Abstract
Background
Variable platelet response to clopidogrel has been widely observed. Studies have shown that the mean aggregation response to clopidogrel can be changed by a higher maintenance dose. However, these studies have not focused on individual changes.
Objectives
This study examined the platelet function effects of increasing the maintenance clopidogrel dose from 75 mg/day to 150 mg/day with a focus on inter-individual response.
Patients/Methods
Twenty patients with known coronary artery disease receiving 75 mg/day clopidogrel were recruited and given 150 mg/day of clopidogrel for 30 days, then returned to 75 mg/day for an additional 30 days. Platelet function was assessed via light-transmittance aggregometry (LTA) and the VerifyNow P2Y12 assay at baseline, 30 days, and 60 days.
Results
Mean platelet inhibition was significantly improved with the increased maintenance dose when measured by the VerifyNow P2Y12 assay (PRU: 191 ± 15 vs. 158 ± 17, p = 0.013), but not when measured by LTA (LTA-ADP5: 40 ± 3 vs. 36 ± 3, p = 0.11; LTA-ADP20: 50 ± 3 vs. 47 ± 3, p = 0.23). However, only 50% of individual patients experienced improved platelet inhibition, as measured by the VerifyNow P2Y12 assay, when treated with the increased maintenance dose. Furthermore, poor baseline platelet response did not predict improved responsiveness at the increased dose.
Conclusions
Despite changing the population's mean antiplatelet response, an increased maintenance dose of clopidogrel did not improve antiplatelet response in a substantial number of patients; nor did baseline platelet function predict response to a higher maintenance dose.
Keywords: clopidogrel, coronary artery disease, blood platelets, platelet aggregation, adenosine diphosphate, purinoceptor P2Y12
Introduction
Combination antiplatelet therapy with aspirin (ASA) and clopidogrel is currently the standard of care to reduce the risk of cardiovascular events in several clinical settings, including acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI).[1, 2] Despite these proven therapeutic benefits of the combination of clopidogrel and ASA, 3-5% of patients presenting with ACS experience a late recurrent thrombotic event (defined as an event occurring a minimum of 30 days after the index event),[1] and stent thrombosis complicates 1-2% of PCI procedures.[3, 4]
Inadequate platelet inhibition is one possible explanation for thrombotic events that occur despite combination clopidogrel and ASA therapy. Indeed, wide inter-individual variability in responsiveness to the standard dose of clopidogrel has been documented extensively with a variety of ex vivo assays.[5-8] Part of this variability may be attributable to the standard maintenance dose of clopidogrel (75 mg/day), which was chosen because it achieved a degree of platelet inhibition similar to that of the initial thienopyridine, ticlopidine. However, the standard ticlopidine dose (500 mg/day) was arbitrarily chosen in the 1970s prior to its use in coronary angioplasty and PCI.[9]
In an attempt to lower the risk of subacute and late stent thrombosis, especially in the setting of drug-eluting stents, alternative strategies of clopidogrel administration are being investigated. In the setting of ACS and elective PCI, a loading dose of 600 mg of clopidogrel has been shown to more rapidly and to a greater degree inhibit platelet function, thereby improving outcomes compared with 300 mg.[10-12] In addition, the current ACC/AHA/SCAI Percutaneous Coronary Intervention guidelines advocate increasing clopidogrel therapy to 150 mg daily in patients deemed to be at high risk for subacute stent thrombosis and in whom platelet function tests demonstrate <50% inhibition of platelet aggregation (Class IIB, level of evidence C).[13]
Studies investigating the biologic effects of higher maintenance doses of clopidogrel on platelet function have found that higher clopidogrel doses can reduce the average platelet aggregation response in a population.[14-17] However, these studies have only minimally described the inter-individual or temporal responses to a higher clopidogrel dose. Thus, it is possible that only a small percentage of patients in a population at any specific time would benefit from a higher clopidogrel dose. Additionally, no study to date has demonstrated the pharmacokinetic effects of an increased maintenance dose, although this is likely due to technical difficulties in measuring the active metabolite of clopidogrel.
In this study, we examined the platelet function effects of increasing the daily maintenance clopidogrel dose from 75 mg/day to 150 mg/day in patients with known coronary artery disease (CAD).
Methods
Subject Enrollment
Patients were recruited from the outpatient cardiology clinic at the University of Kentucky. The study protocol was approved by the Institutional Review Board, and all subjects signed a written consent form. Subjects with documented CAD, as defined angiographically or by history of myocardial infarction, were eligible to participate if they were over the age of 18 and had been treated with dual antiplatelet therapy of aspirin (81 to 325 mg/day) and clopidogrel (75 mg/day) for at least one month. Exclusion criteria included thrombocytopenia (< 100 × 103 platelets/μL), anemia (hemoglobin < 10 mg/dL), recent bleeding diathesis, malignancy, renal insufficiency (creatinine > 2.5 mg/dL), liver dysfunction (bilirubin > 2 mg/dL), or treatment with warfarin or glycoprotein (GP) IIb/IIIa antagonists during the preceding 14 days.
