. Furthermore, EAAT2/GLT1 homozygous knock-out mice are especially vulnerable to acute cortical injury and rarely survive beyond 3 months secondary to spontaneous lethal seizures. Additionally, EAAT1–3 antisense knock-down procedures have been shown to induce excitotoxic effects in both in vitro and in vivo systems. Collectively, the cellular effects induced by disrupting EAAT activity complement the well-established findings of excitotoxic injury induced by brain ischemia. In fact, the two processes are likely to be interdependent as EAATs are very sensitive to inhibition by reactive oxygen species that increase intracellularly as extracellular Glu levels rise [43]. Under normal physiological conditions, Glu activates synaptic NMDA and AMPA receptors, activates trophic downstream effectors, including CREB and BDNF, and preserves neuronal viability, possibly by enhancing intrinsic antioxidant activity within neurons. In contrast, under conditions of Glu spillover, extrasynaptic NMDA receptors are activated which reduces CREB activity and BDNF expression, opposes trophic effects on neurons, and can lead to atrophy and cell death [34,44]. Consequently, enhancing EAAT activity may result in decreased extrasynaptic Glu concentrations and a release from the tonic inhibition of presynaptic neurons by activated mGluR 2/3 receptors. Consequently, pharmacologically increasing Glu clearance by enhancing EAAT activity may be neuroprotective. Indeed, a recent series of pharmacological studies have demonstrated that the selective enhancement of GLT1 function is not only neuroprotective, but is associated with antidepressant effects in both animals and humans.
8. Effects of enhanced GLT1 function in disease models
The first evidence for the pharmacological facilitation of Glu uptake as a viable strategy for treating brain disorders was provided by studies on ceftriaxone, a β-lactam antibiotic that also selectively induces GLT1 gene transcription and increases GLT1 transport activity. This activity of ceftriaxone was initially identified by screening 1040 FDA-approved compounds for the ability to increase GLT1 protein expression in a spinal cord slice culture assay system. Ceftriaxone was then shown to have neuroprotective effects in both in vitro and in vivo models of excitotoxicity. These initial observations were then extended to other excitotoxicity models. For example, the treatment of adult rats with ceftriaxone for 5 days induces protection against neuronal injury from oxygen–glucose deprivation in the CA1 region of hippocampal slices. Furthermore, drug treatment was associated with an increase in the uptake of Glu as revealed by whole-cell patch recordings of CA1 neurons in hippocampal slices [45]. A neuroprotective effect has also been observed in an in vivo rodent ischemia model where ceftriaxone both increased GLT1 expression in the hippocampus and protected CA1 neurons from cellular damage following brief ischemia [46]. In addition, the upregulation of GLT1 mRNA and protein expression induced by ceftriaxone has been associated with decreases in infarct volumes and enhanced ischemic tolerance, an effect that was blocked by the infusion of the selective GLT1 inhibitor dihydrokainate (DHK) [47]. Collectively, these studies have clearly established that the pharmacological facilitation of GLT1 can protect against neural damage in brain ischemia models. However, if disrupted Glu homeostasis is a more generalized mechanism underlying neuropsychiatric disease, then ceftriaxone should have effects in other disease models. Indeed, a recent study has shown that ceftriaxone-induced increases in GLT1 expression are associated with a reversal of the Glu uptake deficit in the striatum of R6/2 mice, a widely used transgenic model for Huntington’s disease (HD) [48]. In addition, drug treatment was associated with an attenuation of the HD-like behaviors exhibited by R6/2 mice. Finally, ceftriaxone has an antidepressant behavioral profile in the tail suspension and forced swim tests supporting the possibility that the enhancement of GLT1 activity represents a new pharmacological strategy for treating MDD [49]. Because ceftriaxone is indispensable to the treatment of infectious disease, it has significant limitations as a practical therapeutic for MDD, although it will likely continue to provide additional insights into the glutamatergic mechanisms underlying MDD. However, riluzole (Rilutek®), an FDA-approved drug for the treatment of ALS, also has antidepressant effects in both preclinical depression models and depressed human subjects. As described below, recent evidence suggests this antidepressant activity may be due to the ability of riluzole to enhance GLT activity.
