Abstract
One-fifth of the tRNAs used in plant mitochondrial translation is coded for by chloroplast-derived tRNA genes. To understand how aminoacyl–tRNA synthetases have adapted to the presence of these tRNAs in mitochondria, we have cloned an Arabidopsis thaliana cDNA coding for a methionyl–tRNA synthetase. This enzyme was chosen because chloroplast-like elongator tRNAMet genes have been described in several plant species, including A. thaliana. We demonstrate here that the isolated cDNA codes for both the chloroplastic and the mitochondrial methionyl–tRNA synthetase (MetRS). The protein is transported into isolated chloroplasts and mitochondria and is processed to its mature form in both organelles. Transient expression assays using the green fluorescent protein demonstrated that the N-terminal region of the MetRS is sufficient to address the protein to both chloroplasts and mitochondria. Moreover, characterization of MetRS activities from mitochondria and chloroplasts of pea showed that only one MetRS activity exists in each organelle and that both are indistinguishable by their behavior on ion exchange and hydrophobic chromatographies. The high degree of sequence similarity between A. thaliana and Synechocystis MetRS strongly suggests that the A. thaliana MetRS gene described here is of chloroplast origin.
In higher plants, protein synthesis takes place in the cytosol, mitochondria, and chloroplasts. Each compartment relies on its own set of aminoacyl–tRNA synthetases (aaRSs) and tRNAs. All aaRSs are encoded in the nucleus, but tRNAs are encoded in nuclear and organellar genomes. Although cytosolic and chloroplast translation systems use tRNAs that are encoded by nuclear and chloroplast genes, respectively (1), the situation is more complex in mitochondria. The mitochondrial genome does not encode a full set of tRNAs, and the translation machinery relies on nuclear-encoded tRNAs that are imported from the cytosol (2, 3). In addition, two kinds of mitochondrially encoded tRNAs are active in translation: the “native” tRNAs, which are encoded by genes derived from the prokaryotic ancestor of mitochondria, and the “chloroplast-like” tRNAs, which are encoded by genes from chloroplast DNA fragments that have been integrated into the mitochondrial genome during evolution (1). As a consequence, mitochondria have several tRNA genes that are 98–100% identical to their chloroplast counterparts.
Although distribution of plant tRNAs has been under considerable investigation over the past decade, very little is known about aaRSs, their genes, and their regulation in higher plant cells. Purification and biochemical characterization of several plant aaRSs have been reported (4–7). These studies established that, in general, plant aaRSs can be classified into two groups, based on their substrate specificity: (i) the cytosolic enzymes that most efficiently aminoacylate plant or yeast cytosolic tRNAs; and (ii) the organellar enzymes that aminoacylate organelle or Escherichia coli tRNAs (8). Exceptions to this rule have been reported. For example, bean (Phaseolus vulgaris) cytosolic and mitochondrial leucyl–tRNA synthetases aminoacylate cytosolic and mitochondrial tRNAs equally but do not function with chloroplast or E. coli tRNAsLeu (5). Additionally, antibodies to cytosolic leucyl–tRNA synthetases (9, 10) specifically inhibit the mitochondrial but not the chloroplastic enzyme (11). These observations were substantiated by the discovery that cytosolic tRNAsLeu are imported into bean mitochondria (11, 12). After the cloning and characterization of an alanyl–tRNA synthetase gene from Arabidopsis thaliana (13), it was observed that a single gene codes for both the cytosolic and the mitochondrial forms of this enzyme. As for tRNAsLeu, A. thaliana cytosolic tRNAAla also is imported into mitochondria (14).
To better understand how aaRSs have adapted to this heterogeneous population of tRNAs that exists in plant mitochondria, we are characterizing a number of plant aaRS genes. In the present work, our attention is focused on methionyl–tRNA synthetase (MetRS) of A. thaliana because mitochondrial elongator tRNAMet is known to be of chloroplast origin in dicotyledonous plants like A. thaliana (15), bean (16), potato (17), and soybean (15) and in monocotyledonous plants like wheat (18) and maize (19). We report now on the isolation and characterization of a MetRS cDNA. We present evidence that the MetRS encoded by this cDNA has a dual destination, to mitochondria and chloroplasts, in plant cells.
MATERIALS AND METHODS
Purification of Chloroplasts and Mitochondria.
Chloroplasts were extracted from pea (Pisum sativum, cv. ‘Douce Provence’) leaves according to reported methods (20). Mitochondria were extracted from potato tubers or from green pea seedlings as described (21). Transfer RNAs were extracted from purified organelles as described (2).