Study Protocol
At the time of enrollment, subjects taking aspirin and clopidogrel were evaluated for baseline platelet function. Following venous blood collection in 3.2% sodium citrate Vacutainer® collection tubes, platelet aggregation was assessed by the VerifyNow P2Y12 point of care test and ADP-induced light transmittance aggregometry (LTA) using 5 and 20 μM ADP. The dose of clopidogrel was then increased to 150 mg/day for 30 days, at which time the platelet function assays were repeated. Subjects then resumed the 75 mg/day dose of clopidogrel for 30 days for a final assessment of platelet function at day 60 of the protocol. Target endpoints for the study were change in platelet function, with both clopidogrel dose and time, as assessed by the VerifyNow P2Y12 assay and LTA. The consistency of results from baseline to 60 days was also assessed.
VerifyNow P2Y12 Assay
An electronic quality control was run daily prior to performing the P2Y12 VerifyNow assay. Citrated whole blood was transferred to 2 mL Greiner® vacuettes and gently inverted 5 times before being inserted onto P2Y12 assay cartridges to determine platelet function. Values are reported as P2Y12 reaction units (PRU) and indicate the extent of ADP-induced platelet aggregation via the P2Y12 receptor. According to the manufacturer, subjects not taking clopidogrel have a mean PRU value of 307, and administration of clopidogrel decreases baseline scores by 185 PRU on average. [18]
Light Transmittance Aggregometry
Platelet rich plasma (PRP) was obtained by low speed centrifugation (100g for 15 minutes at room temperature) of citrated whole blood. After removal of the PRP, the remaining portion of blood was centrifuged at 2400 g for 20 minutes at room temperature to obtain platelet poor plasma (PPP). PRP was diluted with PPP to obtain a final platelet count of 200 to 300 × 103/μL for use in LTA; platelet counts were determined using a Beckman Coulter Counter (Model ACT 10). Aggregation was monitored in a 570VS 4-channel aggregometer (Chrono-Log; Havertown, PA) after the addition of either 5 or 20 μM ADP. Light transmission was continuously recorded for 6 minutes using AGGRO/LINK software, and results were presented as the maximum or residual percent increase in light transmission.
Statistical Analyses
The effects of treatment dose on platelet function were evaluated using one-way repeated measures ANOVAs. Specifically, two linear mixed models were fit to each measure of platelet response (PRU, LTA-ADP5, and LTA-ADP20). The first linear mixed model related mean platelet response to clopidogrel dose (75 mg or 150 mg), while the second related mean platelet response to the time point (baseline, 30 days, or 60 days). Both linear mixed models incorporated subject-specific random effects to capture correlations among repeated measurements on the same individuals. In addition, Pearson correlations (r) were calculated to compare PRU values to LTA values and to determine whether baseline platelet response predicted the change in response after 30 days and/or the final response after 60 days. Subjects whose baseline values on aspirin and clopidogrel 75 mg were more than twice the standard error over mean response were defined as poor responders. For each assay and agonist concentration, poor responders were compared to other subjects by T test to assess whether one group experienced greater change from baseline to 30 days. Agreement of the assays for designating poor responsiveness was assessed with the kappa statistic. All statistical calculations were performed in JMP 7.0 (SAS Institute Inc., Cary, NC). A p-value <0.05 was considered statistically significant. Based on previous data,[16] we anticipated that the mean ± SD level of ADP (5 μM)-induced platelet aggregation would be 47 ± 15% in patients treated with 75 mg of clopidogrel daily and 38 ± 10% in those treated with 150 mg daily. Accordingly, 19 subjects would be needed for a paired comparison with 80% power to detect the aforementioned difference (47% versus 38%) in mean ADP (5 μM)-induced platelet aggregation at the 0.05 significance level (assuming a 0.50 correlation between scores on the same patient at 75 mg and 150 mg).