9. Riluzole
Riluzole (2-amino-6-trifluoromethoxy benzothiazole) was originally developed as an anticonvulsant due to its effects on opposing glutamatergic activity both in vitro and in vivo [50,51]. While the initial studies on the basic pharmacology of riluzole indicated that it acted as an antagonist at a subset of Glu receptors [50], as revealed by functional antagonism of both NMDA and kainate receptors in vitro [52] and, noncompetitive antagonism of AMPA receptors in the rat spinal cord [53] and cortex [54], a direct interaction between riluzole and Glu receptors has never been observed. This suggests that the glutamatergic effects of riluzole are mediated by other mechanisms [55]. For example, riluzole inhibits the release of Glu in vivo [56] and in vitro [57] and this activity may be secondary to effects on a variety of ion channels including voltage-activated sodium channels [58], voltage-gated calcium channels (VGCCs) [59], and voltage-gated potassium channels [60]. While riluzole appears to have complex pharmacological effects, most of these were observed at brain concentrations that are unlikely to be attained in the brains of patients treated with standard oral riluzole dosing of 50 mg twice a day [61]. Consequently, the recent demonstration that riluzole can, at clinically relevant brain concentrations (micromolar), enhance Glu uptake suggests that an additional mechanism underlying the therapeutic effects of riluzole is the facilitation Glu clearance. For example, riluzole reverses the decrease in Glu transport observed in vivo after nerve injury in the rat spinal cord [62]. In addition, in vitro studies have shown that riluzole acutely increases Glu uptake in both rat synaptosomal preparations [63,64] and cultured astrocytes [65], and dose-dependently increases Glu uptake through three rat EAAT subtypes (GLAST, GLT1, EAAC1) when individually expressed in clonal cell lines. This effect may be mediated by an increased affinity of EAATs for Glu in the presence of riluzole [66]. The possibility that riluzole’s activity at EAATs is a clinically relevant mechanism of action is supported by its therapeutic benefit in the treatment of ALS, a serious neurological disease characterized by progressive degeneration of motor neurons. Multiple lines of evidence suggest this motor neurodegeneration is caused by Glu-induced excitotoxicity associated with decreased EAAT activity. Chronic administration of EAAT inhibitors induces the degeneration of motor neurons in organotypic spinal cord cultures [67] and ALS patients exhibit decreased levels of EAAT activity [68]. In addition, a transgenic mouse model of ALS that expresses a mutant form of the human SOD gene identified in a familial form of ALS, exhibits neuropathological changes consistent with increased oxidative stress and reduced EAAT activity [69,70]. While the most clinically relevant pharmacological actions of riluzole remain uncertain, the enhancement of Glu uptake through GLT1 is a plausible mechanism. One experimental approach to identifying whether the facilitation of EAAT activity is a clinically relevant mechanism of riluzole’s antidepressant action is through the use of 13C-MRS.