Fast Protein Liquid Chromatography Purification of MetRS Activities.
Chloroplasts and mitochondria were lysed by sonication in aaRS extraction buffer containing 100 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, 10% (vol/vol) glycerol, 10 mM EDTA, and 5 mM 2-mercaptoethanol and supplemented with a proteinase inhibitor mixture (Complete, Boehringer Mannheim). The lysates were clarified by two centrifugations (27,000 × g for 15 min and 105,000 × g for 2 h, respectively). Protein extracts were loaded on a 5-ml anion exchange column (Bio-Scale Q2, Bio-Rad) and were washed with 20 ml of buffer containing 10 mM Tris⋅HCl (pH 8.0), 10 mM 2-mercaptoethanol, and 1 mM KCl. The preparation was eluted with 30 ml of a linear KCl gradient (from 1 mM to 400 mM) in the washing buffer at a flow rate of 1 ml/min. Fractions of 0.5 ml were collected and tested for MetRS activity. Aminoacylations were performed as described by using E. coli tRNAs as substrates (14). The fractions containing MetRS activity were pooled, were adjusted to a final concentration of 1.7 M (NH4)2SO4, and were loaded onto a 5-ml hydrophobic column (Phenyl-Superose HR-5, Pharmacia). Washing was done by using 20 ml of 50 mM phosphate buffer (pH 7.0) containing 1.7 M (NH4)2SO4. The preparation was eluted with a 30-ml linear gradient of (NH4)2SO4 (from 1.7 M to 0 M) in 50 mM phosphate buffer (pH 7.0) at a flow rate of 0.5 ml/min. Fractions of 0.5 ml were collected and tested for MetRS activity as described above.
Expressed Sequence Tag (EST) Clones, cDNA Library Screening, and Sequencing.
EST clones and λPRL2 cDNA libraries (22) constructed in λZipLox phage (GIBCO/BRL) (23) were obtained from the Arabidopsis Biological Resource Center DNA Stock Center (Ohio State University). Screening of the libraries and all molecular biology techniques were done according to standard procedures. pZL1 plasmids were excised from λ phages according to the manufacturer’s instructions (GIBCO/BRL). DNA sequencing was done by automated sequencing using an Applied Biosystems sequencer. Sequences were analyzed by using the uwgcg software package (Univ. of Wisconsin Genetics Computer Group, Madison). Sequence similarity searches were done by using the blast program (http://www.ncbi.nlm.nih.gov/BLAST/).
Southern Blot Hybridization.
A. thaliana (ecotype columbia) DNA was extracted from rosettes according to ref. 24. Hybridizations and washings were done at 50°C in 6× standard saline citrate, 0.5% SDS, and 2× standard saline citrate, 0.5% SDS, respectively.
In Vitro Protein Synthesis and Mitochondrial and Chloroplast Import.
In vitro protein synthesis was done by using a TNT Coupled Reticulocyte Lysate System (Promega) according to procedures described in the instruction manual. Import of 35S-labeled proteins into purified pea chloroplasts and potato mitochondria was done according to described procedures (20, 25). Proteins were analyzed on 10% polyacrylamide gels in the presence of SDS. Protein sizes were estimated by using low range prestained molecular weight markers (Bio-Rad).
Overexpression in E. coli.
To overexpress the mature form of the A. thaliana MetRS in E. coli, the SacI–HindIII fragment of pMetRS2.2 was cloned into the corresponding sites of pQE52 (Qiagen, Chatsworth, CA). The resulting construct, pQE52—MetRS, was transformed into E. coli SG13009(pREP4). Expression of the construct was induced for 3 hr by adding isopropyl β-d-thiogalactoside (2 mM) to exponentially growing cultures (OD600 nm = 0.6). Cells were collected and were disrupted by sonication in aaRS extraction buffer (see above), and cell debris were eliminated by a 20-min centrifugation at 27,000 × g. tRNAs and amino acids were eliminated from the protein extract by passing successively through a DEAE cellulose column in the presence of 0.15 M NaCl and a Sephadex G75 spin-column, as described (2). Aminoacylation was done as in ref. 26 by using 1 μg of pea mitochondrial and chloroplast tRNAs as substrates in combination with 35S- or 3H-labeled amino acids.
Green Fluorescent Protein (GFP) Expression Vector and Constructs.
