Summary
Lysophosphatidic acid (LPA) and its sphingolipid homolog sphingosine 1-phosphate (S1P) and several other related molecules constitute a family of bioactive lipid phosphoric acids that function as receptor-active mediators with roles in cell growth, differentiation, inflammation, immunomodulation, apoptosis and development. LPA and S1P are present in physiologically relevant concentrations in the circulation. In isolated cell culture systems or animal models, these lipids exert a range of effects that suggest that S1P and LPA could play important roles in maintaining normal vascular homeostasis and in vascular injury responses. LPA and S1P act on a series of G protein–coupled receptors, and LPA may also be an endogenous regulator of PPARγ activity. In this review, we discuss potential roles for lysolipid signaling in the vasculature and mechanisms by which these bioactive lipids could contribute to cardiovascular disease.
Introduction
Lysophospholipids are derivatives of glycero- or sphingo-phospholipids lacking one radyl hydrocarbon chain (Figure 1). The major bioactive lysophospholipids with cell surface receptor-mediated effects are lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P). LPA and S1P circulate in blood, where they are bound to plasma proteins, such as albumin, and found in lipoprotein particles. The biological activities of LPA and S1P are wide-ranging, and in some cases opposing, and include effects on cell growth, apoptosis, adhesion, migration, and invasion. Many of the effects of LPA and S1P are mediated by binding to G-protein-coupled receptors. Because of structural similarities, LPA and SIP receptors were initially classified together as members of the Endothelial Differentiation Gene (Edg) family. Subsequently, these receptors have been more rationally reclassified as LPA receptors and SIP receptors to denote their ligand specificities[1]. To date, five G-protein-coupled LPA receptors have been identified definitively, and evidence for at least three more has been presented [1,2]. LPA1-3 belong to the original Edg class of receptors, whereas most of the more recently identified LPA receptors show greater sequence identity with purinergic receptors. Five documented SIP receptors exist (S1P1-5) [3]. Both LPA and SIP receptor family members activate downstream signals coupled to Gi/o, Gq, or G12/13-mediated pathways[1,4]. LPA may also serve as an endogenous activator of PPARγ [5,6].
Figure 1. Synthesis and inactivation of S1P and LPA.
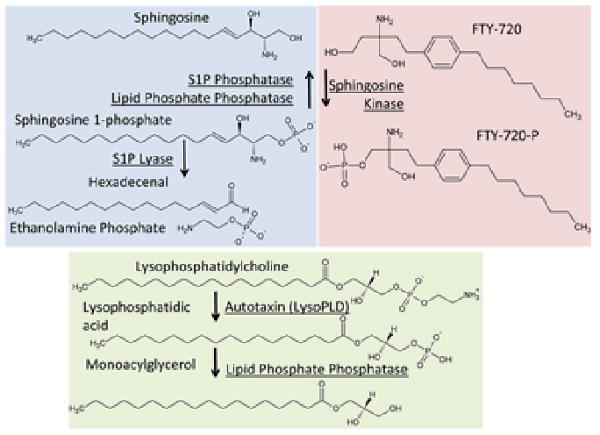
S1P is synthesized intracellularly by sphingosine kinase –catalyzed phosphorylation of sphingosine. Note that dihydrophingosine is also a substrate for this enzyme which forms dihydro S1P. These dihydro derivatives lack the double bond between the C4 and C5 carbon atoms. S1P can be converted to sphingosine by dephosphorylation catalyzed by a selective S1P phosphatase or the broader specificity lipid phosphate phosphatases. S1P can also be degraded by S1P lyase to form hexadecenal and ethanolamine phosphate. The predominant pathway for the production of extracellular LPA is from lysophospholipids, most likely lysophosphatidylcholine by the lysophospholipase D activity of autotaxin. LPA is inactivated by dephosphorylation catalyzed by the broad specificity lipid phosphate phosphatases to form monoacylglycerol. LPA can also be made by phospholipase A2 catalysed hydrolysis of phosphatidic acid. Intracellularly, LPA can be made de novo by acylation of glycerol 3-phosphate for synthesis of di- and tri-glycerides and phospholipids.