Results
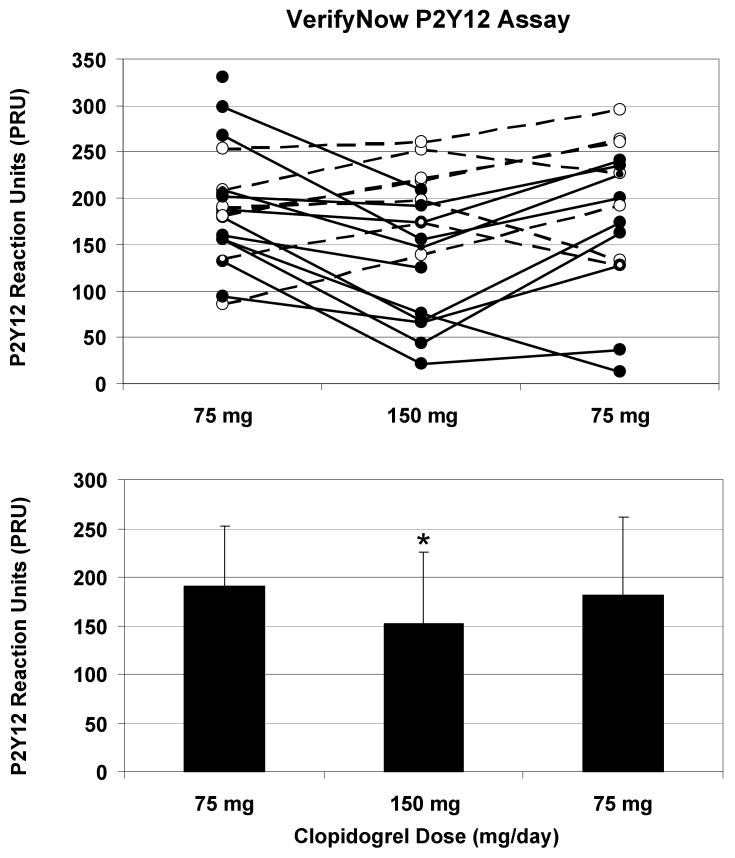
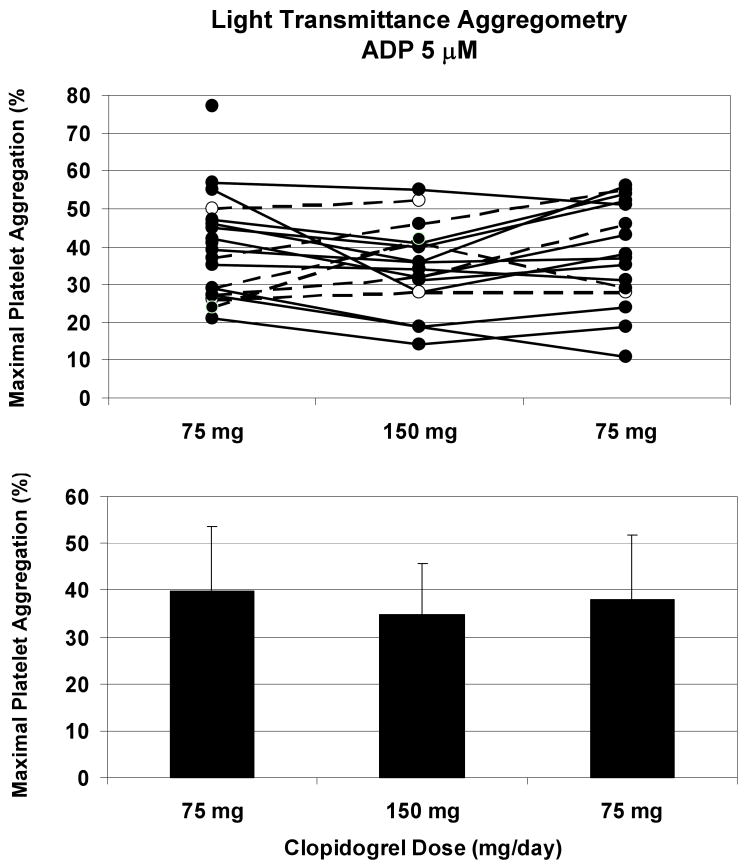
Subject characteristics and baseline laboratory values are depicted in Table 1. Model-based estimates of mean platelet responses and standard errors are shown in Table 2. Platelet function as analyzed by the VerifyNow P2Y12 assay yielded estimated mean PRU scores of 191 with 75 mg clopidogrel (191 at baseline and 191 at 60 days) and 158 with 150 mg clopidogrel at 30 days (Figure 1). The PRU scores were significantly lower when subjects were taking 150 mg/day at 30 days compared to 75 mg/day at baseline alone (p = 0.029) or 60 days alone (p = 0.038) or with these results combined (p=0.013). Even so, there was considerable variability across individuals. Among the 18 subjects with non-missing PRU scores at baseline and 30 days, nine had PRU scores more than 28 points lower at 30 days than at baseline, five had PRU scores more than 28 points higher, and four did not experience changes of more than 28 points in either direction; the frame of reference was set at 28 PRU to match twice the standard error of platelet function at baseline. Analysis of platelet function by light transmittance aggregometry yielded estimated mean LTA-ADP5 values of 40% while subjects were taking 75 mg clopidogrel (39% at baseline and 40% at 60 days) and 36% at 150 mg clopidogrel (Figure 2). Estimated mean LTA-ADP20 scores were 50% at 75 mg clopidogrel (51% at baseline and 49% at 60 days) and 47% at 150 mg (Table 2). For both concentrations of ADP, there were no significant differences by clopidogrel dose or time with LTA. Similar results were obtained with residual platelet aggregation at six minutes (Table 2).