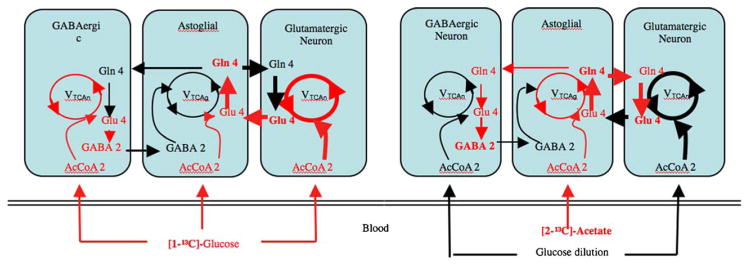
10. 13C-MRS measurements of the glutamate–glutamine cycle
Under normal physiological conditions, the oxidation of glucose through the TCA cycle is the primary source of energy for the brain. As depicted in Fig. 2, experiments that combine the intravenous infusion of 13C-labeled glucose, or other precursors, with MRS allows for the fate of the 13C label to be followed as the isotoptically labeled precursors move through metabolic cycles in different cellular compartments. 13C-MRS has been successfully used in both rodents and human subjects to provide time-resolved observations of label incorporation into the carbon backbones of Glu, Gln, and GABA [71,72] under various physiological conditions. For example, MRS performed on the resting human cortex after the infusion of 13C-labeled glucose has demonstrated that up to 80% of the energy derived from neuronal glucose oxidation is consumed by processes supporting Glu signaling [94]. This tight relationship between cellular energy metabolism and amino acid metabolism gives 13C-MRS the ability to measure dynamic, in vivo, changes in cellular physiology by using labeled TCA cycle precursors. As depicted in Fig. 2, neuronally released Glu and GABA are taken up by tightly juxtaposed astro glia through sodium dependent transporters. When [1-13C] glucose is used as the tracer, it becomes the source for isotopic labeling of intracellular Glu in all three cell types. 13C-MRS measurements of changes in the isotopic-labeling of Glu, Gln and GABA allow for the calculation of the rates of the TCA cycle in GABAergic and glutamatergic cells, which are proportional to their rates of energy production. In contrast to [1-13C] glucose, the infusion of [2-13C] acetate results in selective uptake by glial cells, thereby allowing a direct measurement of the rate of glial metabolism [73] (Fig. 2). The subsequent flow of the isotopic-label from glial-produced Gln into neuronal GABA and Glu potentially allows for separate measurements of the GABA/Gln and Glu/Gln cycles. This approach has been validated in vivo [74,75] and successfully used in 13C-MRS studies of both animals [75] and humans [73]. As described below, the ability of 13C-MRS to selectively measure the flux of Glu through the glial compartment under different experimental conditions allows for hypotheses regarding the effects of stress and pharmacological modulation of glial targets to be directly tested in animal models of depression.
Fig. 2.
Schematic of Glu/Gln and GABA/Gln cycles that interconnect glutamatergic and GABAergic neurons with astrocytes red lines represent primary labeling pathways, and line thickness depicts relative rates of metabolic flux. Isotopic labeleing from [1-13C] glucose (left) and [2-13C] acetate (right) precursors allows for the measurement of metabolic flux in different cellular compartments. The initial rate of isotopic label trapping at Gln 4 after acetate infusion represents astrocytic TCA cycle flux.
11. Effects of chronic stress and riluzole on glial metabolism
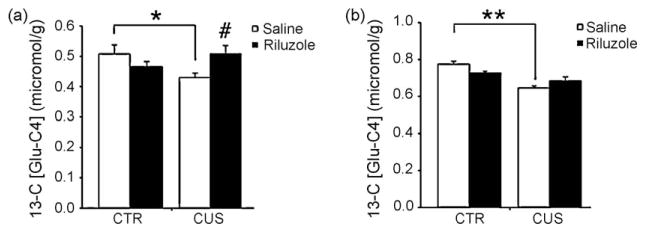
Our group has used 13C-MRS to directly test hypotheses generated from a pathophysiological model of MDD that posits stress-induced changes in glial function as a core component. As shown in Fig. 3, the in vivo effects of chronic unpredictable stress (CUS) and riluzole treatment on glial metabolism and glutamate–glutamine cycling can be examined by 1H–[13C]MRS after infusion of [2-13C] labeled acetate. Adult rats exposed to CUS for 5 weeks exhibit significant decreases in glial cell metabolism as revealed by decreases in 13C-labeled Glu-C4 (Fig. 3a) and Gln-C4 (Fig. 3b) concentrations in ex vivo mPFC extracts [76]. This may be the first experimental evidence for a stress-induced decrease in glial cell metabolism and supports the hypothesis that a consequence of the decreased glial cell proliferation observed in this stress model, is a reduction in cortical glutamate–glutamine cycling. If correct, this hypothesis predicts that the pharmacological facilitation of GLT1 activity would attenuate this stress-induced change in amino acid cycling. Indeed, treatment with riluzole during the final 3 weeks of the5-weekstressprotocolincreasedthepercentage13C-enrichment of Glu-C4 and Gln-C4 in stressed animals when compared to saline-treated controls (Fig. 3a and b). Because of the complex pharmacology exhibited by riluzole, this increase in aminoacid cycling could be secondary to pharmacological effects other than increased EAAT activity. However, the finding that riluzole both reversed stress-induced decreases in GFAP gene expression and increased the expression of GLT1 in the mPFC is consistent with the possibility that increased EAAT activity is both neuro- and glio-protective [76]. In addition, a recent preclinical MRS study has shown that 21 days of riluzole treatment increases Gln-C4 labeling from [1-13C] glucose in healthy adult rats [77]. Collectively, these MRS findings suggest that enhancement of EAAT activity not only increases metabolic flux through the glial compartment, but also indirectly increases neuronal metabolism due to the tight metabolic coupling between glia and neurons. While riluzole has been shown to reduce glutamate release under some experimental conditions, this mechanism has not been observed in MRS studies where decreases in cycling would be predicted instead of the enhancement of glutamatergic metabolism observed in these MRS studies.