The pCK–GFP3 vector was constructed by replacing the GFP coding sequence (NcoI–BamHI fragment) of pCK-GFP S65T (27) with a modified “ultrabright” GFP [Leu64→Phe and Ser65→Tyr; ref. 28 and A. Steinmetz, personal communication). For the MetRS N terminus GFP fusion, the presequence was amplified from the cDNA clone by PCR by using oligonucleotides that contained a NcoI site (ATCCATGGCGGCGAGGATAAAC and ATCCATGGTCTCGCCTTCATCGACGG). For the coxIV–GFP fusions, the yeast coxIV presequence was amplified from plasmid 35S35S-CT-S56TmGFP4 (29) by PCR by using oligonucleotides that contained a NcoI site (ATACATGTTGTCACTACGTCAATCTATA and ATCCATGGGTTTTTGCTGAAG). Amplifications were done as described elsewhere (30). Gel-purified PCR products were digested and cloned into the NcoI site of pCK–GFP3.
Cell Cultures, Transient Expression of GFP Constructs, and Microscopy.
Green tobacco cell suspension cultures were grown in B5c medium (Gamborg salts/20 g/liter sucrose/1 mg/liter nicotinic acid/10 mg/ml thiamine/100 mg/ml inositol/1 mg/ml pyridoxine/1 mg/ml naphthalene acetic acid) and were diluted 1/10 (vol/vol) weekly. Biolistic transformation of 3-day old tobacco cell cultures was done according to ref. 31 by using a helium-driven device. Microscopic observations of GFP in expressing cells were done by using a Nikon ECLIPSE E800 epifluorescence microscope mounted with a GFP(R)–Long Pass (Excitation 460–500, Dichroic Minor 505, Long Pass 510) or a GFP(R)–Band Pass (Excitation 460–500, Dichroic Minor 505, Band Pass 510–560) set of filters. 4-chloromethyltetramethylrosamine staining of mitochondria was done according to ref. 29. Red fluorescence of 4-chloromethyltetramethylrosamine was observed by using a standard tetramethylrhodamine B isothiocyanate filter set (Excitation 540/25, Dichroic Minor 565, Band Pass 605/55). Images were captured with a Power HAD 3CCD video camera (Sony, Tokyo) by using Visiol@b 200 (Biocom, Paris) and were prepared by using Adobe PhotoShop.
RESULTS
Purification of Chloroplast and Mitochondrial MetRS Activities.
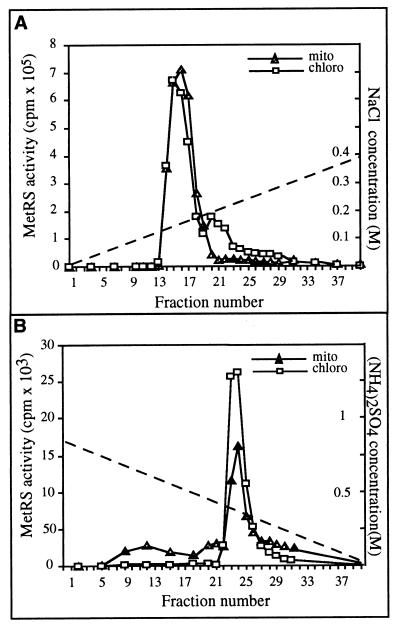
To purify MetRS activities, pea chloroplast and mitochondrial protein extracts (10 mg of proteins for each) were fractionated separately by two successive chromatographic procedures. Protein extracts were first loaded onto an anion exchange column (Bio-Scale Q2, Bio-Rad). Fractions were eluted and tested for MetRS activity by using E. coli tRNAs and [35S]methionine as substrates. The elution profile of the chloroplast proteins showed a major peak of MetRS activity followed by a minor one (Fig. 1A). The small peak is believed to represent a minor contaminant of cytosolic enzyme in the chloroplast preparation (32). Mitochondrial MetRS activity fractionated as a single peak that eluted in the same fractions as the chloroplast MetRS activity (Fig. 1A). These results indicate that chloroplast and mitochondrial MetRS activities are similar and indistinguishable based on their chromatographic behavior on anion exchange chromatography. A comparison also was made to determine whether the two MetRSs activities could be distinguished by their hydrophobic interaction capacities. Fractions from the Bio-Scale column containing MetRS activity were pooled, were loaded onto a Phenyl-Superose column, and were eluted with a gradient of decreasing ammonium sulfate concentration. As with ion exchange chromatography, the elution profiles of MetRS activity were identical for the mitochondrial and chloroplast extracts (Fig. 1B).
Figure 1.