Table 1. Subject demographics and baseline laboratory values.
Numeric data are summarized as the mean plus or minus standard deviation. All 20 subjects had documented coronary artery disease and were treated with aspirin 81 mg to 325 mg and clopidogrel 75 mg for a minimum of one month. PRP = platelet-rich plasma.
| Subject Characteristics (n=20) | Baseline Lab Values |
|---|---|
| Age (years) | 64 ± 11 |
| Race (% Caucasian) | 95% |
| Gender (% male) | 65% |
| Hypertension | 100% |
| Hyperlipidemia | 95% |
| Diabetes | 40% |
| Red blood cells (×106/μL) | 4.5 ± 0.5 |
| Hemoglobin (g/dL) | 12.7 ± 1.3 |
| Hematocrit (%) | 40 ± 4 |
| Platelets (×103/μL) | 247 ± 66 |
| Diluted PRP (×103/μL) | 285 ± 20 |
Table 2. Estimated mean responses by clopidogrel dose and time.
The estimated mean responses and standard errors at the two doses (75 mg and 150 mg) are based on a linear mixed model with dose as the predictor and a random effect for each subject. The estimated mean responses and standard errors at the three times (baseline, 30 days, and 60 days) are based on a linear mixed model with time as the predictor and a random effect for each subject. Superscripts 1, 2, 3, and 4 indicate statistically significant differences in platelet response (p < 0.05) compared to 75 mg dose, baseline, 60 days, and 30 days, respectively. PRU = P2Y12 reaction units, MPA = maximal platelet aggregation, RPA = residual platelet aggregation, and ADP = adenosine diphosphate.
| Dose and Time | PRU | MPA ADP5 |
MPA ADP20 |
RPA ADP5 |
RPA ADP20 |
|---|---|---|---|---|---|
| 75 mg dose | 191 ± 15 | 40 ± 3 | 50 ± 3 | 23 ± 3 | 36 ± 4 |
| 150 mg dose | 158 ± 171 | 36 ± 3 | 47 ± 3 | 19 ± 4 | 33 ± 4 |
| Baseline (75 mg clopidogrel) | 191 ± 16 | 39 ± 3 | 51 ± 3 | 23 ± 4 | 42 ± 43,4 |
| 30 days (150 mg clopidogrel) | 158 ± 172,3 | 36 ± 3 | 47 ± 3 | 18 ± 4 | 33 ± 4 |
| 60 days (75 mg clopidogrel) | 191 ± 17 | 40 ± 3 | 49 ± 4 | 22 ± 4 | 28 ± 4 |
Figure 1. Clopidogrel 150 mg/day significantly decreased ADP-induced platelet function as assessed by the VerifyNow assay.
Subjects with known CAD collectively demonstrated decreased platelet aggregation with 150 mg of clopidogrel at 30 days compared with 75 mg of clopidogrel at baseline and 60 days (p = 0.013). Although extensive variability was present, overall there was no significant difference in platelet response at 60 days compared to baseline. Platelet function was assessed by the VerifyNow point of care device, and values are reported as P2Y12 reaction units (PRU). In the top portion of the figure, individual subject values are plotted across the 3 time points from baseline (75 mg) to 30 days (150 mg) to 60 days (75 mg). Solid lines indicate subjects whose values decreased from baseline to the 30-day time point, and dashed lines denote an increase from baseline to 30 days. In the bottom portion of the figure, aggregate results (mean ± standard deviation) are presented for each time point.
Figure 2. No significant effects of clopidogrel 150 mg/day were observed with ADP-induced platelet aggregation assessed by light transmittance aggregometry.
Platelet function did not differ significantly by clopidogrel dose (75 mg versus 150 mg) when analyzed by aggregometry (p = 0.11). Maximal platelet aggregation was assessed for 6 minutes after stimulation with 5 μM ADP. Solid lines indicate a decrease from baseline to the 30-day time point, and dashed lines denote an increase from baseline to 30 days. Aggregate results (mean ± standard deviation) are presented for each time point in the bottom portion of the figure.