Fig. 3.
Chronic unpredictable stress (CUS) decreases the rate of glial metabolism as compared to non-stressed controls (CTR). (a) Reduced labeling of Glu-C4 indicates a decreased rate of Glu/Gln cycling between neurons and astroglia. (b) Reduced labeling of Gln-C4 reflects a decreased rate of astroglial TCA cycle flux. Stress-induced reductions of glial metabolism are mitigated by chronic treatment with riluzole.
Further in vitro and in vivo studies will be required to better elucidate the pharmacological actions of riluzole at concentrations achievable in the brains of patients and to provide additional evidence for the viability of targeting glial physiology in the development of novel antidepressants.
12. Summary
Despite remarkable advances in the neuroscientific understanding of the brain, it has proven difficult to translate this knowledge into clinically meaningful advances in the treatment of MDD. This failure to innovate may be due to a reliance on overly simplistic models of the pathophysiological mechanisms underlying MDD. Contemporary neurobiological models must now account for the dynamic interaction between genetic, psychological and neurobiological factors that mediate the brain’s response to stressors that threaten homeostasis and promote mental illness. The development of pharmacological agents with improved efficacy and/or a more rapid onset of antidepressant action may be profitably guided by targeting neural systems with more fundamental roles in the pathophysiology of the disorder. While the potential for a model of MDD centered upon dysregulated Glu homeostasis to guide the commercialization of antidepressant compounds has not been realized, it has already contributed to the interpretation of seemingly disparate pharmacological findings, and fits squarely within a larger body of biological evidence for the role of disrupted glutamatergic neurotransmission in brain dysfunction. Data provided by additional MRS studies might provide further evidence for the utility of this model in developing novel psychopharmaceuticals.
Abbreviations used
- AANt
amino acid neurotransmitters
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazole-4-propionate
- BDNF
brain-derived neurotrophic factor
- CREB
cyclic AMP response element binding protein
- CUS
chronic unpredictable stress
- EAAT
excitatory amino acid transporter
- GABA
gamma-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- Gln
glutamine
- Glu
glutamate
- L-beta-THA
L-beta-threohydroxyaspartate
- L-Trans 2
4-PDC, L-trans-2,4-pyrrolidine dicarboxylate
- mGlu
metabotropic glutamate
- MRS
magnetic resonance spectroscopy
- NMDA
N-methyl-D-aspartate
- TCA
tricarboxylic acid
- VGLUT
vesicular glutamate transporters
References
- 1.Olney JW. Excitatory transmitter neurotoxicity. Neurobiol Aging. 1994;15:259–60. doi: 10.1016/0197-4580(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 2.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–37. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6:101–15. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- 4.Witkin JM, Marek GJ, Johnson BG, Schoepp DD. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 5.Bleakman D, Alt A, Witkin JM. AMPA receptors in the therapeutic management of depression. CNS Neurol Disord Drug Targets. 2007;6:117–26. doi: 10.2174/187152707780363258. [DOI] [PubMed] [Google Scholar]
- 6.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–60. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 8.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–84. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- 9.Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectrom. 2005;10:808–19. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 10.Francis PT, Poynton A, Lowe SL, Najlerahim A, Bridges PK, Bartlett JR, et al. Brain amino acid concentrations and Ca2+-dependent release in intractable depression assessed antemortem. Brain Res. 1989;494:315–24. doi: 10.1016/0006-8993(89)90600-8. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–6. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–8. doi: 10.1073/pnas.0507901102. [Epub 2005 Oct 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altar CA, Vawter MP, Ginsberg SD. Target identification for CNS diseases by transcriptional profiling. Neuropsychopharmacology. 2009;34:18–54. doi: 10.1038/npp.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–12. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, et al. Subtype-specific alterations of GABA and glutamate in major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 16.Castillo M, Kwock L, Courvoisie H, Hooper SR. Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. Am J Neuroradiol. 2000;21:832–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- 18.Michael N, Erfurth A, Pecuch P, Ohrmann P, Wolgast M, Arolt V, et al. Clinical response to electroconvulsive therapy (ECT) restores reduced glutamate/glutamine levels in the left anterior cingulum of severely depressed patients. World Congress of Biological Psychiatry; Berlin, Germany. 2001. p. 191S. [Google Scholar]