Generation of lysophospholipids in the circulation
The data suggest roles for multiple vascular cells in maintaining LPA and S1P homeostasis (Figure 1) with a single discrete mechanism of synthesis for each mediator in plasma, although alternative pathways likely occur in certain settings. In the case of LPA, the primary route of production in blood involves hydrolysis of lysophosphatidyl choline (LPC) by the secreted lysopholipase D (lysoPLD) autotaxin. Autotaxin/lysoPLD is a member of the ectonucleotide pyrophosphatase/phosphodiesterase (Enpp) family, designated Enpp2, with a unique ability to hydrolyze LPC. Genetic deletion of Enpp2 in mice (Enpp2-/-) is lethal embryonically due in part to vascular defects and failure of vessel maturation [7,8]. Enpp2+/- mice have approximately half of the normal circulating levels of autotaxin/lysoPLD and LPA. Transgenic overexpression of human Enpp2 using the α1-anti-trypsin inhibitor promoter to drive expression in liver increases plasma autotaxin/lysoPLD activity and LPA levels [9]. These findings establish that autotaxin/lysoPLD regulates LPA levels in the blood. The substrate for autotaxin/lysoPLD activity is likely LPC produced during cholesterol esterification or from the action of A-type phospholipases (PLA) [10]. Activated platelets can produce LPA through the actions of PLAs, and LPA levels in serum prepared from platelet-rich plasma have been reported to be ∼5-10–fold higher than in platelet-poor plasma. Circulating LPA levels can be lowered in rodents by making them thrombocytopenic [11]. Isolated platelets can generate LPA directly, but the quantities involved are too small to make a significant contribution to serum LPA levels. It may be that a primary role of platelets here is in localized LPA production by generation of a pool of LPC that can serve as a substrate for autotaxin/lysoPLD. Autotaxin/lysoPLD has recently been shown to bind leukocyte integrins [12]. We have found that it can associate with activated platelets in a β3 integrin-dependent manner and accumulates in arterial thrombi [9]. This interaction could be very important for localized generation of LPA and/or targeting the enzyme to cells, membrane microvesicles or lipoproteins that are enriched in its substrates. It is notable that an early study identified a critical role for microparticles produced by activated platelets and Ca2+-treated erythrocytes as a key intermediate in LPA production in the blood.
S1P is produced by 2 sphingosphine kinases (Sphk1 and Sphk2) [13]. The two murine sphingosine kinase genes appear redundant because inactivation of either gene produces animals with no discernable phenotype. However, concurrent inactivation of both genes results in early embryonic lethality. Recent genetic studies in mice revealed a role for erythrocytes in maintaining plasma S1P levels. Cyster, Couglin and colleagues [14] generated animals that survival to adulthood with no detectable SIP in circulation by conditional deletion of Sphk1 in Sphk2-/- pups. They demonstrated that plasma S1P levels can be restored in the Sphk1- and Sphk2-deficient animals by transplant of normal bone marrow cells or by transfusion red blood cells but not platelets. This finding is essentially a genetic ratification of a contemporaneously published study conducted using human and mouse models demonstrating a key role for erythrocytes as a “buffer” system in the storage and release of S1P in the blood [15]. It is important to emphasize that erythrocytes are almost certainly not the sole source of circulating S1P, and a very recent report by Hla and colleagues identifies a role for vascular endothelial cells in the production of circulating S1P[16].
Metabolism of lysophospholipids in circulation
As with the synthetic pathway, degradation of LPA and S1P likely proceeds by several pathways including phospholipase-catalyzed deacylation or reacylation to form receptor-inactive free fatty acids or phosphatidic acid. LPA and S1P can also be inactivated by dephosphorylation by cell surface integral membrane enzymes termed lipid phosphate phosphatases (LPPs). LPPs are a family of integral membrane glycoproteins that localize to the cell surface with a topology that orients the active site towards the extracellular space [17,18]. Overexpression studies in mammalian cells and genetic experiments in Drosophila suggest that LPP3, in particular, functions as regulator of both rapid and longer term LPA signaling responses. Whereas overexpression of Lpp1 does not alter circulating LPA levels or cellular responses to LPA [19] and deficiency of Lpp2 [20] in mice has no obvious phenotypic effect, an unexpected role for LPP3 in early vascular development was revealed by genetic inactivation in mice. Lpp3 deletion in mice is embryonically lethal [21], which we have found is recapitulated by targeted deletion of LPP3 in endothelial cells. Targeted-deletion of LPP3 in smooth muscle cells (SMC) reduces LPA degradation and increases migratory potential. A more selective S1P phosphatase enzyme also exists although this enzyme appears to be intracellular and likely plays a role in intracellular sphingolipid metabolism. S1P can also be degraded by an S1P lyase which is a pyridoxal phosphate-dependent enzyme that cleaves S1P at the C2-C3 bond to yield ethanolamine phosphate and hexedecenal [22].