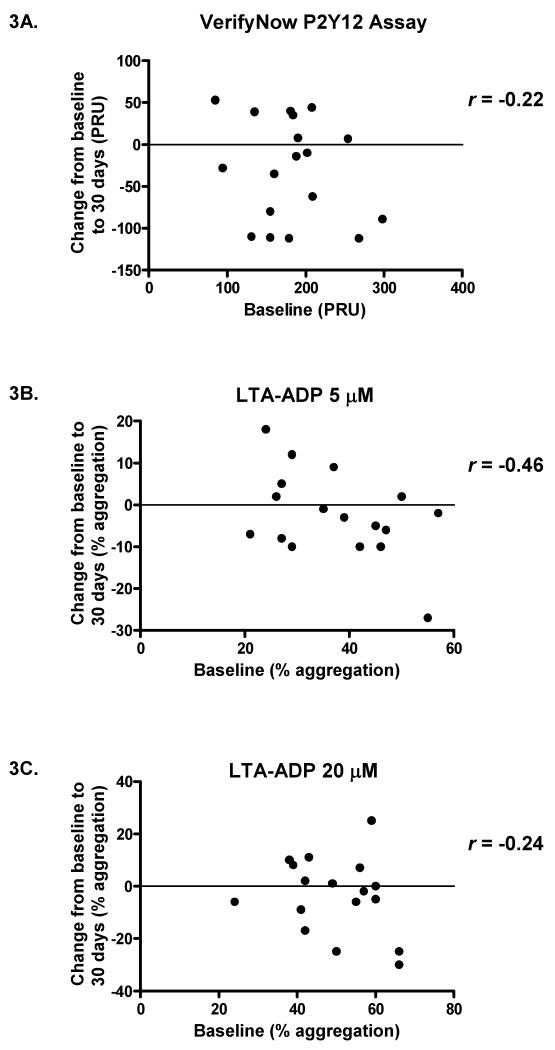
Results from the two platelet aggregation assays were highly correlated at all time points. The Pearson correlations between PRU and LTA-ADP5 values were 0.87, 0.82, and 0.89 at baseline, 30 days, and 60 days, respectively (all p-values < 0.0001); similarly, correlations remained strong between PRU and LTA-ADP20 at baseline (r = 0.82, p < 0.0001), 30 days (r = 0.83, p < 0.0001), and 60 days (r = 0.90, p < 0.0001). On the other hand, baseline platelet function did not predict the change in response at 30 days. The Pearson correlations relating baseline platelet function to the change in response were -0.22, -0.46, and -0.24 based on the VerifyNow P2Y12 assay, LTA with 5 μM ADP, and LTA with 20 μM ADP respectively (Figure 3); these correlations were not significantly different from zero. Moreover, baseline platelet function correlated only modestly with platelet function at 60 days, after subjects were again taking 75 mg clopidogrel for a minimum of 30 days. Statistically significant but moderate correlations were obtained for the VerifyNow P2Y12 assay (r = 0.54, p = 0.031) and light transmittance aggregometry with 5 μM ADP (r = 0.68, p = 0.006). At 20 μM ADP, there was no significant correlation between baseline and 60-day values for light transmittance aggregometry (r = 0.41, p = 0.12). Among the 16 subjects with non-missing PRU scores at baseline and 60 days, four had PRU scores more than 28 points lower at 60 days than at baseline, seven had PRU scores more than 28 points higher, and five had PRU scores within twice the standard error of baseline scores (see Figure 1).
Figure 3. Baseline platelet function at 75 mg clopidogrel did not predict response to the increased dose of clopidogrel.
The baseline results for subjects taking 75 mg clopidogrel were plotted against the changes in response after switching to 150 mg clopidogrel (values at 30-day time point minus values at baseline). Pearson correlations (r) were calculated for the VerifyNow P2Y12 assay (Figure 4A), light transmittance aggregometry stimulated by 5 μM ADP (Figure 4B), and light transmittance aggregometry with 20 μM ADP (Figure 4C). For all three assays, there were no significant correlations between baseline values and changes in platelet response (p = 0.37, 0.063, and 0.35, respectively, for Figures 4A, 4B, and 4C). PRU = P2Y12 reaction units, LTA = light transmittance aggregometry, and ADP = adenosine diphosphate.
Separating the subjects into poor responders and other subjects did not elucidate the change in response from baseline (75 mg clopidogrel) to 30 days (150 mg clopidogrel). Poor responders, whose baseline values were more than twice the standard error over mean response for each assay, were not statistically different from other subjects as indicated by PRU (p = 0.29), MPA (maximal platelet aggregation)-ADP5 (p = 0.11), MPA-ADP20 (p = 0.60), RPA (residual platelet aggregation)-ADP5 (p = 0.11), and RPA-ADP20 (p = 0.42). Using these defined cut-off values for poor responders, the calculated kappa statistics for each assay combination were variable and ranged from 0.30 to 1.0 (Table 3).
Table 3. Agreement between assays on the designation of poor responsiveness.