- 19.Cousins JP, Harper G. Neurobiochemical changes from Taxol/Neupogen chemotherapy for metastatic breast carcinoma corresponds with suicidal depression. Cancer Lett. 1996;110:163–7. doi: 10.1016/s0304-3835(96)04486-2. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–6. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 21.Brown GW. The social etiology of depression: London studies. In: Depue RA, editor. The psychobiology of depressive disorders: implications for the effects of stress. New York: Academic Press; 1979. pp. 263–90. [Google Scholar]
- 22.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–87. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 23.Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- 24.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–33. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 26.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression?: Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–60. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 28.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–70. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–96. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatemi SH, Laurence JA, Araghi-Niknam M, Stary JM, Schulz SC, Lee S, et al. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res. 2004;69:317–23. doi: 10.1016/j.schres.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 32.Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–93. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–98. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–9. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 35.Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–4. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- 36.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 37.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 38.Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71:1273–88. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [Epub 2006 Aug 16] [DOI] [PubMed] [Google Scholar]
- 42.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2007;63:349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 43.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328–34. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 44.Hardingham GE. Pro-survival signalling from the NMDA receptor. Biochem Soc Trans. 2006;34:936–8. doi: 10.1042/BST0340936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–29. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–60. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–82. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 48.Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, et al. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–37. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mineur YS, Picciotto MR, Sanacora G. Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry. 2007;61:250–2. doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 50.Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission. II. Biochemical properties. Neuropharmacology. 1985;24:1085–92. doi: 10.1016/0028-3908(85)90196-0. [DOI] [PubMed] [Google Scholar]
- 51.Mizoule J, Meldrum B, Mazadier M, Croucher M, Ollat C, Uzan A, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission. I. Anticonvulsant properties. Neuropharmacology. 1985;24:767–73. doi: 10.1016/0028-3908(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 52.Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–9. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- 53.Albo F, Pieri M, Zona C. Modulation of AMPA receptors in spinal motor neurons by the neuroprotective agent riluzole. J Neurosci Res. 2004;78:200–7. doi: 10.1002/jnr.20244. [DOI] [PubMed] [Google Scholar]
- 54.Zona C, Cavalcanti S, De Sarro G, Siniscalchi A, Marchetti C, Gaetti C, et al. Kainate-induced currents in rat cortical neurons in culture are modulated by riluzole. Synapse. 2002;43:244–51. doi: 10.1002/syn.10040. [DOI] [PubMed] [Google Scholar]
- 55.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–41. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 56.Cheramy A, Barbeito L, Godeheu G, Glowinski J. Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo. Neurosci Lett. 1992;147:209–12. doi: 10.1016/0304-3940(92)90597-z. [DOI] [PubMed] [Google Scholar]
- 57.Doble A, Hubert JP, Blanchard JC. Pertussis toxin pretreatment abolishes the inhibitory effect of riluzole and carbachol on D-[3H]aspartate release from cultured cerebellar granule cells. Neurosci Lett. 1992;140:251–4. doi: 10.1016/0304-3940(92)90114-m. [DOI] [PubMed] [Google Scholar]
- 58.Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–74. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- 59.Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T. Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997;282:1280–90. [PubMed] [Google Scholar]
- 60.Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, et al. Interaction of riluzole with the closed inactivated state of Kv4.3 channels. J Pharmacol Exp Ther. 2006;319:323–31. doi: 10.1124/jpet.106.106724. [DOI] [PubMed] [Google Scholar]