Regulation of endothelial barrier function by lysophospholipids
The first defined lysolipid receptor S1P1/Edg1 was originally identified as a gene upregulated in endothelial cells in an in vitro model of angiogenesis. The identification of S1P as high-affinity ligand for this receptor provoked great interest in bioactive lysolipids as regulators of endothelial cell function and vasculature dynamics. Genetic inactivation of S1P1 in mice results in embryonic lethality and impaired vessel formation due to a lack of homing of SMC to the developing vessels [23]. In cultured cells, physiologic concentrations of S1P (0.5 – 1 μM) promote endothelial barrier functions via S1P1-mediated activation of the Rho GTPase Rac and cytoskeletal reorganization and stabilization of junctions [24,25]. S1P can restore barrier function after disruption by edemagenic agents such as thrombin. Additionally, many barrier-stabilizing agents, including activated protein C, transactivate S1P1 signaling. The potent protective effects of S1P on endothelial barrier function have led to the proposal that the lipid could serve as a therapy to reduce endothelial permeability in inflammatory settings such as sepsis and acute lung injury. In that regard, S1P is cardioprotective in animal models of ischemia/reperfusion injury [26]. Exposure of endothelial cells to higher concentrations of S1P (∼ 5 mM) triggers S1P3-mediated Rho activation that disrupts barrier function. Recently, a protective effect for Sphk1 in maintaining endothelial barrier function in the setting of LPS- or thrombin-induced pulmonary edema has been demonstrated [27].
The effects of LPA on endothelial cell function have not been studied as extensively as SIP. The preponderance of the data would suggest that LPA elicits a loss of vascular integrity and decreases transendothelial resistance by preventing tight junction formation [28], although some investigators have observed that LPA stabilizes endothelial barrier function [29]. In a lung injury model, the LPA1 receptor mediates vascular leak [30].
Immunomodulatory properties of lysophospholipids
S1P promotes a range of physiologically relevant immunomodulatory effects [31]. The pharmaceutical compound FTY720, a pro-drug that is phosphorylated in vivo to produce an SIP receptor agonist and desensitizes S1P1, S1P2, and S1P5 receptors, prevents lymphocyte egress from thymus and lymphoid organs. Extensive data analyzing effects of FTY720 and results from mice lacking specific S1P receptors have established a role for S1P signaling and the S1P1 receptor, in particular, in regulation of T and B cell trafficking between the lymphatic system and the peripheral circulation. These studies support a model in which a gradient of S1P – high levels in blood and lymph and low levels in lymphoid organs – promotes the outward migration of lymphocytes. Recently Keul et al. demonstrated that FTY720 attenuates atherosclerosis formation in ApoE-/- mice and reduces macrophage accumulation in plaque [32]. It is noteworthy that S1P also prevents macrophage activation [33] and monocyte-endothelial interactions [34].
Less is known about the immunomodulatory/inflammatory effects of LPA [35,36]. LPA receptors are found on lymphocytes, dendritic cells, and in lymphoid organs. Recent work by Rosen and colleagues demonstrated that autotaxin/lysoPLD binds to lymphocytes in an integrin-dependent manner to generate LPA at the cell surface which promotes the entry of lymphocytes into lymphoid organs [12]. LPA also stimulates chemotaxis of human dendritic cells and, in the case of immature murine dendritic cells, the effects are mediated by LPA3. LPA activates innate immune cells, including neutrophils, eosinophils, and mononuclear phagocytes.
Regulation of SMC function and the development of intimal hyperplasia by lysophospholipids
LPA has been proposed to be a key factor in serum that promotes phenotypic modulation of SMCs [37]. When added to cultured SMCs, LPA promotes dedifferentiation, proliferation, and migration Local infusion of LPA in the rodent carotid artery induces vascular remodeling by stimulating neointimal formation that may mediated by PPARγ [38]. LPA1 and LPA2 appear to be to be critical receptors for regulating SMC migration, however, the receptors that are coupled to other aspects of phenotypic modulation of SMC are not known. Additionally, LPA has vasoregulatory properties. For example, intravenous injection of LPA elevates arterial blood pressure in rats and mice and local application causes cerebral vasoconstriction in pigs.
Following arterial injury, an early increase in S1P1 and S1P3 levels occur in the vessel accompanied by a transient decrease in S1P2 levels, which is followed by a late increase in S1P2 levels [39]. Rat pup intimal SMC, which have a higher proliferative capacity, also express greater levels of S1P1 than do adult medial cells [40]. Mouse strains with higher S1P1 expression develop more intimal hyperplasia following arterial injury [41], and pharmacologically targeting of S1P1 and S1P3 reduces the formation of neotintima [39]. S1P1 and S1P3 may mediate their effects in part by promoting dedifferentiation of SMC. In contrast, S1P2 appears to protect from the development of intimal hyperplasia, in that S1p2-/- mice develop more robust neointima after arterial injury and an S1P2 antagonist promotes a more differentiated phenotype in SMC [42]. S1P may also regulate vascular tone, although in some arterial beds, it has vasoconstrictor properties and in other beds promotes vasodilation.