Fast protein liquid chromatography elution profiles of chloroplast (chloro) and mitochondrial (mito) MetRS activities on a Bio-Scale Q2 anion exchange column (A) and on a Phenyl-Superose hydrophobic column (B). MetRS activity was tested by aminoacylation of E. coli tRNAs with 35S-labeled methionine. Acid-insoluble radioactivity was counted after 20 min of reaction. NaCl and (NH4)2SO4 gradients used for elution in A and B, respectively, are indicated by dotted lines.
Isolation and Characterization of a Full Length MetRS cDNA.
To isolate cDNAs coding for an organelle MetRS, we searched databases for ESTs having similarities to prokaryotic MetRS genes. Two A. thaliana ESTs (clone 108M13T7, accession no. T41822 and cloneTAP0385, accession no. F19928) of 1.1 and 0.4 kilobase pairs, respectively, with significant sequence similarities to prokaryotic MetRS were identified. Nucleotide sequence analysis of the two clones revealed that they did not share sequence similarity to each other and they both were used to screen (105 plaques) an A. thaliana cDNA library (λ-PRL2). Two clones, one probed with 108M13T7 and one probed with TAP0385, were isolated from the library. The excised plasmids yielded cDNAs of 1.4 kilobases and 2.2 kilobases that were named pMetRS1.4 and pMetRS2.2, respectively. pMetRS2.2 cDNA (GenBank accession no. U7461) encodes a 616-residue protein with high similarity to known MetRSs whereas pMetRS1.4 was an incomplete form of the same cDNA, indicating that EST clones 108M13T7 and TAP0385 were derived from nonoverlapping parts of the same mRNA. The 616-residue protein encoded by the pMetRS2.2 cDNA possesses two signature sequences (HIGH and KMSKS) characteristic of class I aaRSs. Striking similarities with a putative MetRS of the cyanobacterium Synechocystis (33) were observed for long amino acid stretches (Fig. 2). The sequence alignment also showed that A. thaliana and Synechocystis protein sequences have the same gaps compared with the E. coli MetRS sequence and revealed the presence, in the A. thaliana protein, of an N-terminal extension rich in basic and hydroxylated residues, characteristic of precursors of organellar proteins.
Figure 2.
Alignment of the A. thaliana (A.t.) MetRS amino acid sequence with the Synechocystis (Syn) and E. coli (E.c.) MetRS sequences. Optimal sequence alignment was obtained by using pileup (Univ. of Wisconsin Genetics Computer Group). Amino acids in the A. thaliana sequence that are conserved either in the Synechocystis or in the E. coli protein are highlighted. The class I aminoacyl–tRNA synthetase signatures HIGH and KMSKS are underlined with filled triangles. The C-terminal amino acid of the presequence that was fused to the GFP is indicated by an open arrow. The N-terminal amino acid of the protein expressed in E. coli is indicated by a filled arrow.
To determine whether the gene corresponding to this MetRS cDNA was unique, total DNA of A. thaliana ecotype Columbia was digested with several restriction enzymes and was probed with a 0.9-kilobase EcoRI fragment of pMetRS2.2. The hybridization pattern obtained with each digest suggested that introns are present and that this MetRS gene is unique in the genome of A. thaliana (not shown).
The pMetSR2.2 cDNA Encodes a MetRS Activity.
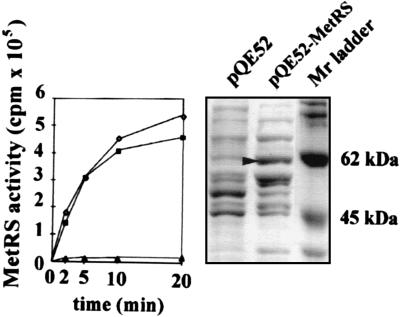
Preparations of aaRS were obtained from isopropyl β-d-thiogalactoside-induced cells containing plasmids pQE52–MetRS or pQE52 without an inserted gene. Overexpression of a 62-kDa protein was observed in the pQE52–MetRS-containing cells (Fig. 3). MetRS activity was highly induced in bacteria containing the pQE52–MetRS construct, thus confirming that the cDNA encodes a functional MetRS. Chloroplast and mitochondrial tRNAs were aminoacylated with approximately the same efficiency (Fig. 3). No significant increase in activity was observed when the same protein extract was tested for another aaRS activity (valyl–tRNA synthetase; data not shown).
Figure 3.