- 61.Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs. 2008;22:761–86. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- 62.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–80. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- 64.Dunlop J, Beal McIlvain H, She Y, Howland DS. Impaired spinal cord glutamate transport capacity and reduced sensitivity to riluzole in a transgenic superoxide dismutase mutant rat model of amyotrophic lateral sclerosis. J Neurosci. 2003;23:1688–96. doi: 10.1523/JNEUROSCI.23-05-01688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24:123–8. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–6. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 67.Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–5. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 69.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 70.Rao SD, Yin HZ, Weiss JH. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J Neurosci. 2003;23:2627–33. doi: 10.1523/JNEUROSCI.23-07-02627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Graaf RA, Mason GF, Patel AB, Behar KL, Rothman DL. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–57. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- 72.Shen J. 13C magnetic resonance spectroscopy studies of alterations in glutamate neurotransmission. Biol Psychiatry. 2006;59:883–7. doi: 10.1016/j.biopsych.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 73.Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–9. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64:2773–82. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- 76.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chowdhury GM, Banasr M, de Graaf RA, Rothman DL, Behar KL, Sanacora G. Chronic riluzole treatment increases glucose metabolism in rat prefrontal cortex and hippocampus. J Cereb Blood Flow Metab. 2008;12:1892–7. doi: 10.1038/jcbfm.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Preskorn S, Baker B, Omo K, Kolluri S, Menniti F, Landen J. A placebo-controlled trial of the NR2B specific NMDA antagonist CP-101,606 plus paroxetine for treatment resistant depression (TRD) San Diego, CA: American Psychiatric Association; 2007. pp. 154–NR362. [Google Scholar]
- 79.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–47. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 81.Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2007;33:1603–10. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- 82.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–44. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 83.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 84.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–4. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 85.Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–2. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 86.Mathew SJ, Keegan K, Smith L. Glutamate modulators as novel interventions for mood disorders. Rev Bras Psiquiatr. 2005;27:243–8. doi: 10.1590/s1516-44462005000300016. [DOI] [PubMed] [Google Scholar]
- 87.Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry. 2005;58:424–8. doi: 10.1016/j.biopsych.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 88.Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007;61:822–5. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine–glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 91.Lafleur DL, Pittenger C, Kelmendi B, Gardner T, Wasylink S, Malison RT, et al. N-Acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 2006;184:254–6. doi: 10.1007/s00213-005-0246-6. [DOI] [PubMed] [Google Scholar]
- 92.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–7. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 93.Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–9. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Tang BL. SNAREs in neurons—beyond synaptic vesicle exocytosis (Review) Mol Membr Biol. 2006;23:377–84. doi: 10.1080/09687860600776734. [DOI] [PubMed] [Google Scholar]
- 95.Lesch KP, Schmitt A. Antidepressants and gene expression profiling: how to SNARE novel drug targets. Pharmacogenomics J. 2002;2:346–8. doi: 10.1038/sj.tpj.6500150. [DOI] [PubMed] [Google Scholar]
- 96.Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 2007;1135:129–35. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–51. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 98.Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, et al. Enhanced anxiety, depressivike behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur J Neurosci. 2007;25:281–90. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- 99.Tordera RM, Pei Q, Sharp T. Evidence for increased expression of the vesicular glutamate transporter, VGLUT1, by a course of antidepressant treatment. J Neurochem. 2005;94:875–83. doi: 10.1111/j.1471-4159.2005.03192.x. [DOI] [PubMed] [Google Scholar]
- 100.Moutsimilli L, Farley S, Dumas S, El Mestikawy S, Giros B, Tzavara ET. Selective cortical VGLUT1 increase as a marker for antidepressant activity. Neuropharmacology. 2005;49:890–900. doi: 10.1016/j.neuropharm.2005.06.017. [DOI] [PubMed] [Google Scholar]