Atherothrombosis
While the studies related above, including those describing the effects of FTY720, suggest that S1P may be atheroprotective, the available data would indicate that LPA signaling could promote the development of atherosclerosis and its complications. Local concentrations of LPA may be increased along inflamed vessels and at sites of platelet adhesion and thrombus formation where autotaxin accumulates. Seiss and Tigyi found that LPA is abundant in the lipid-rich core of human atherosclerotic plaque, where is may be derived from mildly oxidized LDL [43]. We have more recently found that when normalized to total phospholipid phosphorous LPA and compared to healthy tissue, polyunsaturated LPA species are significantly enriched in experimentally induced murine atheromas. Thus, LPA is present or can be formed in the settings where it could influence development and complications of atherosclerosis. LPA triggers an inflammatory response in endothelial cells involving LPA1 and LPA3- mediated expression of leukocyte chemoattractants and adhesion receptors[44,45]. These responses promote monocyte binding to endothelial cells. Moreover, LPA is a weak activator of platelets from most human donors [46], although it lacks stimulatory effects on rodent platelets. Thus, in addition to proinflammatory changes that may promote atherosclerosis, LPA exposure could contribute to the major complication of atherosclerosis, namely acute arterial thrombosis. In this regard, it is interesting that mouse platelets are not activated by physiologically relevant concentrations of LPA and that mice do not display spontaneous thrombosis even in the presence of extensive atherosclerosis.
Summary
The ongoing work discussed above clearly implicates both LPA and S1P in a wide range of cardiovascular functions. However, functional redundancy between different members of the LPA and S1P selective receptor classes coupled with compensatory changes in their expression continues to make it challenging to use mice with targeted inactivation of these receptor genes to provide definitive insights into the roles of these lipids in cardiovascular physiology and disease. Interestingly, the processes involved in LPA and S1P production and metabolism appear to involve fewer non-redundant genes. For example, LPA production in the blood is critically dependent on a single gene product, autotaxin/lysoPLD and manipulation of this enzyme in mouse models has significant effects on circulating LPA levels. Efforts aimed at targeting LPA and S1P synthesis and inactivation may prove to be more effective strategies for both experimental investigations into their roles in cardiovascular function and disease in animal models and eventually for pharmacological intervention in humans.
Figure 2. S1P and LPA homeostasis in the blood.
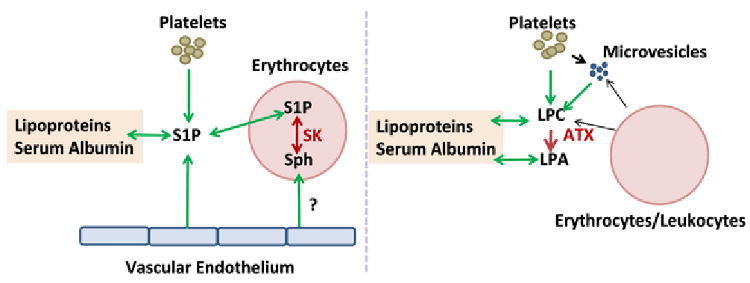
S1P can be produced by platelets, vascular endothelium and erythrocytes. Adoptive transfer and transfusion experiments establish a critical role for erythrocytes in this process in mice. Erythrocytes sphingosine kinase and de novo synthesis of S1P may involve uptake of sphingosine (sph) from an undefined source, possibly vascular endothelium. S1P is carried in the blood bound to lipoproteins and serum albumin. LPA is formed by lysophospholipase D activity of autotaxin (ATX) which hydrolyzes circulating lyosphospholipids, predominantly LPC which may be formed as a by-product of cholesterol esterification or potentially generated by phospholipases acting on lipids in platelets, erythrocytes or membrane microparticles released from these cells. Like S1P, LPA and LPC are bound to serum albumin and lipoproteins
Acknowledgments
This work was supported by NIH grants HL070304, HL078663, HL074219, GM050388, and a VA Merit Award.
Footnotes
Disclosure of conflict of interest
The authors report not conflicts of interest.