The agreement between assays and conditions for categorizing subjects as poor responders was assessed using the kappa statistic. Poor responders were defined as having baseline platelet function more than twice the standard error over mean response. PRU = P2Y12 reaction units, MPA = maximal platelet aggregation, and RPA = residual platelet aggregation.
| Kappa Statistic | ||
|---|---|---|
| PRU | MPA 5 | 0.85 |
| PRU | MPA 20 | 0.46 |
| PRU | RPA 5 | 1.00 |
| PRU | RPA 20 | 0.30 |
| MPA 5 | MPA 20 | 0.62 |
| MPA 5 | RPA 5 | 0.85 |
| MPA 5 | RPA 20 | 0.43 |
| MPA 20 | RPA 5 | 0.46 |
| MPA 20 | RPA 20 | 0.33 |
| RPA 5 | RPA 20 | 0.30 |
Discussion
The main finding of this study is that despite an average higher degree of platelet inhibition as measured by PRU scores in a population of patients taking 150 mg/day versus 75 mg/day of clopidogrel, substantial inter-individual variability exists in response to a higher maintenance dose of clopidogrel. Nine out of 18 patients in this study had similar or enhanced platelet function while taking 150 mg/day of clopidogrel compared to 75 mg/day at baseline, and platelet aggregation at baseline did not predict response to a higher maintenance dose of clopidogrel. Moreover, there is substantial temporal variability in response to the 75 mg/day dose of clopidogrel. Eleven out of 16 patients had PRU scores at least 28 points higher or lower at 60 days compared to baseline. Reproducibility in platelet function testing over time remains an area of concern for evaluating antiplatelet agents. In addition to the modest reproducibility demonstrated after two months with clopidogrel in this study, Harrison et al. have previously documented minimal consistency of aspirin response after one year of follow-up using conventional platelet function testing.[19] Although it is possible that sample processing could contribute to the variability in LTA, the high correlation between the VerifyNow P2Y12 assay and LTA makes this unlikely and suggests that an individual's platelet responsiveness may vary over time.
Recently, there has been increasing interest in the clinical relevance of ex vivo-determined inter-individual variability in the response to clopidogrel, with most studies finding a wide, Gaussian distribution of responses ranging from 0% inhibition of ADP-induced aggregation to almost 100% inhibition.[7, 8, 20] There is mounting evidence to suggest that an inadequate response to clopidogrel, as measured by ex vivo platelet function tests, is associated with adverse clinical events, especially in the setting of PCI.[20-23] However, it is not entirely clear if this is a cause and effect relationship as a poor response to clopidogrel has been associated with high-risk clinical features.[24] Perhaps more convincing evidence for the clinical benefit of higher levels of platelet inhibition comes from studies of more potent P2Y12 receptor antagonists. One such thienopyridine, prasugrel, achieves greater levels of platelet inhibition than clopidogrel with less inter-patient variability.[25] In the pivotal TRITON trial, more rapid and consistent inhibition of ADP-induced platelet aggregation by prasugrel translated into a reduced frequency of ischemic events compared to clopidogrel with a tradeoff of increased bleeding.[26] Likewise, the non-thienopyridines cangrelor and AZD6140, competitive P2Y12 antagonists that elicit a higher level of platelet inhibition than clopidogrel,[27, 28] are currently undergoing Phase III testing (ClinicalTrials.gov Identifiers NCT00385138 and NCT00391872, respectively).
While TRITON demonstrated improved ischemic outcomes and caused more frequent bleeding with a more potent thienopyridine, it is still not clear whether ex vivo platelet function testing can be utilized to optimize antiplatelet therapy. Several studies have shown both biologic and clinical benefit with the use of a higher loading dose of clopidogrel in population based approaches.[12] However, much of the benefit noted in clinical studies from an increased loading dose may be the result of decreased time to peak antiplatelet effect, which appears to be about 2 hours with a 600 mg dose but potentially up to 24 hours with 300 mg.[29, 30] Bonello et al. also recently demonstrated reduced rates of adverse cardiovascular events with an individualized, vasodilator-stimulated phosphoprotein (VASP) index-guided loading dose of clopidogrel prior to PCI in 78 patients compared to a control group receiving a single 600 mg dose.[31] However, the small sample size precludes widespread application of this approach in clinical practice.