Overexpression of A. thaliana MetRS in E. coli. Protein extracts of isopropyl β-d-thiogalactoside-induced E. coli cells containing pQE52 or pQE52–MetRS were analyzed on a 10% polyacrylamide gel in the presence of SDS (Right). Induction of a 62-kDa protein (arrowhead) was obtained in the bacteria containing pQE52–MetRS. MetRS activity in the protein extract from the pQE52–MetRS strain was measured by using 1 μg of mitochondrial (♦) or chloroplast (⋄) tRNAs as a substrate (Left) and was compared with the activity in the control protein extract from the pQE52 strain (▴) by using chloroplast tRNAs as a substrate.
In Vitro Import of MetRS into Isolated Chloroplasts and Mitochondria.
To evaluate whether the characterized MetRS was a mitochondrial or a chloroplast protein, we tested its ability to be transported into organelles in vitro. A 35S-labeled MetRS of ≈66 kDa was synthesized in vitro (Fig. 4, lane 1) and was incubated with purified pea chloroplasts or potato mitochondria in conditions allowing specific transport of proteins across organellar membranes.
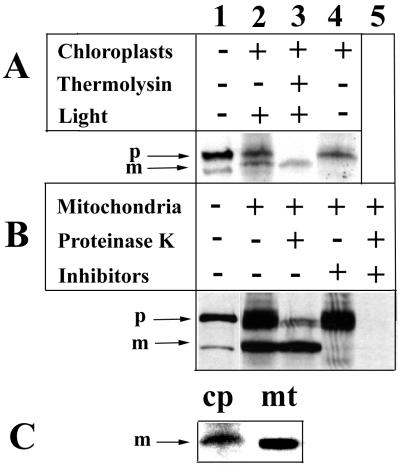
Figure 4.
In vitro import of 35S-labeled MetRS into isolated chloroplasts (A) and mitochondria (B). 35S-labeled MetRS (lane 1) was incubated with purified chloroplasts or mitochondria for 30 min at 25°C (lane 2). On completion of the import reaction, one half of the reaction mix was treated with proteases (lane 3). Transport reaction into chloroplasts was inhibited in the absence of light, and import into mitochondria was inhibited by pretreatment of mitochondria with valinomycin and potassium cyanide (lane 4). For mitochondria, one half of the inhibition assay (lane 4) was treated with proteinase K before loading on gel (lane 5). In C, chloroplast (cp) and mitochondrial (mt) mature forms were compared. (p, precursor; m, mature form).
Transport of the protein into chloroplasts was demonstrated by the appearance of a processed polypeptide associated with chloroplasts in a light-dependent manner (Fig. 4A). The processed protein, which is ≈62 kDa, was protected from proteinase (thermolysin) activity added to the reaction medium after completion of the transport reaction (Fig. 4A, lane 3). Because protein transport across the chloroplast envelope strictly depends on high stromal ATP concentrations (34), which are provided by photosynthesis in our experiments, transport of MetRS into chloroplasts occurred only in the presence of light (Fig. 4A, lane 4). These results demonstrate that this protein is actively imported and processed into its mature form in chloroplasts in vitro.
Likewise, the 35S-labeled MetRS protein also was transported actively into isolated mitochondria (Fig. 4B), as revealed by the appearance of the processed 62-kDa protein in the mitochondrial fraction. This processed protein also was protected from the action of proteinase K added to the medium on completion of the transport reaction (Fig. 4B, lane 3). In addition, transport was inhibited by treating mitochondria with valinomycin (10 μM) and potassium cyanide (1 mM), which are known to disrupt the membrane potential. Processing of the MetRS precursors in chloroplasts and in mitochondria does not appear to occur at the same site, as shown by the size difference observed when the chloroplast and mitochondrial mature forms were run side by side on a 10% polyacrylamide gel (Fig. 4C). Together, these data clearly show that the MetRS protein is transported actively into both mitochondria and chloroplasts in vitro and is processed into its mature form.
Quantification of the protein fraction resistant to the proteinase treatment in the chloroplast and in the mitochondrial in vitro import assays, respectively (Fig. 4 A and B, lane 3), revealed that, in both assays, up to 10% of the labeled precursors could be transported into the organelles. There appeared to be no significant difference in the rate of transport into the two types of organelle in vitro.
The MetRS Presequence Targets GFP into Chloroplasts and Mitochondria in Vivo.