Reference List
- 1.Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Pyne NJ, Pyne S. Sphingosine 1-phosphate, lysophosphatidic acid and growth factor signaling and termination. Biochim Biophys Acta. 2008;1781:467–476. doi: 10.1016/j.bbalip.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology and pathology. J Lipid Res. 2008 doi: 10.1194/jlr.R800047-JLR200. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre TM, Pontsler AV, Silva AR, St HA, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 8.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pamuklar Z, Federico L, Liu S, Umezu-Goto M, Dong A, Panchatcharam M, Fulkerson Z, Berdyshev E, Natarajan V, Fang F, van Meeteren LA, Moolenaar WH, Mills GB, Morris AJ, Smyth SS. Autotaxin/lysopholipase D and Lysophosphatidic Acid Regulate Murine Hemostasis and Thrombosis. J Biol Chem. 2009;284:7385–94. doi: 10.1074/jbc.M807820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milstien S, Gude D, Spiegel S. Sphingosine 1-phosphate in neural signalling and function. Acta Paediatr Suppl. 2007;96:40–43. doi: 10.1111/j.1651-2227.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 14.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 15.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brindley DN. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J Cell Biochem. 2004;92:900–912. doi: 10.1002/jcb.20126. [DOI] [PubMed] [Google Scholar]
- 18.Sciorra VA, Morris AJ. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim Biophys Acta. 2002;1582:45–51. doi: 10.1016/s1388-1981(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 19.Yue J, Yokoyama K, Balazs L, Baker DL, Smalley D, Pilquil C, Brindley DN, Tigyi G. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell Signal. 2004;16:385–399. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Sundberg JP, Gridley T. Mice mutant for Ppap2c, a homolog of the germ cell migration regulator wunen, are viable and fertile. Genesis. 2000;27:137–140. doi: 10.1002/1526-968x(200008)27:4<137::aid-gene10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Escalante-Alcalde D, Hernandez L, Le SH, Maeda R, Lee HS, Jr GC, Sciorra VA, Daar I, Spiegel S, Morris AJ, Stewart CL. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 22.Bandhuvula P, Saba JD. Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends Mol Med. 2007;13:210–217. doi: 10.1016/j.molmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karliner JS. Mechanisms of cardioprotection by lysophospholipids. J Cell Biochem. 2004;92:1095–1103. doi: 10.1002/jcb.20129. [DOI] [PubMed] [Google Scholar]
- 27.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Nieuw Amerongen GP, Vermeer MA, H van V. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:E127–E133. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- 29.Alexander JS, Patton WF, Christman BW, Cuiper LL, Haselton FR. Platelet-derived lysophosphatidic acid decreases endothelial permeability in vitro. Am J Physiol. 1998;274:H115–H122. doi: 10.1152/ajpheart.1998.274.1.H115. [DOI] [PubMed] [Google Scholar]
- 30.Tager AM, Lacamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Poorvu EC, Brooks SF, Bercury SD, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The Lysophosphatidic Acid Receptor LPA1 Links Pulmonary Fibrosis to Lung Injury by Mediating Fibroblast Recruitment and Vascular Leak. Proc Am Thorac Soc. 2008;5:363. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 31.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keul P, Tolle M, Lucke S, von Wnuck LK, Heusch G, Schuchardt M, van der GM, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 33.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolick DT, Srinivasan S, Kim KW, Hatley ME, Clemens JJ, Whetzel A, Ferger N, Macdonald TL, Davis MD, Tsao PS, Lynch KR, Hedrick CC. Sphingosine-1-phosphate prevents tumor necrosis factor-{alpha}-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- 35.Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest. 2004;114:1531–1537. doi: 10.1172/JCI23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Knudsen E, Jin Y, Gessani S, Maghazachi AA. Lysophospholipids and chemokines activate distinct signal transduction pathways in T helper 1 and T helper 2 cells. Cell Signal. 2004;16:991–1000. doi: 10.1016/j.cellsig.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, Aoki J, Arai H, Sobue K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluk MJ, Hla T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ Res. 2001;89:496–502. doi: 10.1161/hh1801.096338. [DOI] [PubMed] [Google Scholar]
- 41.Inoue S, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy M. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: a role for sphingosine-1-phosphate receptors. J Vasc Surg. 2007;46:756–763. doi: 10.1016/j.jvs.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy MA. Sphingosine 1-phosphate receptor 2 negatively regulates neointimal formation in mouse arteries. Circ Res. 2007;101:995–1000. doi: 10.1161/CIRCRESAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 43.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin CI, Chen CN, Lin PW, Chang KJ, Hsieh FJ, Lee H. Lysophosphatidic acid regulates inflammation-related genes in human endothelial cells through LPA1 and LPA3. Biochem Biophys Res Commun. 2007;363:1001–1008. doi: 10.1016/j.bbrc.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 45.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smyth S, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim Biophys Acta. 2008;1781:563–570. doi: 10.1016/j.bbalip.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