The ex vivo effect of a higher maintenance dose was examined in diabetic patients in the OPTIMUS trial, where the mean inhibition of LTA was improved by 150 mg/day of clopidogrel compared to the standard 75 mg/day regimen.[14] Similar findings of a decrease in mean platelet reactivity with a higher maintenance dose of clopidogrel have been reported by von Beckerath et al. and by Angiolillo et al. in patients undergoing PCI.[15, 16]
Despite the data indicating that higher doses of clopidogrel can achieve greater average platelet inhibition in a population of patients, less is known about individual responsiveness; the existing data suggest that many individuals may not benefit from higher doses of clopidogrel. A re-analysis of the OPTIMUS data indicated that 85% (17/20) of the patients assigned to 150 mg/day of clopidogrel failed to achieve platelet inhibition > 50%,[32] although 150 mg/day is the dose recommended by the ACC/AHA/SCAI guidelines for patients undergoing PCI who respond poorly to 75 mg/day. In addition, Neubauer et al. used an aggregometry-guided protocol and found that only 58% (22/38) of the patients identified as low-responders at 75 mg/day of clopidogrel were classified as responders at 150 mg/day clopidogrel.[33] Importantly, there is, as of yet, no clinical evidence that an increased maintenance dose is either efficacious or safe. The CURRENT/OASIS-7 (Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent EveNTs/Optimal Antiplatelet Strategy for InterventionS) trial (ClinicalTrials.gov Identifier: NCT00335452), in which patients with ACS undergoing primary PCI will be randomized to receive either 600 mg/150 mg/day or 300 mg/75 mg/day of clopidogrel for one week, should establish a clinical basis for the use of higher maintenance doses at the population level if results are favorable. Unfortunately, this trial is not evaluating the usefulness of platelet function testing to guide therapy for individual patients. Therefore, validation of a patient-specific approach for optimizing antiplatelet therapy and identification of the best ex vivo method for monitoring therapy are still needed. The GRAVITAS (Gauging Responsiveness With A VerifyNow Assay-Impact On Thrombosis And Safety) trial (ClinicalTrials.gov Identifier: NCT00645918) in which patients will be randomized to increased loading/maintenance doses of clopidogrel versus standard therapy based on platelet response, will help to answer some of the questions surrounding the clinical utility of tailored therapy.
In conclusion, several studies with clopidogrel have found marked variability in ex vivo platelet function responses to both the standard 75 mg/day dose and 300 mg loading dose. In this study, despite an increased mean level of platelet inhibition, substantial inter-patient variability was still present at a dose of 150 mg/day, suggesting that doubling the dose of clopidogrel may have little or no effect in a large percentage of patients. Also, poor baseline platelet reactivity did not predict response to the higher maintenance dose of clopidogrel, casting further doubt upon which factors influence individual patient responsiveness to clopidogrel therapy.
There were several limitations to this study. To promote compliance with clopidogrel therapy, patients received a phone call one week prior to platelet function testing. However, there were no objective means utilized to ensure compliance. Also, patients were advised to take 150 mg of clopidogrel at a single time, preferably in the morning. There was no assessment, however, of whether it would be more efficacious to administer the dose as 75 mg twice daily, although this could be a reasonable hypothesis based on pharmacokinetic limitations such as absorption. Additionally, we did not enroll or separately study a group of clopidogrel naïve patients, which may have provided further data about clopidogrel responsiveness from a true physiologic baseline. Finally, while there were no adverse bleeding or ischemic events, the small sample size precluded formal assessment of bleeding risk or efficacy of a higher maintenance dose of clopidogrel.
While sub-optimal platelet inhibition with clopidogrel may be associated with adverse clinical events, we have demonstrated that a population-based approach of an increased maintenance dose will not improve antiplatelet response in a substantial number of patients as measured by the VerifyNow assay and LTA. Although the results of the TRITON trial strengthen the argument that more intensive ADP inhibition and by extension, platelet function monitoring, may serve as a surrogate marker for clinical outcomes, additional research is needed to define and examine clinical applications of ex vivo platelet monitoring and to identify optimal individual- and population-based approaches for improving outcomes with antiplatelet therapy.
Acknowledgments
This research was supported in part by a grant from the University of Kentucky General Clinical Research Center M01 RR02602 and awards from the American Heart Association and the PhRMA Foundation.
References
- 1.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002 Nov 20;288(19):2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001 Aug 16;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 3.Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001 Apr 17;103(15):1967–71. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 4.Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G. Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart. 2006 May;92(5):641–9. doi: 10.1136/hrt.2005.061622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003 Jun 17;107(23):2908–13. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 6.Hochholzer W, Trenk D, Frundi D, et al. Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005 May 24;111(20):2560–4. doi: 10.1161/01.CIR.0000160869.75810.98. [DOI] [PubMed] [Google Scholar]