To confirm that the characterized MetRS is targeted to both types of organelles in vivo, we fused the MetRS presequence to a reporter GFP and microscopically followed the destination of GFP in plant cells. The sequence coding for the first 70 amino acids of the MetRS was fused, in frame, to the 5′ end of a GFP coding sequence to yield a construct named pCK-MetPS-GFP3. This construct was expressed transiently in cultured tobacco cells after biolistic delivery. After 24 hr of culture, GFP could be observed both in mitochondria and in chloroplasts (Fig. 5 B and D). Chloroplasts could be distinguished by their red autofluorescence, because of chlorophyll, when using a filter that did not cut fluorescence emission >600 nm (Fig. 5A). Mitochondria were identified by staining with 4-chloromethyltetramethylrosamine, a mitochondria-specific stain (Fig. 5C). Controls using mitochondria- (yeast COXIV; ref. 29) and chloroplast- (RecA; ref. 35) specific presequences fused to the GFP were expressed to show that fluorescence was restricted to the target organelle (Figs. 5E and 6F). Our transient expression experiments revealed that the MetRS transit peptide was sufficient to target reporter GFP into mitochondria and chloroplasts in vivo.
Figure 5.
Transient expression of pCK-MetPS-GFP3 (A–D), RecA–GFP (E), and COXIV–GFP (F) in tobacco cells. A and B, and C and D, respectively, are two views of the same cell. GFP was observed by using a GFP(R)-BP set of filters (A and C), GFP plus chlorophyll fluorescence with a GFP(R)-LP set of filters (B, E, and F), and 4-chloromethyltetramethylrosamine staining of mitochondria with a tetramethylrhodamine B isothiocyanate filter set (D). Examples of plastids (p) and mitochondria (m) are pointed out. (×100; bars = 1 μm.)
DISCUSSION
A cDNA for an A. thaliana MetRS was characterized and was shown to encode a MetRS that is transported into both chloroplasts and mitochondria. These conclusions are based on in vitro import experiments and in vivo expression studies using GFP fusions. Because of the small size of A. thaliana, it was not possible to purify organelle MetRS activities from this plant; however, we could demonstrate that, in good correlation with the data obtained with the A. thaliana cDNA, pea mitochondrial and chloroplast MetRS activities cannot be distinguished on the basis of their chromatographic properties. These results agree with earlier work (32) that demonstrated that chloroplast and mitochondrial MetRS activities from bean (P. vulgaris) cannot be separated by ion exchange or adsorption chromatography by using DEAE-cellulose, hydroxylapatite, or CM-Sepharose columns. This suggests that, in plants other than A. thaliana (namely, pea and bean), there also may be only one gene coding for chloroplast and mitochondrial MetRS.
Correct targeting of precursor proteins to their respective cell compartments is crucial for organelle biogenesis. In contrast to yeast or animal cells, plant cells need to discriminate between the mitochondrial and the chloroplast precursor polypeptides, which are synthesized on cytosolic ribosomes. Precursors of organelle proteins possess transit peptides that specifically recognize sites on the surface of their target organelles. Despite the high specificity of mitochondrial and chloroplast transit peptides, there is no conservation in their primary structure or their length (for reviews, see refs. 36–38). Moreover, chloroplast and mitochondrial transit peptides have very similar amino acid compositions (rich in hydroxylated and basic residues and deficient in acidic residues). It is accepted that the consensus for mitochondrial transit peptides is their ability to form an amphiphilic α-helix whereas chloroplast transit peptides may adopt more predominantly random coil conformations, although this may not be true in all cases. The transit peptide of the MetRS described here is sufficient to target GFP to both types of organelle in vivo; therefore, we can infer that the peptide contains targeting information for chloroplasts and mitochondria. This transit peptide represents a class of “ambiguous targeting signals” having structures allowing them to interact with receptors on the surface of both mitochondria and chloroplasts. Two other plant proteins described so far have transit peptides that belong to this class: the pea glutathione reductase (39) and ferrochelatase I, an enzyme involved in the last step of heme biosynthesis (40). These two proteins were shown to be transported into both chloroplasts and mitochondria. However, there is no obvious similarity between the presequences of these proteins and that of the MetRS.
It has been shown that a chloroplast-like elongator tRNAMet gene is present in the mitochondrial genome of A. thaliana (15, 41), suggesting that the tRNAsMet used in chloroplast and mitochondrial protein synthesis are almost identical. We now demonstrate that the same MetRS is also present in both organelles. A high similarity with the MetRS sequence of Synechocystis, a bacterium belonging to the group of cyanobacteria that are considered to be the ancestors of chloroplasts, strongly suggests that the gene coding for this enzyme is of chloroplast origin. Nonetheless, the presence in mitochondria of tRNAMet and a MetRS of chloroplast origin is not necessarily linked in evolution because there is still a “native” initiator tRNAMet of genuine mitochondrial origin in plant mitochondria (16) and in A. thaliana in particular (42). Presumably, this mitochondrial tRNA has to be aminoacylated by the MetRS of chloroplast origin that we have characterized because we have identified only one peak of MetRS activity in mitochondrial extracts (because MetRS purifications were done with pea organelles, we cannot rule out completely the presence, in A. thaliana, of a MetRS gene of mitochondrial origin that was undetected on our Southern blot). The use of a chloroplast enzyme in mitochondrial translation is made possible because chloroplast and mitochondrial tRNAs and aaRSs are prokaryotic in nature and can work efficiently together in aminoacylation (8, 32). Therefore, the presence of the chloroplast MetRS in mitochondria may simply be caused by its prokaryotic nature, and characterization of more plant genes coding for organelle aaRSs will probably reveal other examples of dually targeted enzymes.
Acknowledgments
We thank Anne Cosset for technical assistance, Ralph Backhaus for proofreading the manuscript, Rainer Köhler for the gift of the coxIV–GFP construct, and Ian Small for the gift of the RecA–GFP construct. We also thank F. Gaire for help with microscopy and J. Gualberto for help with fast protein liquid chromatography purifications. This work was supported by the Centre National de la Recherche Scientifique and by a grant from the Groupement d’Etudes des Génomes.
ABBREVIATIONS
- MetRS
methionyl–tRNA synthetase
- aaRS
aminoacyl–tRNA synthetase
- EST
expressed sequence tag
- GFP
green fluorescent protein
Footnotes
References
- 1.Maréchal-Drouard L, Weil J H, Dietrich A. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:13–32. [Google Scholar]
- 2.Maréchal-Drouard L, Small I, Weil J H, Dietrich A. Methods Enzymol. 1995;260:310–327. doi: 10.1016/0076-6879(95)60148-1. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Maréchal-Drouard L, Akama K, Small I. Mol Gen Genet. 1996;252:404–411. doi: 10.1007/BF02173005. [DOI] [PubMed] [Google Scholar]
- 4.Carias J R, Mouricourt M, Julien R. Biochem Biophys Res Commun. 1981;98:735–742. doi: 10.1016/0006-291x(81)91174-8. [DOI] [PubMed] [Google Scholar]
- 5.Guillemaut P, Steinmetz A, Burkard G, Weil J H. Biochim Biophys Acta. 1975;378:64–72. doi: 10.1016/0005-2787(75)90137-9. [DOI] [PubMed] [Google Scholar]
- 6.Dardel F, Fayat G, Blanquet S. J Bacteriol. 1984;160:1115–1122. doi: 10.1128/jb.160.3.1115-1122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauhut R, Gabius H J, Cramer F. J Biol Chem. 1986;261:2799–2803. [PubMed] [Google Scholar]
- 8.Steinmetz A, Weil J H. Methods Enzymol. 1986;118:212–231. [Google Scholar]
- 9.Dietrich A, Souciet G, Colas B, Weil J H. J Biol Chem. 1983;258:12386–13393. [PubMed] [Google Scholar]
- 10.Dietrich A, Souciet G, Weil J H. J Biol Chem. 1987;262:4248–4251. [PubMed] [Google Scholar]
- 11.Maréchal-Drouard L, Weil J H, Guillemaut P. Nucleic Acids Res. 1988;16:4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green G, Maréchal L, Weil J H, Guillemaut P. Plant Mol Biol. 1987;10:13–19. doi: 10.1007/BF00014182. [DOI] [PubMed] [Google Scholar]