- 7.Muller I, Besta F, Schulz C, Massberg S, Schonig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003 May;89(5):783–7. [PubMed] [Google Scholar]
- 8.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005 Jan 18;45(2):246–51. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 9.Thebault JJ, Kieffer G, Lowe GD, Nimmo WS, Cariou R. Repeated-dose pharmacodynamics of clopidogrel in healthy subjects. Semin Thromb Hemost. 1999;25 2:9–14. [PubMed] [Google Scholar]
- 10.Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. 2006 Oct 3;48(7):1339–45. doi: 10.1016/j.jacc.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Kastrati A, von Beckerath N, Joost A, Pogatsa-Murray G, Gorchakova O, Schomig A. Loading with 600 mg clopidogrel in patients with coronary artery disease with and without chronic clopidogrel therapy. Circulation. 2004 Oct 5;110(14):1916–9. doi: 10.1161/01.CIR.0000137972.74120.12. [DOI] [PubMed] [Google Scholar]
- 12.Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2005 Apr 26;111(16):2099–106. doi: 10.1161/01.CIR.0000161383.06692.D4. [DOI] [PubMed] [Google Scholar]
- 13.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006 Feb 21;113(7):e166–286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 14.Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007 Feb 13;115(6):708–16. doi: 10.1161/CIRCULATIONAHA.106.667741. [DOI] [PubMed] [Google Scholar]
- 15.von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J. 2007 Aug;28(15):1814–9. doi: 10.1093/eurheartj/ehl489. [DOI] [PubMed] [Google Scholar]
- 16.Angiolillo DJ, Bernardo E, Palazuelos J, et al. Functional impact of high clopidogrel maintenance dosing in patients undergoing elective percutaneous coronary interventions. Results of a randomized study. Thromb Haemost. 2008 Jan;99(1):161–8. doi: 10.1160/TH07-09-0562. [DOI] [PubMed] [Google Scholar]
- 17.Fontana P, Senouf D, Mach F. Biological effect of increased maintenance dose of clopidogrel in cardiovascular outpatients and influence of the cytochrome P450 2C19*2 allele on clopidogrel responsiveness. Thromb Res. 2008;121(4):463–8. doi: 10.1016/j.thromres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Steinhubl S, Roe MT. Optimizing platelet P2Y12 inhibition for patients undergoing PCI. Cardiovasc Drug Rev. 2007 Summer;25(2):188–203. doi: 10.1111/j.1527-3466.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 19.Harrison P, Segal H, Silver L, Syed A, Cuthbertson FC, Rothwell PM. Lack of reproducibility of assessment of aspirin responsiveness by optical aggregometry and two platelet function tests. Platelets. 2008 Mar;19(2):119–24. doi: 10.1080/09537100701771736. [DOI] [PubMed] [Google Scholar]
- 20.Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol. 2005 Nov 15;46(10):1827–32. doi: 10.1016/j.jacc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 21.Cuisset T, Frere C, Quilici J, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006 Mar;4(3):542–9. doi: 10.1111/j.1538-7836.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 22.Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005 Mar 8;111(9):1153–9. doi: 10.1161/01.CIR.0000157138.02645.11. [DOI] [PubMed] [Google Scholar]
- 23.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004 Jun 29;109(25):3171–5. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 24.Geisler T, Grass D, Bigalke B, et al. The Residual Platelet Aggregation after Deployment of Intracoronary Stent (PREDICT) score. J Thromb Haemost. 2008 Jan;6(1):54–61. doi: 10.1111/j.1538-7836.2007.02812.x. [DOI] [PubMed] [Google Scholar]
- 25.Weerakkody GJ, Jakubowski JA, Brandt JT, Payne CD, Naganuma H, Winters KJ. Greater inhibition of platelet aggregation and reduced response variability with prasugrel versus clopidogrel: an integrated analysis. J Cardiovasc Pharmacol Ther. 2007 Sep;12(3):205–12. doi: 10.1177/1074248407304731. [DOI] [PubMed] [Google Scholar]
- 26.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15;357(20):2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 27.Storey RF, Wilcox RG, Heptinstall S. Comparison of the pharmacodynamic effects of the platelet ADP receptor antagonists clopidogrel and AR-C69931MX in patients with ischaemic heart disease. Platelets. 2002 Nov;13(7):407–13. doi: 10.1080/0953710021000024402. [DOI] [PubMed] [Google Scholar]
- 28.Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007 Nov 6;50(19):1852–6. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 29.Kandzari DE, Berger PB, Kastrati A, et al. Influence of treatment duration with a 600-mg dose of clopidogrel before percutaneous coronary revascularization. J Am Coll Cardiol. 2004 Dec 7;44(11):2133–6. doi: 10.1016/j.jacc.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 30.Steinhubl SR, Berger PB, Brennan DM, Topol EJ. Optimal timing for the initiation of pre-treatment with 300 mg clopidogrel before percutaneous coronary intervention. J Am Coll Cardiol. 2006 Mar 7;47(5):939–43. doi: 10.1016/j.jacc.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008 Apr 8;51(14):1404–11. doi: 10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 32.Angiolillo DJ, Costa MA, Shoemaker SB, et al. Functional effects of high clopidogrel maintenance dosing in patients with inadequate platelet inhibition on standard dose treatment. Am J Cardiol. 2008 Feb 15;101(4):440–5. doi: 10.1016/j.amjcard.2007.09.087. [DOI] [PubMed] [Google Scholar]
- 33.Neubauer H, Lask S, Engelhardt A, Mugge A. How to optimise clopidogrel therapy? Reducing the low-response incidence by aggregometry-guided therapy modification. Thromb Haemost. 2008 Feb;99(2):357–62. doi: 10.1160/TH07-10-0624. [DOI] [PubMed] [Google Scholar]