- 13.Mireau H, Lancelin D, Small I. Plant Cell. 1996;8:1027–1039. doi: 10.1105/tpc.8.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich A, Maréchal-Drouard L, Carneiro V, Cosset A, Small I. Plant J. 1996;10:913–918. doi: 10.1046/j.1365-313x.1996.10050913.x. [DOI] [PubMed] [Google Scholar]
- 15.Wintz H, Chen H C, Pillay D T N. Curr Genet. 1988;13:255–260. doi: 10.1007/BF00387772. [DOI] [PubMed] [Google Scholar]
- 16.Maréchal L, Guillemaut P, Grienenberger J M, Jeannin G, Weil J H. Plant Mol Biol. 1986;7:245–253. doi: 10.1007/BF00752898. [DOI] [PubMed] [Google Scholar]
- 17.Maréchal-Drouard L, Guillemaut P, Cosset A, Arbogast M, Weber F, Weil J H, Dietrich A. Nucleic Acids Res. 1990;18:3689–3696. doi: 10.1093/nar/18.13.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joyce P B M, Gray M W. Nucleic Acids Res. 1989;17:5461–5476. doi: 10.1093/nar/17.14.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangare A, Weil J-H, Grienenberger J M, Fauron C, Lonsdale D. Mol Gen Genet. 1990;223:224–232. doi: 10.1007/BF00265058. [DOI] [PubMed] [Google Scholar]
- 20.Robinson C, Barnett L K. In: Plant Molecular Biology: A Practical Approach. Shaw C H, editor. Oxford: IRL Press; 1988. pp. 67–78. [Google Scholar]
- 21.Gualberto J M, Wintz H, Weil J H, Grienenberger J M. Mol Gen Genet. 1988;215:118–127. doi: 10.1007/BF00331312. [DOI] [PubMed] [Google Scholar]
- 22.Newman T, de Buijn F J, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M, et al. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Alessio J M, Bebee R, Hartley J L, Noon M C, Polayes D. Focus (Rochester, NY) 1992;14:76. [Google Scholar]
- 24.Dellaporta S L, Wood J, Hicks J B. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- 25.Whelan J, Knorpp C, Glaser E. Plant Mol Biol. 1990;14:977–982. doi: 10.1007/BF00019394. [DOI] [PubMed] [Google Scholar]
- 26.Carneiro V T C, Dietrich A, Maréchal-Drouard L, Cosset A, Pelletier G, Small I. Plant Mol Biol. 1994;26:1843–1853. doi: 10.1007/BF00019497. [DOI] [PubMed] [Google Scholar]
- 27.Reichel C, Methur J, Eckes P, Langenkemper K, Koncz C, Schell J, Reiss B, Maas C. Proc Natl Acad Sci USA. 1996;93:5888–5893. doi: 10.1073/pnas.93.12.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youvan D C, Michel-Beyerle M E. Nat Biotechnol. 1996;14:1219–1220. doi: 10.1038/nbt1096-1219. [DOI] [PubMed] [Google Scholar]
- 29.Köhler R H, Zipfel W R, Webb W W, Hanson M R. Plant J. 1997;11:613–621. doi: 10.1046/j.1365-313x.1997.11030613.x. [DOI] [PubMed] [Google Scholar]
- 30.Wintz H. Plant Physiol Biochem. 1994;32:649–653. [Google Scholar]
- 31.Russel J A, Roy M K, Sanford J C. In Vitro Cell Dev Biol. 1992;28:97–105. [Google Scholar]
- 32.Guillemaut P, Weil J H. Biochim Biophys Acta. 1975;407:240–248. doi: 10.1016/0005-2787(75)90288-9. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 34.Theg S M, Scott S V. J Biol Chem. 1989;264:6730–6736. [PubMed] [Google Scholar]
- 35.Köhler R H, Cao J, Zipfel W R, Webb W W, Hanson M R. Science. 1997;276:2039–2042. doi: 10.1126/science.276.5321.2039. [DOI] [PubMed] [Google Scholar]
- 36.Whelan J, Glaser E. Plant Mol Biol. 1997;33:771–789. doi: 10.1023/a:1005755505738. [DOI] [PubMed] [Google Scholar]
- 37.Lübeck J, Heins L, Soll J. Physiol Plant. 1997;100:53–64. [Google Scholar]
- 38.Moore A L, Wood C K, Watts F Z. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:545–575. [Google Scholar]
- 39.Creissen G, Reynolds H, Xue Y B, Mullineaux P. Plant J. 1995;8:167–175. doi: 10.1046/j.1365-313x.1995.08020167.x. [DOI] [PubMed] [Google Scholar]
- 40.Chow K S, Signh P, Roper J, Smith A. J Biol Chem. 1997;272:27565–27571. doi: 10.1074/jbc.272.44.27565. [DOI] [PubMed] [Google Scholar]
- 41.Chen H C, Wintz H, Weil J H, Pillay D T N. Nucleic Acids Res. 1988;16:10372. doi: 10.1093/nar/16.21.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unseld M, Marienfeld J, Brandt P, Brennicke A. Nat Genet. 1997;15:57–61. doi: 10.1038/ng0197-57. [DOI] [PubMed] [Google Scholar]